Abstract
The effects of highly purified natural porcine cholecystokinin (CCK) and synthetic caerulein on the rate of flow of pancreatic juice, the rate of output of amylase, and the rate of release of immunoreactive insulin (IRI) and immunoreactive glucagon (IRG) were simultaneously investigated in the isolated perfused rat pancreas.
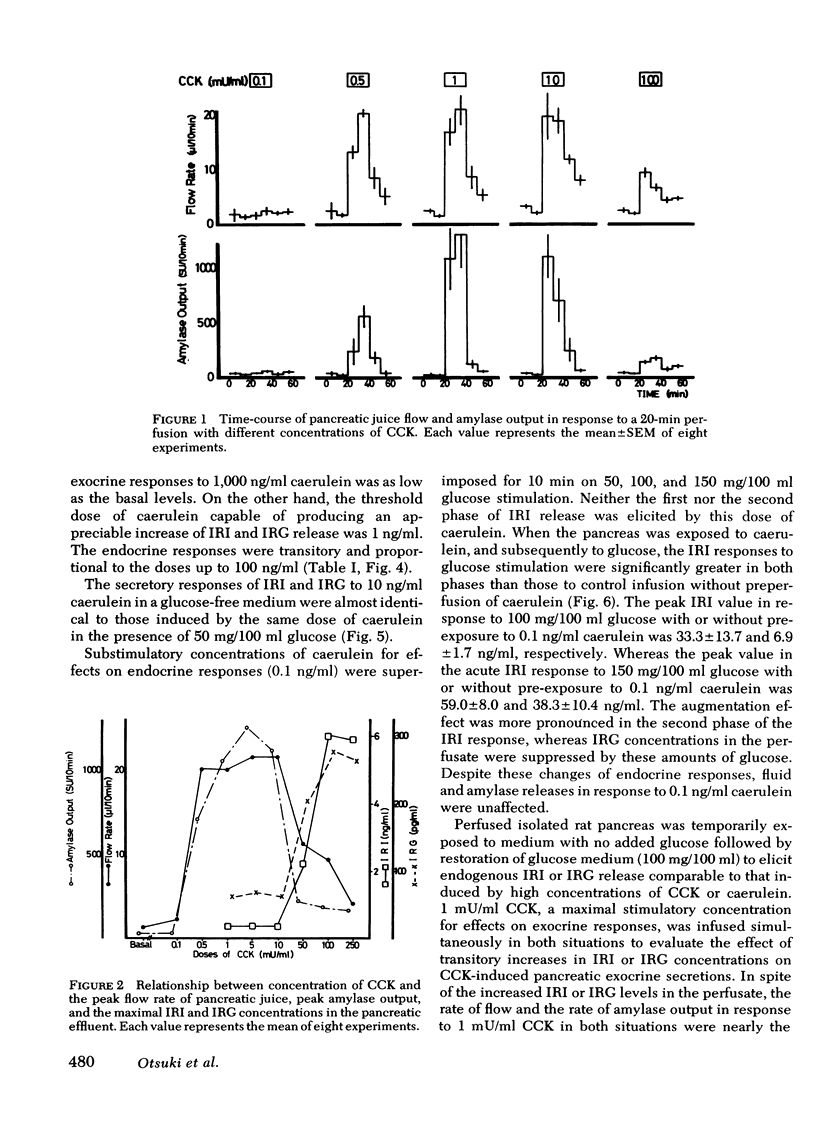
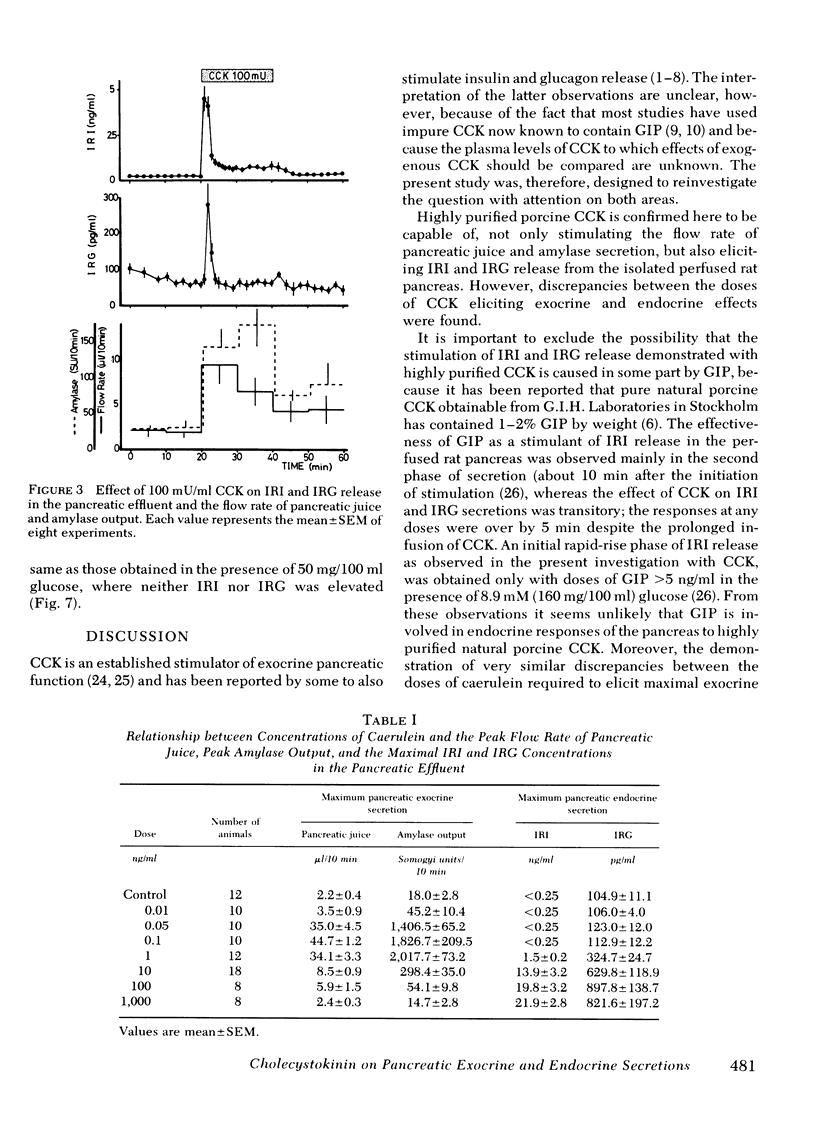
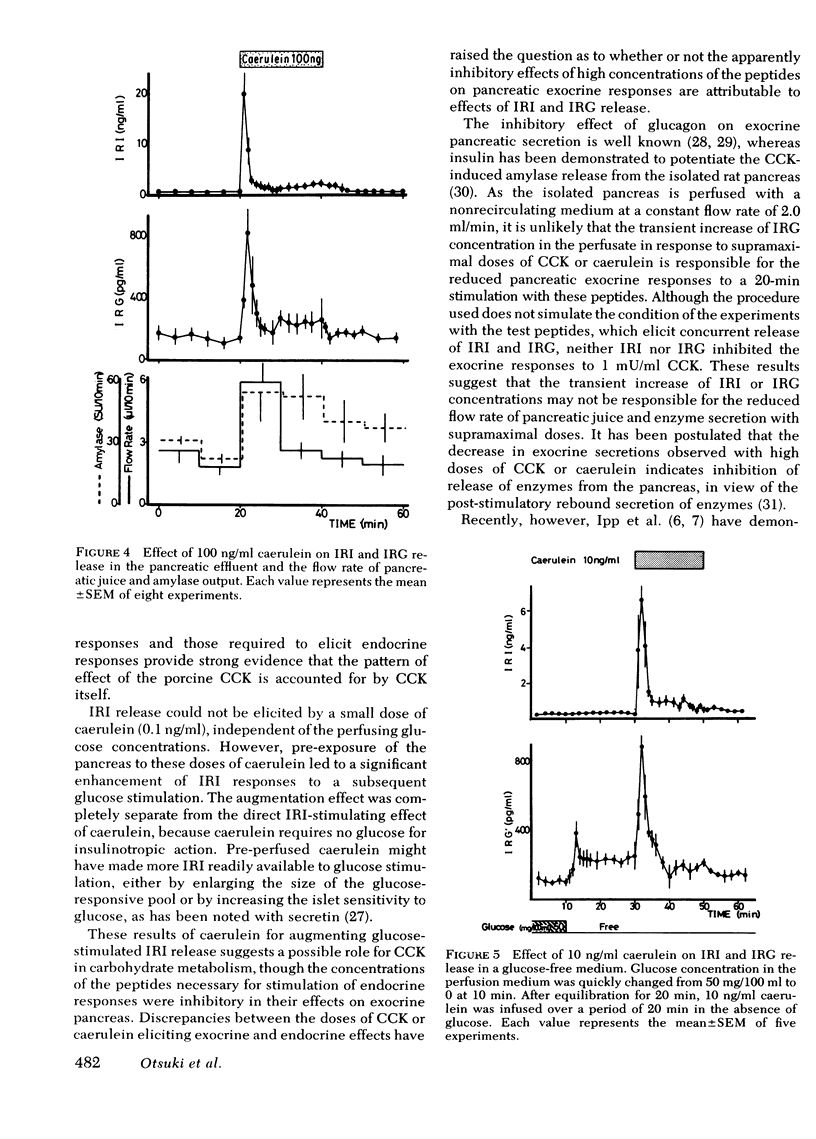
The maximal flow rate of pancreatic juice was obtained with concentrations of CCK ranging from 0.5 to 10 mU/ml, whereas amylase output was maximal at CCK concentrations from 1 to 10 mU/ml. Caerulein at concentrations of 0.05-1 ng/ml induced a similar maximal flow rate and amylase secretion. Supramaximal stimulatory concentrations of these peptides resulted in lower rates of release of fluid and amylase than with the maximally effective concentrations. Stimulation of IRI and IRG release was elicited only with concentrations of peptides supramaximal for effects on the exocrine responses.
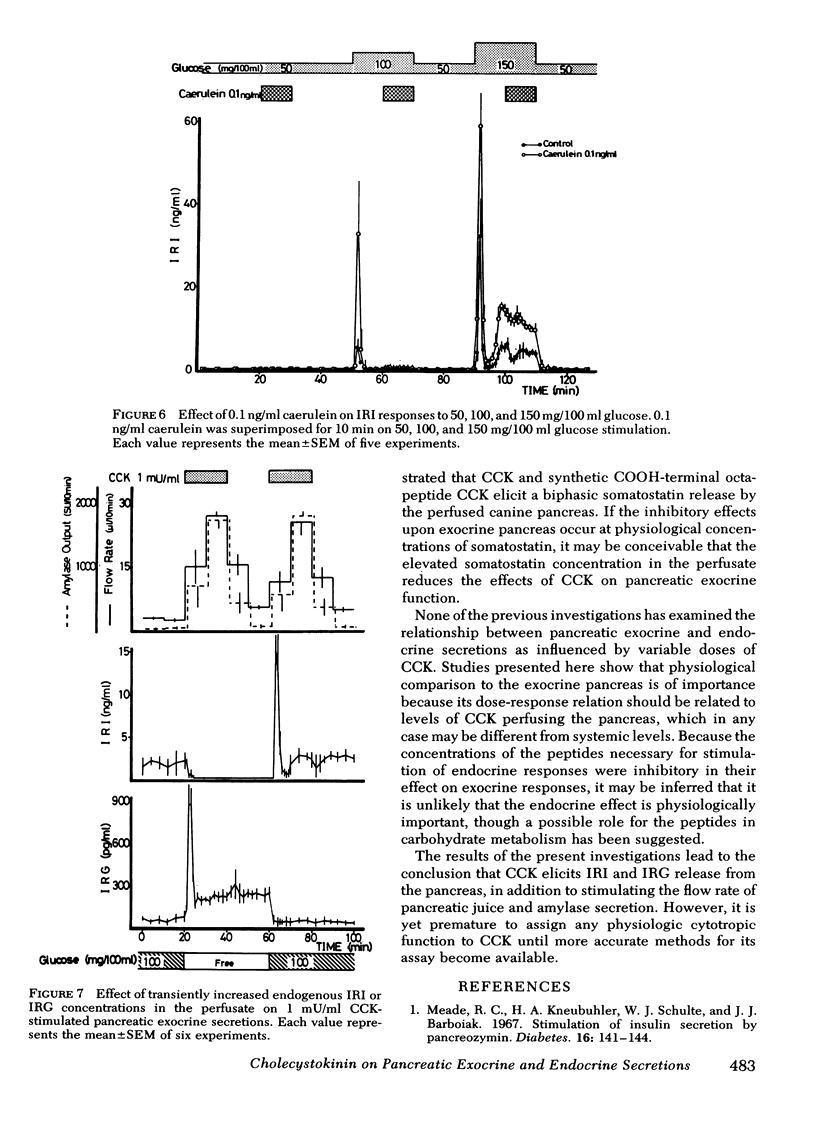
The demonstration of very similar discrepancies between the doses of caerulein required to elicit maximal exocrine responses and those required to elicit endocrine responses provide strong evidence that the pattern of the effect of the porcine CCK is accounted for by CCK itself.
Although caerulein had no influence on IRI response when superimposed on 100 or 150 mg/100 ml glucose stimulation, preperfusion of caerulein led to a significant enhancement of IRI response to a subsequent glucose stimulation in both phases. The augmentation effect was completely separate from the direct IRI-stimulating effect of caerulein, because the CCK-like peptide requires no glucose for insulinotropic action.
Because the concentrations of the peptides necessary for stimulation of endocrine responses were inhibitory in their effects on exocrine responses, it may be inferred that it is unlikely that the endocrine effect is physiologically important, though the results of caerulein for augmenting glucose-stimulated IRI release suggests a possible role for CCK in carbohydrate metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Bertaccini G., De Caro G., Endean R., Erspamer V., Impicciatore M. The action of caerulein on pancreatic secretion of the dog and biliary secretion of the dog and the rat. Br J Pharmacol. 1969 Sep;37(1):185–197. doi: 10.1111/j.1476-5381.1969.tb09537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini G., De Caro G., Endean R., Erspamer V., Impicciatore M. The actions of caerulein on the smooth muscle of the gastrointestinal tract and the gall bladder. Br J Pharmacol. 1968 Oct;34(2):291–310. doi: 10.1111/j.1476-5381.1968.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini G., Endean R., Erspamer V., Impicciatore M. The actions of caerulein on gastric secretion of the dog and the rat. Br J Pharmacol. 1968 Oct;34(2):311–329. doi: 10.1111/j.1476-5381.1968.tb07053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyns D. R., Jarrett R. J., Keen H. Intestinal hormones and plasma insulin: an insulinotropic action of secretin. Br Med J. 1967 Jun 10;2(5553):676–678. doi: 10.1136/bmj.2.5553.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Morgan A., Williams R. H. Effect of pancreozymin on insulin and glucagon levels in blood and bile. Am J Physiol. 1968 Dec;215(6):1293–1298. doi: 10.1152/ajplegacy.1968.215.6.1293. [DOI] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Dupre J., Curtis J. D., Unger R. H., Waddell R. W., Beck J. C. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969 Apr;48(4):745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- Dupré J., Beck J. C. Stimulation of release of insulin by an extract of intestinal mucosa. Diabetes. 1966 Aug;15(8):555–559. doi: 10.2337/diab.15.8.555. [DOI] [PubMed] [Google Scholar]
- Dyck W. P., Rudick J., Hoexter B., Janowitz H. D. Influence of glucagon on pancreatic exocrine secretion. Gastroenterology. 1969 Mar;56(3):531–537. [PubMed] [Google Scholar]
- Fontana G., Costa P. L., Tessari R., Labo G. Effect of glucagon on pure human exocrine pancretic secretion. Am J Gastroenterol. 1975 Jun;63(6):490–494. [PubMed] [Google Scholar]
- Frame C. M., Davidson M. B., Sturdevant R. A. Effects of the octapeptide of cholecystokinin on insulin and glucagon secretion in the dog. Endocrinology. 1975 Sep;97(3):549–553. doi: 10.1210/endo-97-3-549. [DOI] [PubMed] [Google Scholar]
- Fussgänger R. D., Straub K., Goberna R., Jaros P., Schröder K. E., Raptis S., Pfeiffer E. F. Primary secretion of insulin and secondary release of glucagon from the isolated perfused rat pancreas following stimulation with pancreozymin. Horm Metab Res. 1969 Jul;1(4):224–227. doi: 10.1055/s-0028-1095139. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner P., Persson G., Ursing D. Insulin release in fasting man induced by impure but not by pure preparations of cholecystokinin. Acta Med Scand. 1975 Jan-Feb;197(1-2):109–112. doi: 10.1111/j.0954-6820.1975.tb04886.x. [DOI] [PubMed] [Google Scholar]
- IVY A. C., JANECEK H. M. Assay of Jorpes-Mutt secretin and cholecystokinin. Acta Physiol Scand. 1959 Mar 31;45:220–230. doi: 10.1111/j.1748-1716.1959.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Ipp E., Dobbs R. E., Arimura A., Vale W., Harris V., Unger R. H. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. J Clin Invest. 1977 Sep;60(3):760–765. doi: 10.1172/JCI108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipp E., Dobbs R. E., Harris V., Arimura A., Vale W., Unger R. H. The effects of gastrin, gastric inhibitory polypeptide, secretin, and the octapeptide of cholecystokinin upon immunoreactive somatostatin release by the perfused canine pancreas. J Clin Invest. 1977 Nov;60(5):1216–1219. doi: 10.1172/JCI108875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen J. Secretion of glucagon from the isolated, perfused canine pancreas. J Clin Invest. 1971 Oct;50(10):2123–2136. doi: 10.1172/JCI106706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol. 1976 Oct;261(3):505–521. doi: 10.1113/jphysiol.1976.sp011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. L. The augmentation effects of secretin on the insulin responses to known stimuli: specificity for glucose. J Clin Endocrinol Metab. 1977 Jul;45(1):1–9. doi: 10.1210/jcem-45-1-1. [DOI] [PubMed] [Google Scholar]
- Meade R. C., Kneubuhler H. A., Schulte W. J., Barboriak J. J. Stimulation of insulin secretion by pancreozymin. Diabetes. 1967 Mar;16(3):141–144. doi: 10.2337/diab.16.3.141. [DOI] [PubMed] [Google Scholar]
- Pederson R. A., Brown J. C. The insulinotropic action of gastric inhibitory polypeptide in the perfused isolated rat pancreas. Endocrinology. 1976 Sep;99(3):780–785. doi: 10.1210/endo-99-3-780. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Dupré J. Effects of the gastric inhibitory polypeptide present in impure pancreozymin-cholecystokinin on plasma insulin and glucagon in the rat. Endocrinology. 1974 Apr;94(4):1139–1144. doi: 10.1210/endo-94-4-1139. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormsley K. G. A comparison of the response to secretin, pancreozymin and a combination of these hormones, in man. Scand J Gastroenterol. 1969;4(5):411–417. [PubMed] [Google Scholar]


