Abstract
Neuroprotective and neurorescue effects after neural stem/precursor cell (NPC) transplantation have been reported, but the mechanisms underlying such phenomena are not well understood. Our recent findings in a rat Parkinson’s disease (PD) model indicate that transplantation of NPCs before a 6-hydroxydopamine (6-OHDA) insult can result in nigrostriatal protection which is associated with endogenous NPC proliferation, migration and neurogenesis. Here, we sought to determine whether the observed endogenous NPC response (1) contributes to transplanted NPC - mediated neuroprotection and/or (2) affects graft phenotype and function. Host Fischer 344 rats were administered the antimitotic agent cytosine-β-D-arabinofuranoside (Ara-C) to eliminate actively proliferating endogenous neural precursors before being transplanted with NPCs and treated with 6-OHDA to induce nigrostriatal degeneration. Behavioral and histological analyses demonstrate that the neuroprotective response observed in NPC transplanted animals which had not received Ara-C was significantly attenuated in animals which did receive pre-transplant Ara-C. Also, while grafts in Ara-C treated animals showed no decrease in cell number, they exhibited significantly reduced expression of the neural stem cell regulators nestin and sonic hedgehog. In addition, inhibition of the endogenous NPC response resulted in an exaggerated host glial reaction. Overall, the study establishes for the first time that endogenous NPCs contribute to transplanted NPC-mediated therapeutic effects by affecting both grafted and mature host cells in unique ways. Thus, both endogenous and transplanted NPCs are important in creating an environment suitable for neural protection and rescue, and harnessing their synergistic interaction may lead to the optimization of cell-based therapies for PD.
Keywords: Neural precursor cells, Parkinson’s disease, Neurogenesis, Transplantation, Subventricular zone
INTRODUCTION
Neural stem cell based strategies focusing on either endogenous (resident) or exogenous (grafted) NPCs have shown potential to regenerate and repair the central nervous system (CNS) in a variety of animal models of brain injury and neurodegeneration. In Parkinson’s disease (PD) models, it has been reported that transplanted NPCs can integrate, differentiate, express plasticity-inducing factors and produce functional effects (Studer et al., 1998; Ourednik et al., 2002; Yasuhara et al., 2006; Madhavan et al., 2009). Endogenous NPCs recruited through growth factor infusion in parkinsonian environments have also been demonstrated to differentiate into and rescue injured dopamine neurons stimulating improvement in motor behavior (Fallon et al., 2000; Cooper & Isacson, 2004; Androutsellis-Theotokis et al., 2009). Nevertheless, inherent problems with both these stem cell - based approaches such as poor survival and stable differentiation of NPCs after grafting, and inability to stimulate a therapeutically significant endogenous NPC response have to date prevented optimal clinical applications (Madhavan & Collier, 2010).
Studies, including those from our laboratory, indicate that transplanted stem cells interact with endogenous stem cells. In particular, it has been shown that transplantation of stem cells, including NPCs, into the naive, pathologic, or aged brain can result in stimulation of endogenous NPCs, neurogenesis, and functional effects (Mahmood et al., 2004; Taguchi et al., 2004; Munoz et al., 2005; Cova et al., 2010; Kan et al., 2010; van Velthoven et al., 2010). Naturally in light of these studies, it has been proposed that endogenous NPCs plausibly contribute to transplant-induced therapeutic effects, but this proposition has not been confirmed to date. Endogenous neural precursors are part of the ‘niche’ or local environment of transplanted stem cells, which is critical in determining grafted cell behavior and fate (Doetsch, 2003; Riquelme et al., 2008). The abovementioned studies emphasize the importance of ‘niche’ effects on transplantation, and argue that niche interactions in the form of reciprocal influences between grafted stem cells and endogenous neural precursors could be important for the observed neuroprotection and regeneration effects observed in several pathological CNS scenarios.
We have recently reported that the phenomenon of ‘transplantation-induced neurogenesis’ occurs in a rat PD model in association with nigrostriatal neuroprotection (Madhavan et al., 2009). The present study follows up on this report, and explores the mechanisms underlying NPC-mediated neuroprotection. Particularly, the study examines whether stimulation of endogenous neural precursors following NPC grafting contributes to dopamine system neuroprotection, and graft survival, differentiation, and function. We postulated that a reduction in endogenous NPC numbers would alter exogenous NPC behavior and affect their ability to protect the nigrostriatal system. Host NPC proliferation was inhibited by infusion of Ara-C (mitotic inhibitor that eliminates f a s t proliferating endogenous precursors) (Doetsch et al., 1999), and consequences of the loss of endogenous precursors on grafted cells and the host nigrostriatal system were investigated. Analyses indicate that endogenous NPCs do in fact contribute to transplantation-induced therapeutic effects by influencing donor and mature host cell function in multiple ways.
MATERIALS AND METHODS
NPC culture:NPCs were isolated from subventricular zones (SVZs) of newborn human placental alkaline phosphatase (hPAP) transgenic Fischer 344 rats (Madhavan et al, 2009). They express hPAP in a stable manner in culture and when transplanted in vivo (Han et al., 2002; Mujtaba et al., 2002). The NPCs were grown under standard conditions in uncoated dishes and serum-free neurobasal-A medium (Invitrogen, Carlsbad, CA) supplemented with 2% B27 (Invitrogen, Carlsbad, CA), 20 ng/ml epidermal growth factor (EGF, Cell Sciences, Canton, MA), 10 ng/ml basic fibroblast growth factor (bFGF, Cell Sciences, Canton, MA), 5 ug/ml heparin (Stemcell Technologies, Vancouver, BC, Canada), 500 μm glutamax, 200 units/ml penicillin, and 200 μg/ml streptomycin (Invitrogen, Carlsbad, CA), at 37°C (Madhavan et al., 2009).
Animals and Surgery
The rats were housed and treated according to the rules and regulations of NIH and Institutional Guidelines on the Care and Use of Animals. University of Cincinnati Institutional Animal Care and Use Committee approved all experimental procedures.
The hPAP transgenic rat colony (from which the NPCs were isolated) was housed at the laboratory animal medical services (LAMS) facility at University of Cincinnati.
Experimental subjects were male Fischer 344 rats (250 - 275g, Harlan, Indianapolis, IN) which were maintained on a reverse 12 hour light-dark cycle with ad libitum food and water. The number of animals in each of the experimental groups were as follows: Longer term NPC transplantation experiment (results depicted in figure 1): NPCs + 6-OHDA, 2 wks (n=8); NPCs + 6-OHDA, 7 wks (n=8); Hanks balanced salt solution (HBSS) + 6-OHDA, 2 wks (n=8); HBSS + 6-OHDA, 7 wks (n=7); HBSS only, 2 wks (n=8), HBSS only, 7 wks (n=8). Ara-C Experiment: 0.9% Saline + NPCs + 6-OHDA (n=15); Ara-C + NPCs + 6-OHDA (n=14); 0.9% Saline + 6-OHDA (n=8); NPCs + 6-OHDA (n=8); 0.9% Saline only (n=5); Ara-C only (n=8).
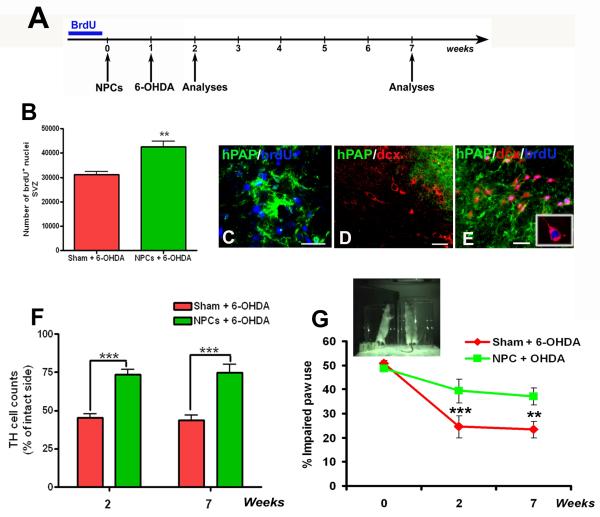
Figure 1.
Transplanted NPC mediated nigrostriatal protection and endogenous precursor activation
Schematic showing the timeline for the ‘long-term NPC transplantation experiment’ (A). After 3 days of brdU adminstration, 100,000 hPAP+ NPCs were transplanted into the striatum and SN of host rats. One week later 4μg/μL of 6-OHDA was infused into the MFB, and animals were analyzed behaviorally and histologically at 2 weeks and 7 weeks post transplantation. As reported previously (Madhavan et al, 2009), at 2 weeks after implantation, stereological counts through the dorsolateral SVZ confirmed the presence of a greater number of brdU nuclei in NPC transplanted animals compared to controls (B). Also, brdU labeled endogenous cells were found adjacent to grafted cells (C), many of which were dcx-positive but hPAP-negative suggesting stimulation of host neurogenesis (D, E). We found significantly (p<0.001) increased preservation of TH expressing dopamine neurons in the SN of NPC transplanted animals through stereological estimation, at both 2 and 7 weeks after transplantation (F, raw cell counts presented in the results section). Also behaviorally, the NPC transplanted rats performed significantly (p<0.05) better in the spontaneous paw placement cylinder task as compared to non-transplanted controls (image and graph in G) at both time intervals. [**p<0.05, ***p<0.001, one way ANOVA for both F and G]. Scale bars: C, D, E – 20 μm
AraC infusions
Cytosine-β-D-arabinofuranoside (Ara-C, 2%; Sigma-Aldrich, St. Louis, MS, U.S.A.), a mitotic inhibitor that eliminates fast proliferating endogenous precursors in vehicle (0.9% saline) was continuously infused onto the surface of rat brains for 10 days with a mini-osmotic pump (model 2002; Alzet, Palo Alto, CA, U.S.A.) at rate of 0.5 μL/h, according to guidelines provided in Doetsch et al. (1999). Cannulas were implanted on the brain surface at AP 0, ML 1, and DV −1.1 mm relative to bregma and surface of the brain. Ara-C infusion was ceased 24 hours prior to NPC transplantation.
NPC transplants
Infusion of undifferentiated hPAP+ NPC’s (50,000 cells/μL of HBSS, 2 μL/site) in the striatum (AP +0.2, ML +3, DV −5.5 from bregma) and substantia nigra (AP −5.3, ML +2.5, DV −7 from bregma) in order to provide NPC influence at both the source and target structure of the relevant nigrostriatal system was conducted as described previously (Madhavan et al., 2009) [see Fig 4B, C for transplant locations]. NPC transplantation from hPAP rats does not require immune suppression as these rats are raised on the same Fischer 344 background as the host rats (Han et al., 2002; Mujtaba et al., 2002).
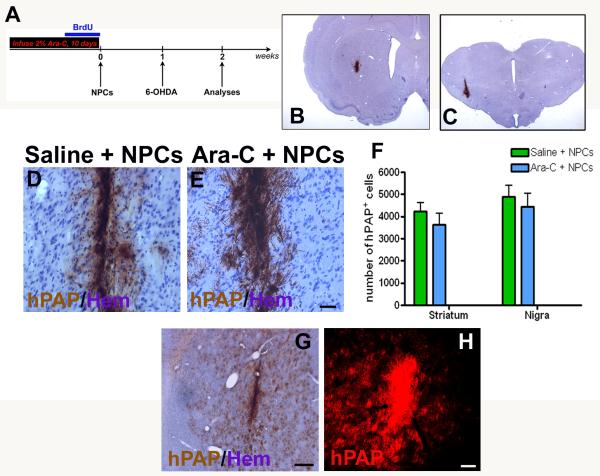
Figure 4.
Effects of Ara-C induced inhibition on grafted NPC survival and migration
Two weeks after NPC transplantation (timeline in A) we assessed graft survival and migration in both the striatum and nigra (B, C). Grafts in both the Ara-C (E) and no Ara-C (D) treated rats appeared healthy. Grafted cell counts of hPAP+ cells counterstained with hematoxylin showed that transplanted NPC survival was somewhat better in the nigral region as compared with the striatum (F). Also, animals receiving Ara-C showed generally lower cell counts than those not infused with Ara-C although not significantly. In terms of graft migration, transplanted cells in animals which did not receive Ara-C seemed restricted to the injection site with minimal spread (D). However, in animals which were infused with Ara-C prior to transplantation grafts appeared more spread out with irregular borders (E). Also, in three Ara-C infused animals a particularly widespread migration of hPAP - NPCs in the striatum was observed (G, H). Scale bars: D, E, H – 100 μm, G – 500 μm
6-OHDA lesions
1 μl of 6-OHDA (4μg/μl) in a 0.2% solution of ascorbic acid in saline was infused into the median forebrain bundle (MFB, AP −4, ML +1.2, DV −7.5) at 1 week after NPC transplantation as described previously (Madhavan et al., 2009). Infusion of 6-OHDA into the MFB results in bilateral neural degeneration towards both the striatum and nigra. In our model, the choice of this particular site of injection had the advantage that the toxin would not be in direct proximity to grafted cells that had previously been implanted in the striatum and substantia nigra (Madhavan et al, 2009).
At the prescribed time points after NPC grafting, animals were sacrificed via barbiturate overdose, perfused with 4% paraformaldehyde (PFA), extracted brains post-fixed in 4% PFA solution, and sectioned (40 μm) on a freezing sliding microtome for morphological studies.
BrdU administration
Bromodeoxyuridine (brdU), the thymidine analog that is incorporated into the DNA of dividing cells during S-phase, was used to label proliferating endogenous NPCs. Rats were injected intraperitoneally (i.p) with brdU (50 mg/kg/12 hrs) for 3 days before transplantation. Administration of brdU before transplantation labels pre-existing naïve resident NPCs present in the germinal niches (SVZ and dentate gyrus of hippocampus), which later respond to ensuing NPC transplantation and 6-OHDA application.
Experimental paradigm
For the Longer term NPC transplantation experiment, (timeline in Figure 1A) NPCs were transplanted 1 week before 6-OHDA injections, and the animals were sacrificed at either 2 weeks or 7 weeks after grafting. For the Ara-C experiment, (timeline in Figure 3A) the animals were infused with 2% Ara-C for 10 days, prior to the NPC transplantation and 6-OHDA application. Ara-C infusion during this time-period would mainly affect proliferating cells in the germinal zones, with no direct effect expected on the to-be implanted NPCs or the parenchymal cell response to grafting. NPC transplantation occurred 24 hours after the Ara-C infusion was stopped to allow a ‘wash out’ period for systemic elimination of Ara-C. Ara-C bioavailability after administration has been documented to be less than six hours (Groothuis et al., 2000). The animals were subsequently sacrificed at 2 weeks after grafting.
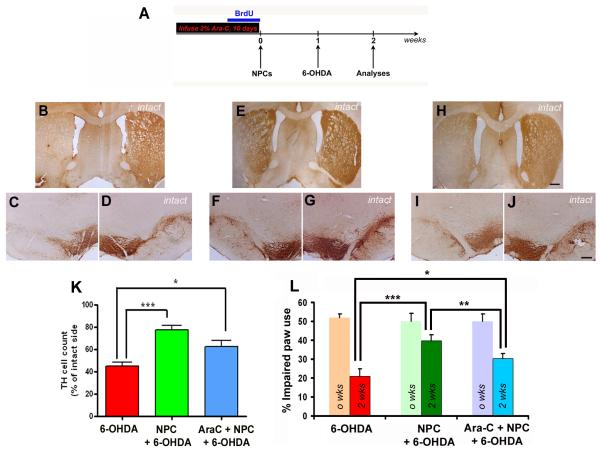
Figure 3.
Effects of Ara-C induced endogenous NPC inhibition on nigrostriatal neuroprotection
Two weeks after transplantation (schema in A), in 6-OHDA treated control animals which had not received any Ara-C or NPCs, we observed a significant loss of TH immunoreactivity in the striatum (B) and SN (C) on the ipsilateral side. In contrast, animals which had received NPC transplants prior to 6-OHDA but no Ara-C displayed increased preservation of TH immunoreactivity in both the striatum (E) and SN (F) as observed in our previous study. The animals which had been given both Ara-C and NPCs prior to 6-OHDA showed greater loss of TH in the striatum (H) and in the SN (I) when compared the transplanted animals not infused with Ara-C. Behavioral analysis using spontaneous paw placement in the cylinder task (L), NPC transplanted animals which had received no Ara-C performed significantly (p <0.001) better than 6-OHDA controls at 2 weeks as shown before. Ara-C treated transplanted animals on the other hand performed significantly (p<0.05) better than the 6-OHDA controls, but also significantly worse than the no Ara-C treatment transplanted animals (p<0.05). The lighter colored bars on the graph show the baseline behavior scores before grafting. Data expressed have been calculated by using the formula (total ipsilateral limb use + 1/2 bilateral) / total limb use × 100. [*p<0.05, ***p<0.001, two way RM-ANOVA]. Stereological counts of TH neurons in the SN (K) supported these behavioral observations, and showed that Ara-C treated transplanted rats possessed significantly (p<0.05) greater number of TH neurons than 6-OHDA controls, but strongly lower numbers (though not significant in this case, p=0.059) than NPC transplanted animals receiving no Ara-C. [*p<0.05, ***p<0.001, one way ANOVA]. Scale bar: 500 μm
In terms of the relationship between the timing of NPC transplantation and 6-OHDA infusion, NPCs were transplanted before the 6-OHDA insult in order to study their neuroprotective potential particularly with respect to ongoing degenerative processes still at work in the parkinsonian brain. Since the time course of 6-OHDA degeneration is quite short, the only dependable way to examine NPC effects during degeneration was to have the cells present prior to the 6-OHDA application.
Behavioral analysis
Rats were evaluated for spontaneous forepaw use through the cylinder task (Schallert et al., 2000). Briefly, each rat was placed in a clear plexiglass cylinder and the number of forepaw contacts with the cylinder walls during exploration was recorded for each paw during a 5 minute period. Analyses were run during the rat’s dark cycle to enhance spontaneous exploratory behaviors. The forelimb asymmetry score was calculated using the formula: (total ipsilateral limb use + 1/2 bilateral) / total limb use × 100.
Immunohistochemistry
Sections were blocked [(10% goat serum, 0.5% triton-X in tris buffered saline (TBS)] and incubated with the primary antibody overnight at room temperature (RT) or at 4°C for 48 hrs. Primary antibodies were detected in a 2-hr-incubation at RT with secondary antibodies (1:250) coupled to the fluorochromes Alexa Fluor 488, 555, 594, or 647 (Invitrogen-probes, Carlsbad, CA) for immunofluorescence. Alternatively, 3,3′ diaminobenzidine (DAB) or vector blue-staining with biotinylated secondaries and ABC peroxidase kit (Vector labs, Burlingame, CA) was performed. Primary antibodies used were as follows: BrdU (1:200), PSA-NCAM (1:50), Nestin (1:200), RIP (1:500), TH (1:4000), GFAP (1:500) all from Millipore-Chemicon, Danvers, MA; Tuj1 (1:300) from Covance, Princeton, NJ; GDNF (1:500), SHH (1:200), Dcx (1:400) from Santacruz Biotechnology, Santacruz, CA; hPAP (1:500) from Accurate, Westbury, NY; S100β (1:500) from Sigma, St Louis, MO; SDF1α (1:250) from Abcam; CD11b (1:50) and CD68 (1:50) from Serotec, Raleigh, NC. Primary antibody replaced with matched serum or non-immune IgG1 isotype specific controls and secondary antibody deleted controls were performed. All antibodies (except for CD11b and CD68) have been utilized previously and their properties documented in detail in Madhavan et al, 2009 in the antibody characterization subdivision within the Methods section. Details pertaining to CD11b and CD68 antibodies have been provided below.
CD11b (clone OX-42; ABD Serotec, Raleigh, NC):This is a monoclonal antibody of mouse origin and targets rat tissue. It specifically recognizes the equivalent of the human CD11b receptor for the iC3b component of the complement. The antibody (purified IgG) was prepared by affinity chromatography on protein G from tissue culture supernatant, and rat peritoneal macrophages were used as the immunogen.
CD68 (clone ED-1; ABD Serotec, Raleigh, NC):CD68 is a mouse monoclonal antibody targeting rat tissue. It was prepared as a purified IgG by affinity chromatography on Protein A from tissue culture supernatant, with rat spleen cells as the immunogen. In terms of specificity it recognizes an antigen (homologue of human CD68), which is a single chain glycoprotein of 110kD that is expressed predominantly on the lysosomal membrane of myeloid cells, and also by a majority of tissue macrophages.
Stereology and Cell counts
Stereology
Stereological probes were applied using a BX52 Olympus microscope (Olympus America Inc.) equipped with Microbrightfield stereological software and a Microfire CCD camera (Optronics, Goleta, CA) by the optical fractionator method according previously published methods (Madhavan et al., 2009). Briefly, the substantia nigra and SVZ contours were drawn at low magnification (1.25x), and a systematic sample of the region of interest was made from a random starting point. Once section thickness was determined, guard zones were set (2μm at the very top and very bottom of the section that are not included in the counting area) which prevent double counting of cells. Cells were counted under the 60X oil immersion objective by lowering the counting frame through the tissue at 1-2 μm intervals and marking each cell that is counted. TH cells were counted in sections 480 μm apart using a grid size of 170 × 100 μm and counting frame size of 50 × 50 μm. For brdU, counts were conducted through the dorsolateral SVZ in sections at 480 μm intervals between the genu of the corpus callosum and anterior commissure crossing as outlined by Gotts and Chesselet (2005). The grid size used was 100 × 100 μm and the counting frame was 75 × 75 μm. The Gundersen method for calculating the coefficient of error was used to estimate the accuracy of the optical fractionator results. Co-efficients were generally less than 0.1.
Cell counts
Cell counts for estimating the number of Tuj1, S100ß, nestin, sonic hedgehog (SHH), glial cell line derived neurotrophic factor (GDNF) and stromal derived factor 1 alpha (SDF1α) cells, among the hPAP expressing cells within NPC grafts were conducted using confocal microscopy. Utilizing a 63X lens, 8 regions (4 in graft center, and 4 in the graft periphery) were evaluated in 3 adjacent sections containing grafted cells in each animal. Grafted cells in both the striatum and substantia nigra were counted in 5 representative animals per group. Data was expressed as mean ± SEM of percent of hPAP+ cells expressing either Tuj1, S100ß, nestin, SHH, GDNF and SDF1α.
CD11b, CD68 and GFAP expressing cells adjacent to the hPAP positive grafts were quantified using confocal microscopy. In 3 adjacent sections containing grafted cells in each animal, 4 regions in the graft periphery were evaluated under a 100X lens. Cells were quantified in five representative animals per group, and the data was expressed as mean ± SEM of number of cells/section.
Graft survival was estimated semi-quantitatively. Counts of hPAP+ grafted cells were performed using Microbrightfield’s Stereoinvestigator software but without the optical fractionator. Under a 60X lens, 8 regions (4 in graft center, and 4 in the graft periphery) were evaluated in 3 adjacent sections containing grafted cells in each animal. hPAP expressing transplanted cells in both the striatum and nigra were counted in 5 animals in Ara-C treated and untreated groups. Data was expressed as mean ± SEM of total hPAP+ cells per animal.
Microscopy
Sections were analyzed on an OlympusBX60 microscope with a NikonDXM1200 digital camera (non-fluorescent) or a Zeiss Axioplan 2 microscope with an Orca-ER cooled, B&W CCD camera (fluorescent). Confocal imaging (Zeiss LSM510 with Zeiss LSM software) and Z sectioning was performed at 1-2 μm intervals in order to verify co-localization of markers. Images were acquired and reconstructed using the Zeiss LSM software.
Statistical analyses
Sigmaplot 11 and Graphpad prism 5 software were used for statistical analyses. For comparisons between 6-OHDA, Ara-C treated and non-Ara-C treated groups when analyzing the stereological TH nigral cell counts, one way analysis of variance (ANOVA) was performed. This was followed by Dunnett’s post hoc test for multiple comparisons with the control, or Bonferoni’s test for multiple comparisons between treatment groups. For analysis of number of hPAP+ grafted cells expressing either differentiation markers (Tuj1, S100ß, nestin), growth factors (GDNF, SHH, SDF1α), or glial markers (CD11b, CD68, GFAP), one way ANOVA followed by Bonferroni’s post-hoc test was conducted. With respect to the grafted hPAP cell counts, one way ANOVA followed by Tukey’s test was used. Two-way repeated measure ANOVAs (RM-ANOVA) followed by a Tukey’s post-hoc test were used to analyze and determine significant differences between groups for spontaneous paw placement in cylinder task. For comparisons of brdU cell counts in the SVZ between Ara-C treated and non-Ara-C treated groups, unpaired t-test with Welch’s correction was performed. Differences were accepted as significant at p < 0.05.
RESULTS
Neural precursor cell transplantation induces an endogenous precursor response and sustained nigrostriatal protection
In our previous study, we reported that transplantation of hPAP expressing NPCs prior to a 6-OHDA insult induced endogenous subventricular zone (SVZ) neurogenesis and nigrostriatal neuroprotection, at 2 weeks following transplantation (Madhavan et al., 2009). In order to investigate whether the observed neuroprotection (1) had behavioral consequences, and (2) was stable over an extended period of time, the present study examined animals both at 2 and 7 weeks after transplantation (timeline in Fig 1A).
As reported previously (Madhavan et al., 2009), at 2 weeks after implantation, stereological counts through the dorsolateral SVZ confirmed the presence of a greater number of bromodeoxyuridine (brdU, used to label endogenous NPCs) nuclei in NPC transplanted animals compared to controls (Fig 1B, p<0.01, t=4.275, df=9, unpaired t-test with Welch’s correction). BrdU+ precursors, many of which were also positive for doublecortin (dcx, marker of migrating neuroblasts) but hPAP negative (not of graft origin), were also detected in the region around the grafted NPCs, indicating that host neurogenesis and migration had occurred (C, D, image and inset in E).
The endogenous NPC activation events outlined above were associated with protection of TH+ cells in the SN (stereological cell counts in Fig 1F, p<0.001, F2, 28 =20.64). It was determined that NPC transplanted animals had an average of 11,224.7 ± 786.7 TH+ cells in the lesioned hemisphere (15,308.7 ± 968.3 cells in the non-lesioned hemisphere) when compared to saline infused controls which had only 6,896.0 ± 738.4 TH+ cells in the lesioned hemisphere (15,078.0 ± 1,276.2 in the non-lesioned hemisphere). Behaviorally, the NPC transplanted animals exhibited significantly less forelimb akinesia in the cylinder task when compared to saline controls (Fig 1G, p<0.001, F1, 14 =7.84). Further, neuroprotection of TH+ neurons, both at the histological and behavioral level, persisted at 7 weeks after transplantation (Fig 1F, G). At this point, NPC transplanted animals had 10,245.6 ± 1,094.5 TH+ cells in the lesioned hemisphere (13,805.2 ± 1,113.8 cells in the non-lesioned hemisphere) whereas saline controls had 6,483.8 ± 601.4 TH+ cells in the lesioned hemisphere (14,681.8 ± 515.7 cells in the non-lesioned hemisphere).
Ara-C infusion depletes the SVZ of proliferating precursors and prevents endogenous NPC activation in reaction to NPC transplantation
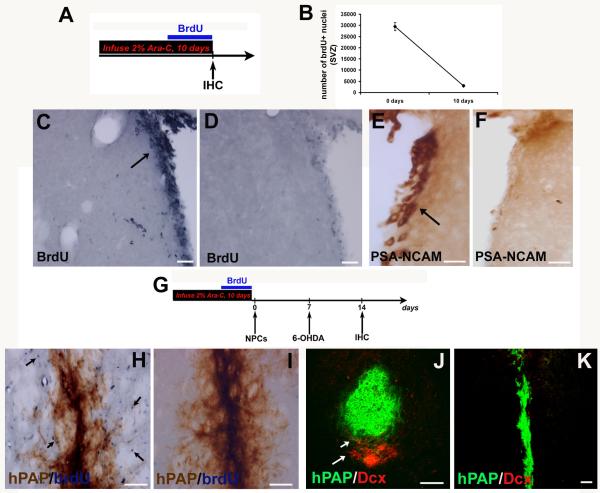
In order to delineate the role of endogenous neural precursors in the observed neuroprotective response to NPC-transplantation, we infused 2% Ara-C into host rat brains [timeline in Fig 2A] (Doetsch et al., 1999; Zhang et al., 2004). Infusion of Ara-C at 0.5μL/hr for 10 days resulted in the depletion of proliferating NPCs in the SVZ, as refglected by the virtual absence of brdU nuclei (Fig 2C - Ara-C, D – no Ara-C) and PSA-NCAM+ neuroblasts in this region (Fig 2E – Ara-C, F – no Ara-C), and negligible SVZ NPC numbers (stereology, Fig 2B).
Figure 2.
Inhibition of endogenous precursors by Ara-C infusion
To inhibit proliferating precursors in the host rat brains we infused the mitotic inhibitor 2% Ara-C, 0.5 μL/hr according to Doetsch et al (1999) for 10 days (timeline in A). As depicted, brdU labeled cells were severely depleted in the SVZ of Ara-C treated animals after 10 days (D) compared to controls (C), and stereological counts of brdU+ nuclei before and after Ara-C confirmed this (B). The inhibition of endogenous precursors was also supported by the observation that there was virtual disappearance of precursor cells expressing PSA-NCAM (E - no Ara-C, F - infusion of Ara-C) in the SVZ soon after treatment. After Ara-C infusion for 10 days, the osmotic pumps were removed and rats were transplanted with hPAP+ NPCs to then be followed by 6-OHDA treatment a week later (timeline in G). When analyzed at 2 weeks after transplantation, almost no brdU labeled or dcx+ cells were found near the graft in the Ara-C treated transplanted rats (I, K) but were observed in the transplanted animals which did not receive Ara-C (arrows in H, J) as noted in the Madhavan et al (2009) study. Scale bars: C-F – 50 μm; H, I – 100 μm; J, K – 50 μm
After Ara-C infusion, the osmotic pumps were removed and rats were transplanted with hPAP+ NPCs followed by 6-OHDA treatment a week later (timeline in 2G). Although there were almost no proliferating endogenous precursors at the time of NPC transplantation, these cells were expected to repopulate the SVZ by 14 days after the completion of Ara-C infusion as already established by our preliminary studies and others (Doetsch et al., 1999; Zhang et al., 2004). Two weeks after transplantation, as observed before, several brdU+ and dcx+ cells flanked grafted cells in NPC transplanted animals that had not received Ara-C (arrows in Fig 2H, J). In contrast, in animals that received Ara-C prior to NPC transplants, brdU and dcx labeled cells adjacent to the graft were absent (Fig 2I, K). All these data indicated that endogenous NPCs had been successfully inhibited by Ara-C infusion, now providing us with a system in which endogenous NPC roles in grafted NPC-mediated neuroprotection could be explored.
Inhibition of endogenous NPCs diminishes NPC graft-mediated nigrostriatal neuroprotection
We analyzed the effect of reduced endogenous precursor cell number on rat motor behavior, and the ability of grafted NPCs to protect the integrity of striatal terminals and TH+ neurons in the SN at 2 weeks after transplantation. Non-Ara-C infused transplanted animals performed significantly (p<0.001) better in the cylinder task than their 6-OHDA only treated counterparts as reported previously (Fig 3L). Ara-C infused grafted animals also performed significantly better than the 6-OHDA only controls (p<0.05), but interestingly in a significantly (p<0.01) worse manner than non-Ara-C treated grafted animals (F2, 16 =5.98, two way RM-ANOVA).
Histological examination revealed a significant preservation of striatal terminal and nigral neuron TH expression in 6-OHDA treated animals which had received previous NPC transplants, when compared to non-transplanted (and saline infused) animals as noted previously (Fig 3B, C, D and E, F, G). On the other hand 6-OHDA treated animals which had received prior NPC transplants but also Ara-C infusions, showed reduced striatal and nigral TH expression (Fig 3H, I, J). Stereological TH cell counts through the central SN (Fig 3K) confirmed that the cells had been significantly preserved in Ara-C infused grafted animals (p<0.05, 7,856.0 ± 1,084.6 TH+ cells in the lesioned hemisphere compared to 12,674.9 ± 1,414.0 cells in the non-lesioned hemisphere) although not to the extent observed in non-Ara-C treated grafted animals (p<0.001, 10,505.2 ± 1,380.3 TH+ cells in the lesioned hemisphere compared to 13,635.7 ± 1,939.8 cells in the non-lesioned hemisphere). Surprisingly, the Ara-C animal group also displayed a near significant (p=0.059) difference in TH neuroprotection efficacy when compared to the non-AraC infused transplanted group (Fig 3K, F2, 19 =15.36, one way ANOVA). Ara-C treated animals which received no subsequent grafts or 6-OHDA were similar to intact animals and did not show any alteration in behavior or SN TH cell counts attributable to Ara-C treatment alone. These analyses affirm that in addition to an autonomous contribution by grafted NPCs, endogenous NPCs also took part in the observed nigrostriatal neuroprotection.
Effects of endogenous NPC reduction on grafted NPC survival and migration
Two weeks after NPC transplantation (timeline in Fig 4A) we assessed grafted cell survival and migration in both the striatum and nigra (Fig 4B, C). Grafts in both the Ara-C (Fig 4E) and no Ara-C (4D) treated rats appeared healthy. Counts of hPAP+ grafted cells showed that transplanted NPC survival was somewhat better in the nigral region as compared with the striatum (Fig 4F). Overall, there was no significant difference between grafted cell counts in animals receiving Ara-C and those that did not (p > 0.05). In terms of grafted cell migration, NPCs in animals which did not receive Ara-C seemed restricted to the injection site with minimal spread (Fig 4D). In contrast, in Ara-C treated animals, grafted cells appeared to be more broadly distributed around the graft core, especially in the striatum (Fig 4E). In three of the fourteen animals in the Ara-C group, a pronounced cell migration covering a large area of the striatum (up to 1.5-2.0 mm away from the graft) was observed (4G, H).
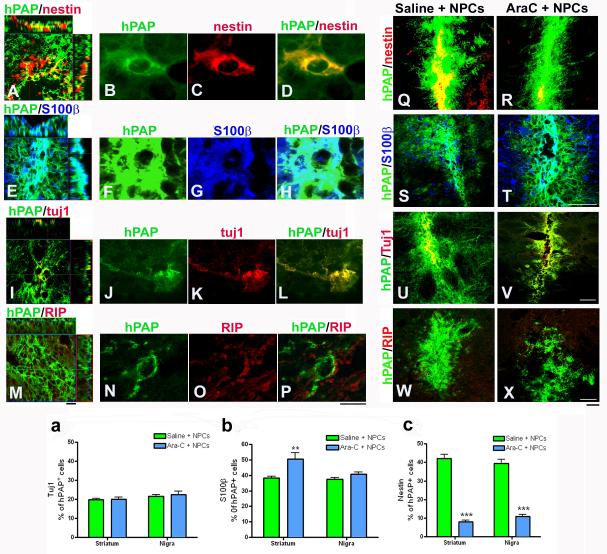
Decline in endogenous neural precursor cells alters the phenotypic fate of grafted NPCs
The differentiation patterns of grafts in both Ara-C and non Ara-C treated animals were evaluated. We observed strong nestin (Fig 5A, B, C, D) and S100β (Fig 5E, F, G, H) expression by grafted NPCs, with lower Tuj1 (Fig 5I, J, K, L) and negligible RIP (Fig 5M, N, O, P) expression as previously reported. On comparing Ara-C treated and non Ara-C treated animals, it was observed that among the hPAP+ grafted cells, S100β (Fig 5S, T), Tuj1 (Fig 5U, V) and RIP (Fig 5W, X) signals appeared quite comparable, but nestin (Fig 5Q, R) expression was drastically reduced in the Ara-C treated rats. Confocal cell counts of the number of hPAP+ NPCs also positive for S100β, Tuj1 or nestin were conducted. No significant differences were revealed between Ara-C and non-Ara-C infused animals in the percent of Tuj1 expressing hPAP+ cells in either the striatum or nigra (Fig 5a) [number of Tuji+ cells among hPAP cells per section analyzed (mean ± SEM): Non-AraC treated, Striatum - 100.3 ± 5.6 in 509.9 ± 20.8; Non-AraC treated, Nigra – 114.9 ± 6.2 in 529.6 ± 22.0; AraC treated, Striatum – 100.5 ± 6.7 in 504.7 ± 20.2; AraC treated, Nigra – 110.2 ± 9.8 in 492 ± 28.0]. S100β astrocyte percents were similar between Ara-C treated and untreated groups in the nigra but significantly (p<0.05) greater in Ara-C treated animals in the striatum (Fig 5b, F3, 56=6.63, one way ANOVA) [number of S100β+ cells among hPAP cells per section analyzed (mean ± SEM): Non-AraC treated, Striatum - 211.5 ± 11.4 in 550.5 ± 21.7; Non-AraC treated, Nigra - 188.8 ± 8.5 in 504 ± 17.9; AraC treated, Striatum – 234.8 ± 25.2 in 472.6 ± 19.3; AraC treated, Nigra – 189.6 ± 8.5 in 471.9 ± 24.9]. Percentage of nestin+ cells among grafted hPAP NPCs, exhibited the most prominent decline (p<0.001) between Ara-C treated and untreated animals in both the striatum and nigra (Fig 5c, F3, 56 =97.73, one way ANOVA) [number of nestin+ cells among hPAP cells per section analyzed (mean ± SEM): Non-AraC treated, Striatum – 245.5 ± 20.4 in 570.4 ± 20.9; Non-AraC treated, Nigra – 213.8 ± 18.0 in 534.7 ± 25.5; AraC treated, Striatum – 32.8 ± 4.3 in 425.2 ± 18.9; AraC treated, Nigra – 48.1 ± 5.4 in 439.8 ± 17.6].
Figure 5.
Effects of Ara-C induced inhibition of endogenous NPCs on graft differentiation
The differentiation pattern of grafts in both Ara-C and non Ara-C treated animals were compared. We observed strong nestin (A) and S100β (E) expression by grafted NPCs, with lower Tuj1 (I) and minimal RIP (M) expression as previously reported. High magnification images showing co-localization of hPAP/nestin (B-D), hPAP/ S100β (F-H), hPAP/tuj1 (J-L), and hPAP/RIP (N-P) are also depicted. On evaluating Ara-C treated and non Ara-C treated animals through confocal microscopy, it was observed that among the hPAP+ cells, S100β (S, T), Tuj1 (U, V) and RIP (W, X) signals appeared quite comparable, but nestin (Q, R) expression was drastically reduced in the Ara-C treated transplanted rats. We counted the number of hPAP+ NPCs also positive for S100β, Tuj1 or nestin in both the striatum and SN. There were no significant differences between Ara-C and non-Ara-C infused animals in the percent of Tuj1 expressing hPAP+ cells in either the striatum or nigra as indicated in the graph in a. S100β astrocyte percents were similar between Ara-C treated and untreated groups in the nigra but significantly (p<0.05) greater in Ara-C treated animals in the striatum as shown in panel b. [*p<0.05, one way ANOVA]. Percentage of nestin+ cells among grafted hPAP NPCs, exhibited the most prominent difference (p<0.001) between Ara-C treated and untreated animals in both the striatum and nigra as illustrated in panel c. [***p<0.001, one way ANOVA]. All raw data for the number of hPAP cells double labeled with either Tuj1, S100β or nestin have been stated within the results section. Scale bars: A,E,I,M - 20 μm; B-D, F-H, J-L, N-P – 10 μm; Q-X – 50 μm
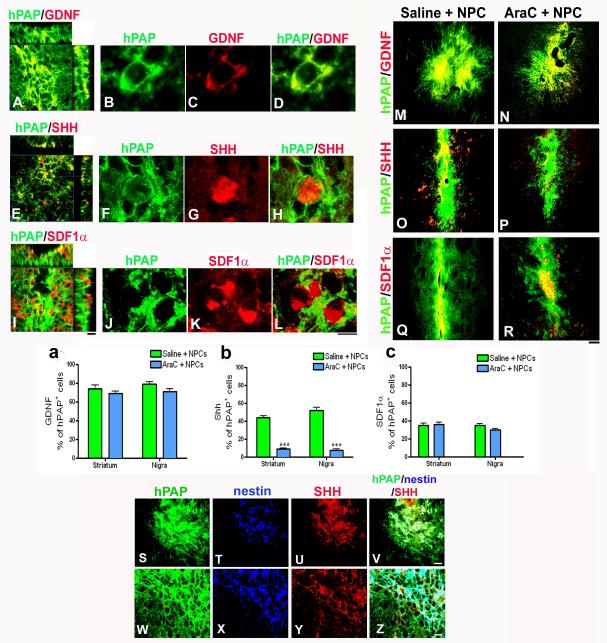
Inhibition of endogenous precursors leads to decreased sonic hedgehog expression in grafted cells
Comparisons of factors expressed by the grafted NPCs in our previous study were conducted between the Ara-C treated and untreated groups (Madhavan et al., 2009). As shown in the confocal images (Fig 6A-L), in the non Ara-C receiving grafted animals, transplants were found to strongly express GDNF (Fig 6A, B, C, D), SHH (Fig 6E, F, G, H) and SDF1α (Fig 6I, J, K, L) as established before. In Ara-C infused animals, GDNF (Fig 6M, N) and SDF1α (Fig 6Q, R) expression appeared equally strong, but SHH expression was reduced (Fig 6O, P). We counted using confocal microscopy the number of GDNF, SHH and SDF1α positive cells present among the hPAP+ grafted NPCs. As shown in figure 6a and c, GDNF and SDF1α percentages were not significantly different between the Ara-C treated and untreated groups in both the striatum and SN. {A - [number of GDNF+ cells among hPAP cells per section analyzed (mean ± SEM): Non-AraC treated, Striatum – 318.5 ± 21.4 in 428.6 ± 14.3; Non-AraC treated, Nigra - 288.8 ± 15.8 in 366.1 ± 16.5; AraC treated, Striatum – 245 ± 12.4 in 353.5 ± 14.8; AraC treated, Nigra – 265.2 ± 22.1 in 366.8 ± 19.9]. B - [number of SDF1α+ cells among hPAP cells per section analyzed (mean ± SEM): Non- AraC treated, Striatum – 150.2 ± 11.9 in 429.4 ± 13.0; Non-AraC treated, Nigra – 156.5 ± 7.6 in 451.0 ± 15.8; AraC treated, Striatum – 155.5 ± 9.4 in 444.6 ± 19.4; AraC treated, Nigra – 127.3 ± 10.4 in 411.2 ± 18.9]}. However, the SHH fraction of grafted cells was significantly (p<0.001) reduced in the Ara-C receiving rats in both the striatum and SN (Fig 6b, F3, 56 =118.5, one way ANOVA) [number of SHH+ cells among hPAP cells per section analyzed (mean ± SEM): Non-AraC treated, Striatum – 165.2 ± 7.0 in 375.9 ± 13.6; Non-AraC treated, Nigra – 193.7 ± 12.8 in 365.8 ± 13.6; AraC treated, Striatum - 29.6 ± 3.0 in 331.8 ± 11.3; AraC treated, Nigra – 23.8 ± 4.9 in 299.2 ± 14.5].
Figure 6.
Effects of Ara-C induced endogenous NPC inhibition on growth factor expression by grafted cells
Comparisons of factors observed to be expressed by the grafted NPCs in our previous study were conducted between the Ara-C treated and untreated transplanted groups. As shown in the confocal images (A-L), in the non Ara-C receiving grafted animals, transplants were found to strongly express GDNF (A, high mag images in B-D), SHH (E, high mag images in F-H) and SDF1α (I, high mag image in J-L) as established in Madhavan et al, 2009. When expression of these factors between the Ara-C receiving and non Ara-C infused animals was studied, GDNF (M, N) and SDF1α (Q, R) expression appeared to be equally strong, but the SHH expression seemed particularly reduced in the Ara-C treated animals (O, P). We counted the number of GDNF, SHH and SDF1α positive cells present among the hPAP expressing grafted NPCs. These are reported in P, Q and R as percentage of total hPAP+ cells counted (raw numbers have been stated in the results section). As shown in a and c, GDNF and SDF1α percentages were not significantly different between the Ara-C treated and untreated groups. However, the SHH fraction of grafted cells was significantly (p<0.001) reduced in the Ara-C receiving rats in both the striatum and SN (b). [***p<0.001, one way ANOVA]. Further immunophenotyping revealed that to a large extent, nestin (blue) expressing hPAP+ NPCs (green) also strongly expressed SHH (red, S-Z). Scale bars: A, E, I – 20 μm; B-D, F-H, J-L – 10 μm; M-R – 50 μm; S-V – 20 μm; W-Z – 10 μm.
We also looked more closely at the expression of nestin and SHH in grafted animals by immunostaining for both these molecules simultaneously in the hPAP grafts. High resolution confocal imaging (Fig 6S-Z) confirmed that most hPAP+ cells which strongly expressed nestin, also expressed SHH. This cell population was virtually absent in the Ara-C treated grafted animals.
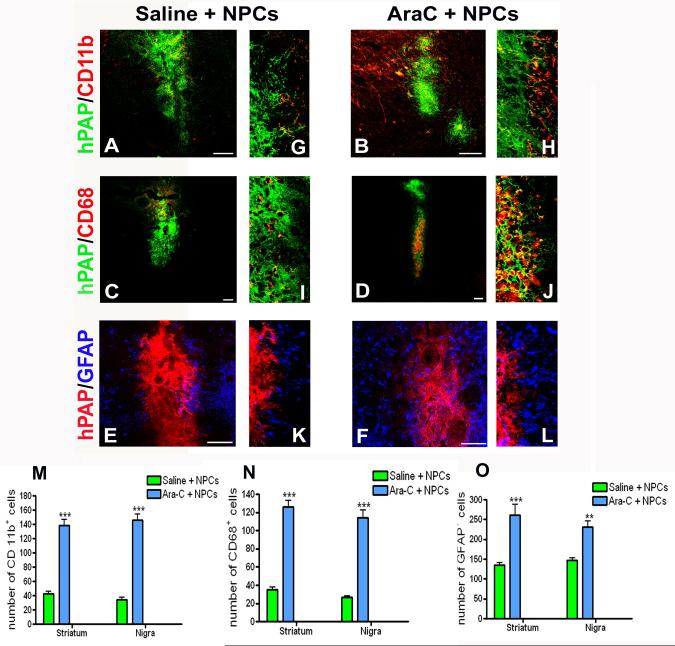
Diminished endogenous precursor cell numbers alter the glial response to NPC grafting
In addition to studying the effect of endogenous NPC inhibition on grafted cells, we also examined their effects on mature host CNS cells involved in immune modulation and inflammation with qualitative observations. Overall, there was a reduced glial reaction around the grafts in non-Ara-C infused animals suggesting that endogenous NPC presence could be exerting a dampening influence on host reactive glia. Firstly, CD11b expressing microglia (red) were noticed in significant numbers around grafts (green) in Ara-C infused animals (Fig 7B, magnified view of graft periphery in H). Additionally, CD68 expressing activated microglia (red) also appeared to be present in greater numbers in Ara-C treated rats when compared with saline infused animals (Fig 7C and 7D, magnified views in I, J). The CD68 and CD11b expressing microglia were predominantly host derived although a few appeared to originate from the graft (being hPAP positive). In addition to microglia, the presence of GFAP+ astrocytes was increased around grafts in animals in which endogenous NPCs had been inhibited (Fig 7F, L) as compared to non-Ara-C treated animals (Fig 7E, K). Quantification of glial populations in regions adjacent to the grafted cells confirmed that the inhibition of endogenous NPCs had in fact resulted in increased numbers of CD11b (M), CD68 (N), and GFAP (O) expressing cells around grafts. Since Ara-C was infused into the naïve host brain where the presence of proliferating glia is negligible it was not expected to directly influence this population, and glial cell profiles in Ara-C only (no NPCs or 6-OHDA) treated animals were observed to be similar to intact animals.
Figure 7.
Effects of Ara-C induced inhibition of endogenous NPCs on host glia.
We studied the glial response of host brains in both Ara-C treated and untreated rats. Specifically we looked at CD11b (microglia, A, G and B, H), CD68 (activated microglia, C, I and D, J) and GFAP (activated glia, E, K and F, L) immunoreactivity, surrounding the NPC grafts. As depicted in A, B and C, D, both CD11b and CD68 cells respectively, were present in greater numbers in Ara-C receiving animals than the non Ara-C infused animals. Magnified views of the graft periphery showing green hPAP grafted cells and red host glial cells are displayed in G-J. Also, the GFAP immunoreactivity (blue) appeared stronger around grafts (red) in Ara-C treated animals (F, L) in contrast to animals not administered Ara-C (E, K). M, N, and O compare the number of CD11b+, CD68+ and GFAP+ cells observed in the periphery of NPC grafts in Ara-C treated and untreated animals. Data has been expressed as the total number of cells in four high power fields (100x, bordering the graft) per section analyzed. Overall, significantly greater numbers of glial cells were present around NPC grafts in Ara-C receiving animals. [***p<0.001, ** p<0.01, one way ANOVA]. Scale bars: 100 μm
DISCUSSION
The fundamental contribution of the presented work is that host-derived NPCs play a significant role in therapeutic effects induced by grafted NPCs. The study demonstrates that endogenous NPCs can affect grafted NPC survival, differentiation, growth factor expression, and interactions with the resident glia to shape the nature of therapeutic consequences, specifically in dopamine system neuroprotection. This novel information may revise interpretation of the mechanisms of graft-induced therapeutic phenomena observed in many models of nervous system injury and disease, and contribute towards development of future therapies.
NPC transplantation-induced neuroprotection and neurorescue have been observed in many neurological disease models, but the mechanisms underlying these phenomena are not well understood. Both host- and graft-mediated events are probably involved. In fact, reciprocal interactions between the graft and host are recognized as being critical in determining the consequences of NPC transplantation (Imitola et al., 2004a; Ourednik & Ourednik, 2005; Ninkovic & Gotz, 2007; Madhavan & Collier, 2010; Pluchino et al., 2010). In support of this concept, host neurogenesis in response to stem cell grafting has been reported to occur in both the adult and aged brain (Ogawa et al., 2002; Taguchi et al., 2004; Yasuhara et al., 2006; Hattiangady et al., 2007; Park et al., 2009b). This endogenous NPC reaction although linked, has not been confirmed as contributing to any functional effects after donor NPC implantation. Our findings for the first time establish an important role for endogenous NPCs in neuroprotection observed after stem cell transplantation. In this study, the absence of endogenous neural precursors at the time of grafting, and by extension the absence of subsequent augmented neurogenesis, is associated with significant reduction of nigrostriatal neuroprotection and behavioral recovery. This result highlights that in addition to grafted NPC effects, resident NPCs play an important role in determining the therapeutic outcome.
In addition to confirming that endogenous NPCs can play a role in graft-mediated therapeutic effects, our study makes inroads into mechanisms by which endogenous NPCs may influence the neuroprotective process. First, the presence of endogenous NPCs affected differentiation of grafted cells. Our study shows that the absence of augmented SVZ neurogenesis and endogenous NPCs at the graft site specifically caused a significant reduction in the number of undifferentiated nestin expressing cells within the graft, indicating that such immature NPCs may be key players in neuroprotection. Nestin is recognized as being necessary for the proper self-renewal and survival of NSCs (Park et al., 2010) and the persistence of nestin positive cells in the graft may perpetuate its function. Second, SHH expression by the graft was noted to be significantly reduced in animals lacking in endogenous neural precursors at the time of grafting. SHH is known to be important for the survival, differentiation, and protection of dopaminergic neurons from toxic insults, and is a natural suspect for a role in the observed neuroprotective process (Hynes et al., 1995; Miao et al., 1997; Tsuboi & Shults, 2002). Furthermore, SHH is known to be critically involved in the maintenance and regulation of endogenous neural stem cell niches (Machold et al., 2003). Third, the fact that transplanted nestin expressing cells also strongly expressed SHH, and this population was significantly lost in Ara-C treated grafted animals, suggests that this subset of cells probably played a significant role in the observed neuroprotection and endogenous precursor activation. Finally, Ara-C infused animals showed elevated host glial activity, signified by greater numbers of reactive astrocytes, CD11b+ microglia, and CD68+ activated microglia surrounding the graft. This inflammatory response may affect grafted cell function and protection of the host dopamine system. Mainly, the activated glial cells could have contributed to the heightened migration but somewhat reduced survival of the grafted NPCs, and attenuated neuroprotection observed in the Ara-C infused animals through the expression of injury-induced cues (Russo et al.; Imitola et al., 2004b; Ekdahl et al., 2009; Park et al., 2009a; Rota Nodari et al., 2010).
Endogenous NPCs affected graft phenotype and function but did not significantly impact the survival of transplanted cells. There was no significant difference in grafted hPAP+ cell numbers between Ara-C and non-Ara-C treated animals. This result also negates any indirect effects (cessation of Ara-C application before NPC transplantation rules out direct effects) that Ara-C might have had on the survival and/or proliferation of the grafted cells. Additionally, given that there was no significant change in total grafted cell numbers between Ara-C treated and untreated animals, any possible down-regulation of hPAP expression by the grafted cells would not be a concern since the effect would be expected to be uniform across all groups. Studies from the lab show robust maintenance of hPAP expression by grafted cells in vivo over time upto three months after transplantation (unpublished observations).
Close communication between exogenous and endogenous NPCs was facilitated by the migration of endogenous precursors into graft areas in our study. In addition, ‘long-distance’ communication between these two NPC types may have also transpired by way of growth factors (namely GDNF, SHH and SDF1α) found expressed by the NPCs, especially since such factors have been found to have the capacity to diffuse significant distances in the brain (Charytoniuk et al., 2002; Kobayashi et al., 2006). Resulting interactions between the two pools of NPCs could be expected to affect both donor and endogenous cell behavior to ultimately determine the consequences of transplantation. Furthermore, Androutsellis-Theotokis et al (2009) propose quite intriguingly that neurogenesis occurring in the SVZ may have direct influence on SN TH neurons. This idea is very interesting considering that SN dopaminergic neurons project to the SVZ and are known to affect the size of the precursor pool (Hoglinger et al., 2004; O’Keeffe et al., 2009). Perhaps in return, stimulation of precursor cells (neurogenesis) can also influence TH neurons in the SN. In our study, NPCs were transplanted both into the striatum and SN, making it unclear whether a direct SVZ precursor influence on the SN TH neuron preservation existed, but prospective studies will investigate the issue.
The report also comments on the timing of grafting. Particularly, it appears that ‘early’ grafting may be crucial in determining the ability of exogenous NPCs to recruit endogenous NPCs, and exert therapeutic effects such as neuroprotection. To this end, unpublished studies from the lab indicate that NPC grafting at a later time (one week after the 6-OHDA application) results in the absence of endogenous neurogenesis and nigrostriatal neuroprotection. On the other hand, a same day grafting and 6-OHDA lesioning paradigm is associated with some endogenous NPC activation, and neuroprotection [L Madhavan, unpublished observations; Paumier K et al. 2011, submitted; Paumier K, abstract in Experimental Neurology (2006)]. Conceptually, the therapeutic importance of neuroprotection in PD is sometimes questioned since patients become symptomatic only after a significant proportion of the DA neurons have degenerated. But it is also well accepted that when symptoms begin to appear in PD, a substantial number of SN DA neurons still exist. Also an unknown number of TH neurons which are dysfunctional (exhibit decreased phenotype expression) may also be present at this stage of the disease. In this context, “proactive” neurotransplantation could become useful in PD by protecting future degeneration of the existing cells and restoring the health of those that have lost their phenotype.
In conclusion, it is clear that both exogenous and endogenous neural precursors are important in determining the outcome of NPC transplantation: both NPC populations can interact synergistically to achieve therapeutic ends. In view of these findings, we propose that endogenous NPCs can not only proliferate and migrate out of the SVZ stem cell niche, but in fact carry with them certain inherent features of the niche such as nestin and SHH, to then influence the transplanted NPCs. This ‘transfer’ of the niche to the microenvironment of grafted cells appears to maintain these cells in a more uncommitted stem-like state that is conducive to neuroprotection. In essence, in our model transplanted and endogenous neural precursors together may be perceived to create an additional ectopic stem cell niche (Ourednik & Ourednik, 2007; Pluchino et al., 2010), which through its regenerative abilities promotes CNS plasticity and resistance to pathological insults.
Acknowledgements
We thank Dr. Itzhak Fischer for providing the hPAP Fischer 344 transgenic founder rats that allowed us to establish our colony, and Nate Levine and Birgit Ehmer for their technical assistance. The work was supported by: NIH grant NS055295, NS58830 the Udall Center of Excellence in Parkinson’s Disease Research at Michigan State University (TJC), the Millennium Scholars Fund at the University of Cincinnati (TJC), and the Gardner Family Foundation at the University of Cincinnati (TJC and LM).
REFERENCES
- Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, Walbridge S, Munasinghe J, Koretsky AP, Lonser RR, McKay RD. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci U S A. 2009;106:13570–13575. doi: 10.1073/pnas.0905125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci. 2002;16:2351–2357. doi: 10.1046/j.1460-9568.2002.02412.x. [DOI] [PubMed] [Google Scholar]
- Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis DR, Benalcazar H, Allen CV, Wise RM, Dills C, Dobrescu C, Rothholtz V, Levy RM. Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res. 2000;856:281–290. doi: 10.1016/s0006-8993(99)02089-2. [DOI] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177:360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Imitola J, Park KI, Teng YD, Nisim S, Lachyankar M, Ourednik J, Mueller FJ, Yiou R, Atala A, Sidman RL, Tuszynski M, Khoury SJ, Snyder EY. Stem cells: cross-talk and developmental programs. Philos.Trans.R.Soc.Lond B Biol.Sci. 2004a:359–823. doi: 10.1098/rstb.2004.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004b;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan I, Barhum Y, Melamed E, Offen D. Mesenchymal Stem Cells Stimulate Endogenous Neurogenesis in the Subventricular Zone of Adult Mice. Stem Cell Rev. 2010;7:404–12. doi: 10.1007/s12015-010-9190-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Collier TJ. A synergistic approach for neural repair: cell transplantation and induction of endogenous precursor cell activity. Neuropharmacology. 2010;58:835–844. doi: 10.1016/j.neuropharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Daley BF, Paumier KL, Collier TJ. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson’s disease. J Comp Neurol. 2009;515:102–115. doi: 10.1002/cne.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- Miao N, Wang M, Ott JA, D’Alessandro JS, Woolf TM, Bumcrot DA, Mahanthappa NK, Pang K. Sonic hedgehog promotes the survival of specific CNS neuron populations and protects these cells from toxic insult In vitro. J Neurosci. 1997;17:5891–5899. doi: 10.1523/JNEUROSCI.17-15-05891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba T, Han SS, Fischer I, Sandgren EP, Rao MS. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Exp Neurol. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Gotz M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17:338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GC, Barker RA, Caldwell MA. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009;8:2888–2894. doi: 10.4161/cc.8.18.9512. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J. Graft/Host relationships in the developing and regenerating CNS of mammals. Ann N Y Acad Sci. 2005;1049:172–184. doi: 10.1196/annals.1334.016. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J. Plasticity of the central nervous system and formation of “auxiliary niches” after stem cell grafting: an essay. Cell Transplant. 2007;16:263–271. doi: 10.3727/000000007783464696. [DOI] [PubMed] [Google Scholar]
- Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, Walton N, Lahn BT. Nestin is Required for the Proper Self-Renewal of Neural Stem Cells. Stem Cells. 2010;28:2162–71. doi: 10.1002/stem.541. [DOI] [PubMed] [Google Scholar]
- Park DH, Eve DJ, Musso J, 3rd, Klasko SK, Cruz E, Borlongan CV, Sanberg PR. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009a;18:693–702. doi: 10.1089/scd.2009.0008. [DOI] [PubMed] [Google Scholar]
- Park DH, Eve DJ, Sanberg PR, Musso J, Iii, Bachstetter AD, Wolfson A, Schlunk A, Baradez MO, Sinden JD, Bickford PC, Gemma C. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 2009b;19:175–80. doi: 10.1089/scd.2009.0172. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Cusimano M, Bacigaluppi M, Martino G. Remodelling the injured CNS through the establishment of atypical ectopic perivascular neural stem cell niches. Arch Ital Biol. 2010;148:173–183. [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota Nodari L, Ferrari D, Giani F, Bossi M, Rodriguez-Menendez V, Tredici G, Delia D, Vescovi AL, De Filippis L. Long-Term Survival of Human Neural Stem Cells in the Ischemic Rat Brain upon Transient Immunosuppression. PLoS One. 2010;5:e14035. doi: 10.1371/journal.pone.0014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Shults CW. Intrastriatal injection of sonic hedgehog reduces behavioral impairment in a rat model of Parkinson’s disease. Exp Neurol. 2002;173:95–104. doi: 10.1006/exnr.2001.7825. [DOI] [PubMed] [Google Scholar]
- van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24:387–393. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J Neurosci. 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]