Abstract
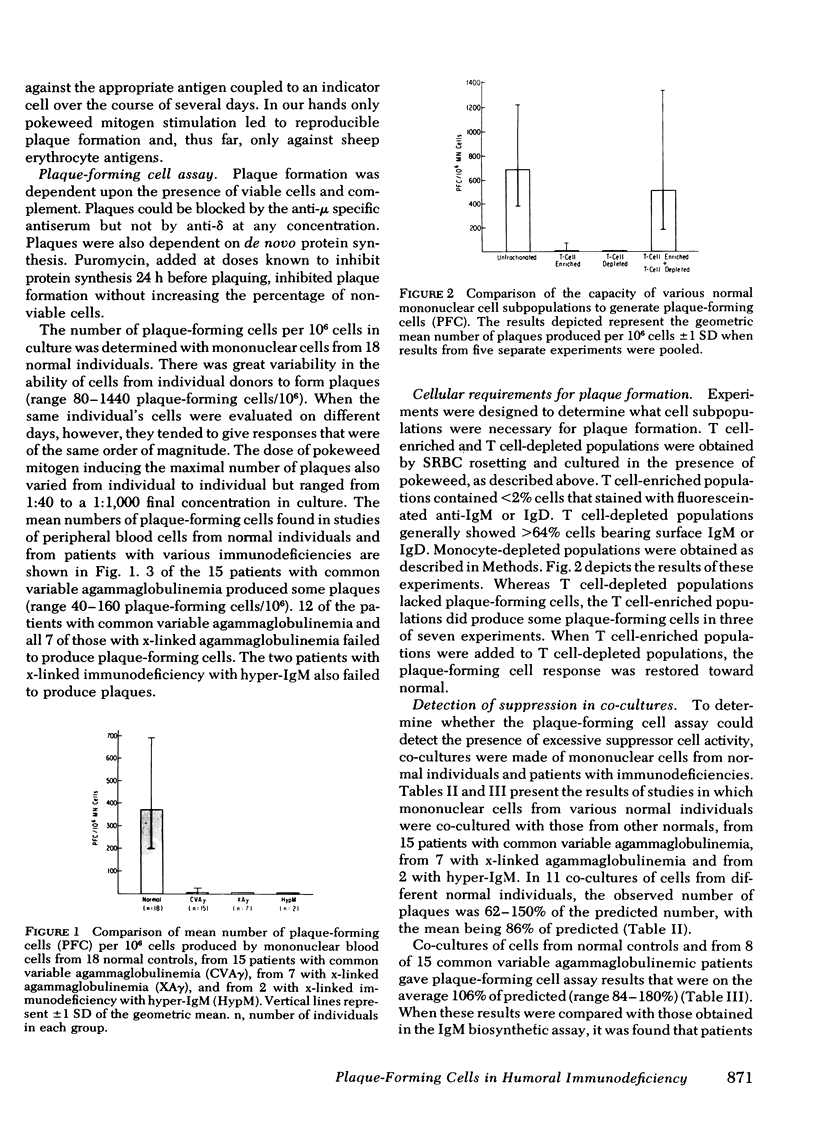
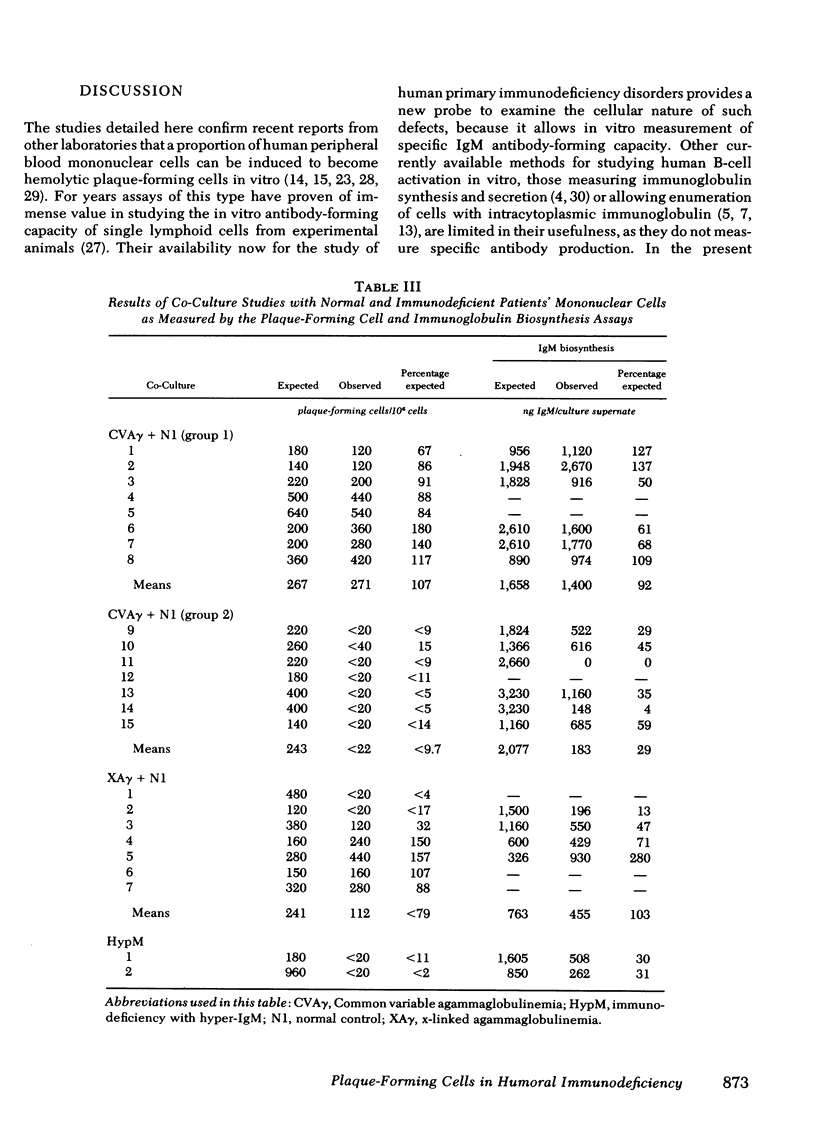
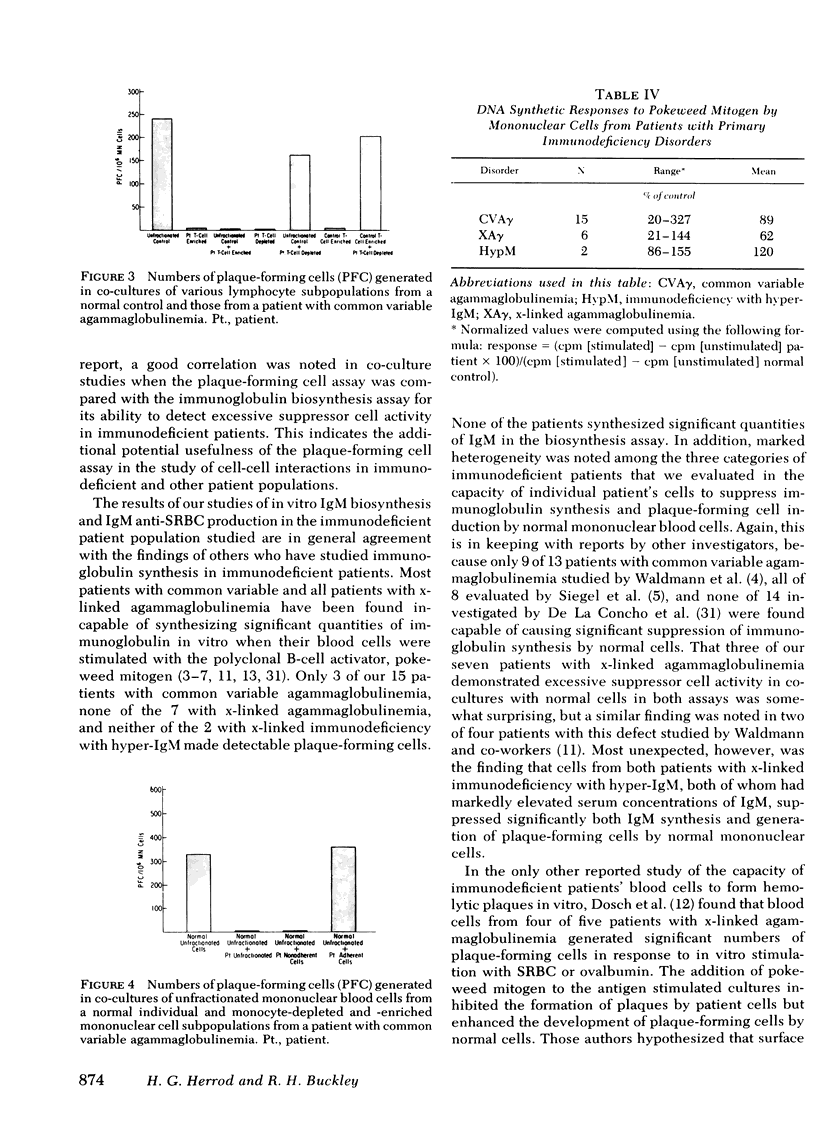
A plaque assay that detects human mononuclear blood cells producing immunoglobulin (Ig)M antibody to sheep erythrocytes was investigated for its usefulness in studying B-cell activation and regulation in 24 patients with humoral immunodeficiency. Cells from 3 of 15 patients with common variable agammaglobulinemia produced some plaques (range 40--160/10(6) cells; normal range 80--1240/10(6)), but those from the other 12, from all 7 with x-linked agammaglobulinemia and from the 2 with x-linked immunodeficiency with hyper-IgM failed to produce any detectable plaques. In co-cultures of patient and normal cells a very good correlation was seen between results of the plaque assay and an IgM biosynthesis assay in detecting excessive suppressor cell activity. Cells from 7 of 15 common variable agammaglobulinemics, from 3 of 7 x-linked agammaglobulinemics, and from both patients with hyper-IgM caused significant suppression of IgM biosynthesis and(or) plaque formation by normal cells. The observations in the last two groups and discordance for excess suppressor activity in identical twins with common variable agammaglobulinemia suggest that the activity develops secondarily to whatever their primary defects may be. Culturing non-T cells from common variable agammaglobulinemics exhibiting excessive suppressor cell activity with normal T cells resulted in plaque formation in four of five patients so studied; in all five the suppressor activity was found in the T-cell population. The availability of a plaque assay for the study of blood cells from immunodeficient patients provides a new probe to examine the cellular nature of such defects.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Broom B. C., De la Concha E. G., Webster A. D., Janossy G. J., Asherson G. L. Intracellular immunoglobulin production in vitro by lymphocytes from patients with hypogammaglobulinaemia and their effect on normal lymphocytes. Clin Exp Immunol. 1976 Jan;23(1):73–77. [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Gilbertsen R. B., Schiff R. I., Ferreira E., Sanal S. O., Waldmann T. A. Heterogeneity of lymphocyte subpopulations in severe combined immunodeficiency. Evidence against a stem cell defect. J Clin Invest. 1976 Jul;58(1):130–136. doi: 10.1172/JCI108441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cooper M. D., Faulk W. P., Fudenberg H. H., Good R. A., Hitzig W., Kunkel H., Rosen F. S., Seligmann M., Soothill J., Wedgwood R. J. Classification of primary immunodeficiencies. N Engl J Med. 1973 May 3;288(18):966–967. doi: 10.1056/NEJM197305032881814. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Primary in vitro antibody response from human peripheral blood lymphocytes. J Immunol. 1977 Feb;118(2):630–635. [PubMed] [Google Scholar]
- Dosch H. M., Gelfand E. W. In vitro induction and measurement of hemolytic plaque forming cells in man. J Immunol Methods. 1976;11(2):107–116. doi: 10.1016/0022-1759(76)90138-1. [DOI] [PubMed] [Google Scholar]
- Dosch H. M., Percy M. E., Gelfand E. W. Functional differentiation of B lymphocytes in congenital agammaglobulinemia. I. Generation of hemolytic plaque-forming cells. J Immunol. 1977 Dec;119(6):1959–1964. [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Averbeck A. K., Swedlund H. A. Measurement of IgE in normal and allergic serum by radioimmunoassay. J Lab Clin Med. 1971 Apr;77(4):690–698. [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kolb J. P., Arrian S., Zolla-Pazner S. Suppression of the humoral immune response by plasmacytomas: mediation by adherent mononuclear cells. J Immunol. 1977 Feb;118(2):702–709. [PubMed] [Google Scholar]
- Luzzati A. L., Taussig M. J., Meo T., Pernis B. Induction of an antibody response in cultures of human peripheral blood lymphocytes. J Exp Med. 1976 Sep 1;144(3):573–585. doi: 10.1084/jem.144.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Cooper M. D. Characterization of human T-cell subpopulations as defined by specific receptors for immunoglobulins. Contemp Top Immunobiol. 1978;8:19–53. doi: 10.1007/978-1-4684-0922-2_2. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Koski I., Dooley N., Blaese R. M. Artifactual plaque formation in vitro and in vivo to passive transfer of specific antibody. J Immunol. 1976 Apr;116(4):1016–1019. [PubMed] [Google Scholar]
- Sanal S. O., Buckley R. H. Antibody-dependent cellular cytotoxicity in primary immunodeficiency diseases and with normal leukocyte subpopulations. Importance of the type of target. J Clin Invest. 1978 Jan;61(1):1–10. doi: 10.1172/JCI108907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. I., Buckley R. H., Gilbertsen R. B., Metzgar R. S. Membrane receptors and in vitro responsiveness of lymphocytes in human immunodeficiency. J Immunol. 1974 Jan;112(1):376–386. [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler L. B., Pearl E. R., Gathings W. E., Lawton A. R., Cooper M. D. Letter: B lymphocyte precursors in bone-marrow in immunoglobulin deficiency diseases. Lancet. 1976 Aug 14;2(7981):376–376. doi: 10.1016/s0140-6736(76)92640-4. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Durm M., Blackman M., Krakauer R., Meade B. Suppressor T cells in the pathogenesis of hypogammaglobulinemia associated with a thymoma. Trans Assoc Am Physicians. 1975;88:120–134. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Krakauer R., Durm M., Meade B., Goldman C. Defect in IgA secretion and in IgA specific suppressor cells in patients with selective IgA deficiency. Trans Assoc Am Physicians. 1976;89:215–224. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Krakauer R., MacDermott R. P., Durm M., Goldman C., Meade B. The role of suppressor cells in the pathogenesis of common variable hypogammaglobulinemia and the immunodeficiency associated with myeloma. Fed Proc. 1976 Jul;35(9):2067–2072. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Suppressor cells in the regulation of the immune response. Prog Clin Immunol. 1977;3:155–199. [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Wilfert C. M., Buckley R. H., Mohanakumar T., Griffith J. F., Katz S. L., Whisnant J. K., Eggleston P. A., Moore M., Treadwell E., Oxman M. N. Persistent and fatal central-nervous-system ECHOvirus infections in patients with agammaglobulinemia. N Engl J Med. 1977 Jun 30;296(26):1485–1489. doi: 10.1056/NEJM197706302962601. [DOI] [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]


