Abstract
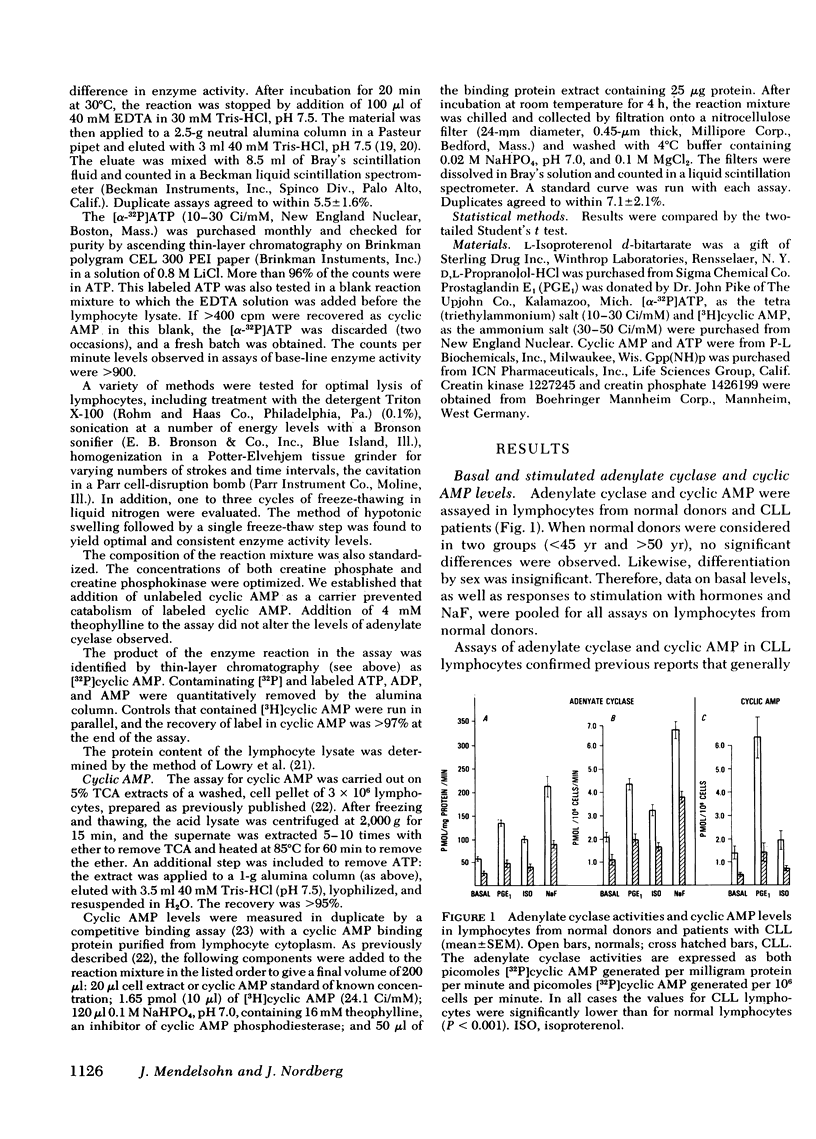
Lymphocytes were purified from peripheral blood of normal donors and patients with chronic lymphocytic leukemia (CLL) by Ficoll-Hypaque centrifugation. Adenylate cyclase activity, expressed as picomoles [32P]cyclic AMP generated per milligram protein per minute, was 57±4 in normals and 26±4 in CLL patients. Enzyme activity, expressed as picomoles [32P]cyclic AMP generated per 106 lymphocytes per minute, was 2.09±0.19 for normal lymphocytes and 1.10±0.16 for CLL lymphocytes. The differences between normal and CLL peripheral lymphocytes are highly significant (P < 0.001) with either method of calculating activity.
Cyclic AMP levels (picomoles per 106 lymphocytes) also differed significantly: 1.38±0.29 for normals and 0.45±0.08 for CLL lymphocytes.
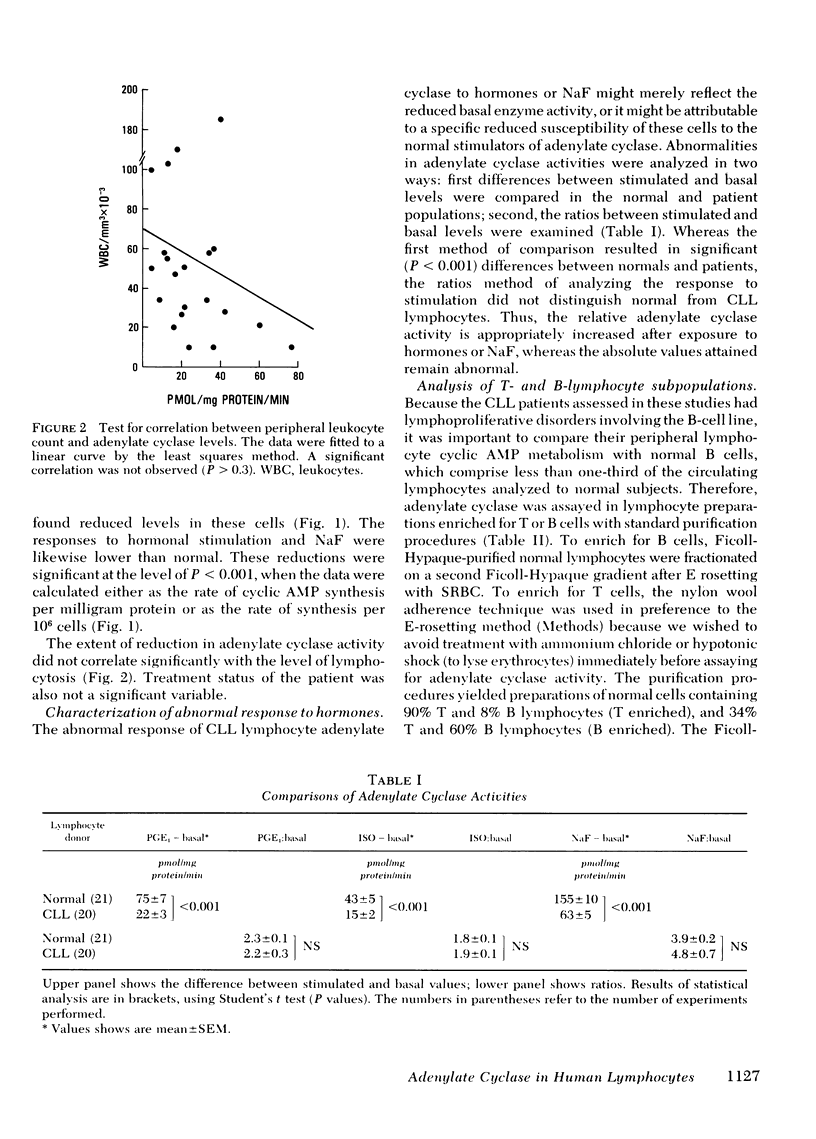
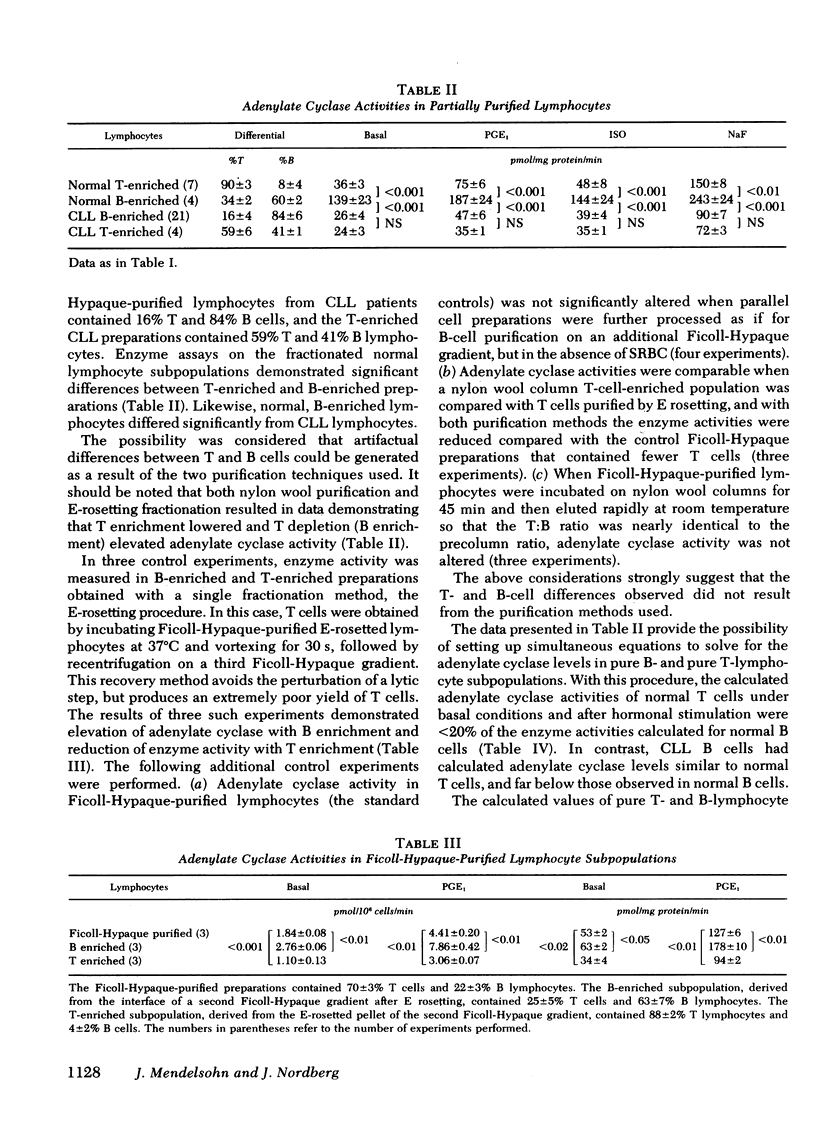
Adenylate cyclase was assayed in lymphocytes enriched for bone marrow-derived (B) cells by removing E-rosetted thymus-derived (T) cells, and enriched for T cells by harvesting E-rosetted lymphocytes or by removing B cells with nylon wool absorption. Solutions to simultaneous equations gave the following calculated enzyme activities for pure B- and T-cell subpopulations (in picomoles [32P]cyclic AMP generated per milligram mg protein per minute): normal B, 196±22; normal T, 30±10; CLL B, 34±6; CLL T, 19±4. Thus. normal B-lymphocyte adenylate cyclase exceeds normal T-lymphocyte activity by more than sixfold, whereas in the case of CLL the enzyme activity in B lymphocytes is markedly reduced to levels comparable to T lymphocytes.
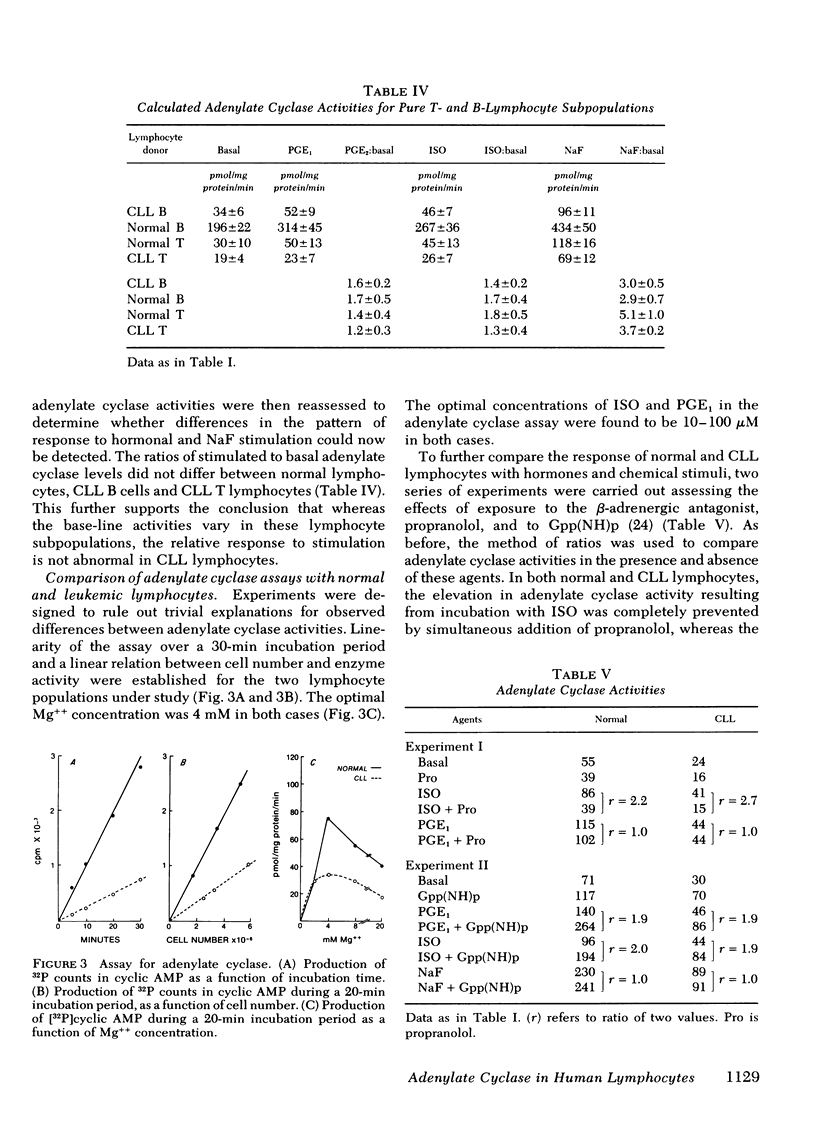
The responses of lymphocytes to stimulation with the hormones prostaglandin E1 and isoproterenol, and with NaF, were assessed. Compared with normal lymphocytes, enzyme activities were reduced in CLL lymphocytes incubated with these agents, but to a degree paralleling the reduced basal activities. Thus, the ratios between stimulated and basal adenylate cyclase levels in Ficoll-Hypaque-purified, normal lymphocytes were 2.3±0.1 after incubation with 10 μM isoproterenol, and 3.9±0.2 with 10 mM NaF, values which did not differ significantly from those obtained with CLL lymphocytes. When the enzyme activities calculated for purified T- and B-lymphocyte subpopulations were used to derive the stimulation ratios, the responses of normal and CLL T and B cells to these agents were also indistinguishable. The simplest explanation for these findings is a reduced number of normally responsive enzyme sites on the surface membranes of CLL lymphocytes, although alternative explanations are possible.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., Wedner H. J., Parker C. W. Two novel stimuli of cyclic adenosine 3',5'-monophosphate (cAMP) in human lymphocytes. J Immunol. 1975 Oct;115(4):1023–1027. [PubMed] [Google Scholar]
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Siegal F. P., Bentwich Z. H., Kunkel H. G. Lymphocyte binding of aggregated IgG and surface Ig staining in chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 May;14(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Hait W. N., Weiss B. Characteristics of the cyclic nucleotide phosphodiesterases of normal and leukemic lymphocytes. Biochim Biophys Acta. 1977 Mar 29;497(1):86–100. doi: 10.1016/0304-4165(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Hepburn B., Ritts R. E., Jr Human T lymphocytes: assay method using permanently fixed slides. Mayo Clin Proc. 1974 Nov;49(11):866–869. [PubMed] [Google Scholar]
- Jondal M. SRBC rosette formation as a human T lymphocyte marker. Scand J Immunol. 1976 Jun;Suppl 5:69–76. doi: 10.1111/j.1365-3083.1976.tb03857.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Decreased phytohemagglutinin receptor sites in chronic lymphocytic leukemia. Biochim Biophys Acta. 1969 Dec 30;192(3):542–545. doi: 10.1016/0304-4165(69)90409-7. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahan L. A., Kemp R. G. Cyclic 3',5'-adenosine monophosphate phosphodiesterase in the thymus of normal and leukemic mice. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):417–425. [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Boone R. F. Enhanced effects of prostaglandin E1 and dibutyryl cyclic AMP upon human lymphocytes in the presence of cortisol. J Clin Invest. 1973 Sep;52(9):2129–2137. doi: 10.1172/JCI107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan T. M., Marchand N. W., Fritz R. R., Abell C. W. Cyclic adenosine 3':5'-monophosphate levels and activities of related enzymes in normal and leukemic lymphocytes. Cancer Res. 1975 Sep;35(9):2540–2547. [PubMed] [Google Scholar]
- Niaudet P., Beaurain G., Bach M. A. Differences in effect of isoproterenol stimulation on levels of cyclic AMP in human B and T lymphocytes. Eur J Immunol. 1976 Nov;6(11):834–836. doi: 10.1002/eji.1830061117. [DOI] [PubMed] [Google Scholar]
- Polgar P., Vera J. C., Kelley P. R., Rutenburg A. M. Adenylate cyclase activity in normal and leukemic human leukocytes as determined by a radioimmunoassay for cyclic AMP. Biochim Biophys Acta. 1973 Feb 28;297(2):378–383. doi: 10.1016/0304-4165(73)90085-8. [DOI] [PubMed] [Google Scholar]
- Polgar P., Vera J. C., Rutenburg A. M. An altered response to cyclic AMP stimulating hormones in intact human leukemic lymphocytes. Proc Soc Exp Biol Med. 1977 Apr;154(4):493–495. doi: 10.3181/00379727-154-39701. [DOI] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Scher N. S., Quagliata F., Malathi V. G., Faig D., Melton R. A., Silber R. Cyclic adenosine 3':5'-monophosphate phosphodiesterase activity in normal and chronic lymphocytic leukemia lymphocytes. Cancer Res. 1976 Nov;36(11 Pt 1):3958–3962. [PubMed] [Google Scholar]
- Sheppard J. R., Gormus R., Moldow C. F. Catecholamine hormone receptors are reduced on chronic lymphocytic leukaemic lymphocytes. Nature. 1977 Oct 20;269(5630):693–695. doi: 10.1038/269693a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryckmans P. A., Debusscher L., Collard E. Cell kinetics in chronic lymphocytic leukaemia (CLL). Clin Haematol. 1977 Feb;6(1):159–167. [PubMed] [Google Scholar]
- Themi H., Trepel F., Schick P., Kaboth W., Begemann H. Kinetics of lymphocytes in chronic lymphocytic leukemia: studies using continuous 3H-thymidine infusion in two patients. Blood. 1973 Oct;42(4):623–636. [PubMed] [Google Scholar]
- Walton G. M., Garren L. D. An assay for adenosine 3',5'-cyclic monophosphate based on the association of the nucleotide with a partially purified binding protein. Biochemistry. 1970 Oct 13;9(21):4223–4229. doi: 10.1021/bi00823a026. [DOI] [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Lymphocyte activation. Prog Allergy. 1976;20:195–300. [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


