Abstract
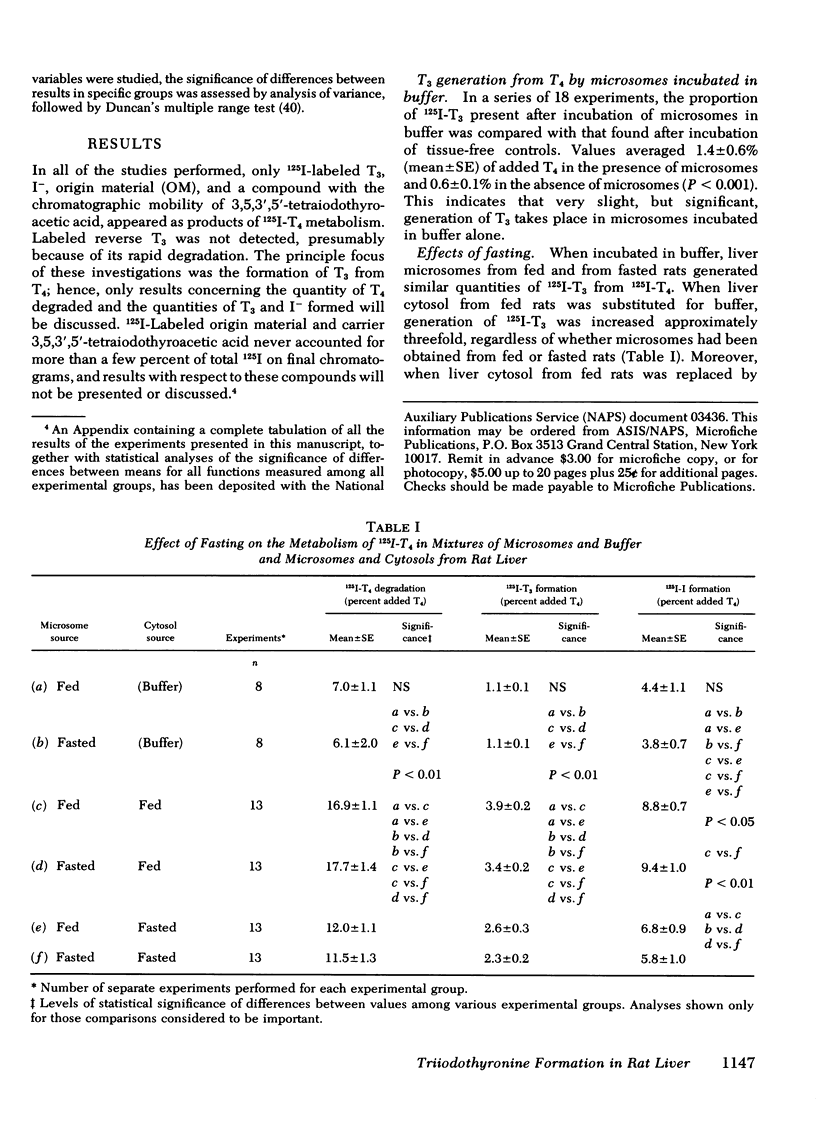
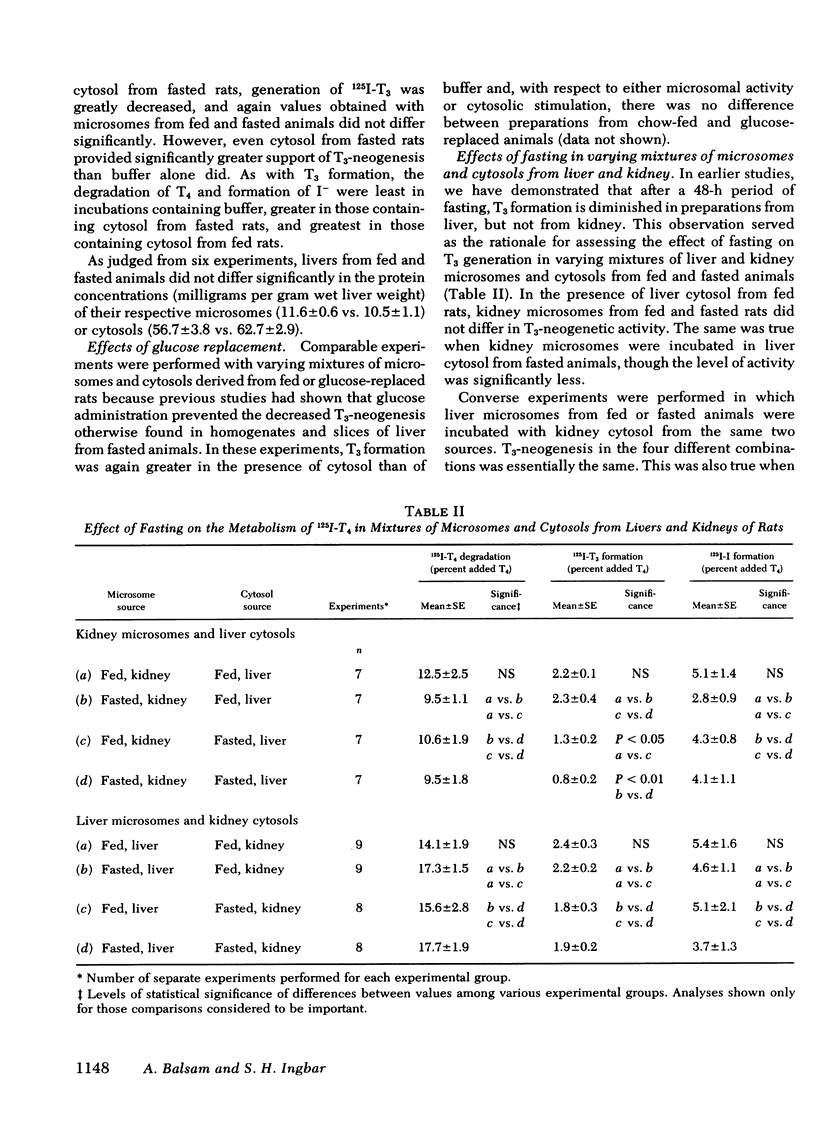
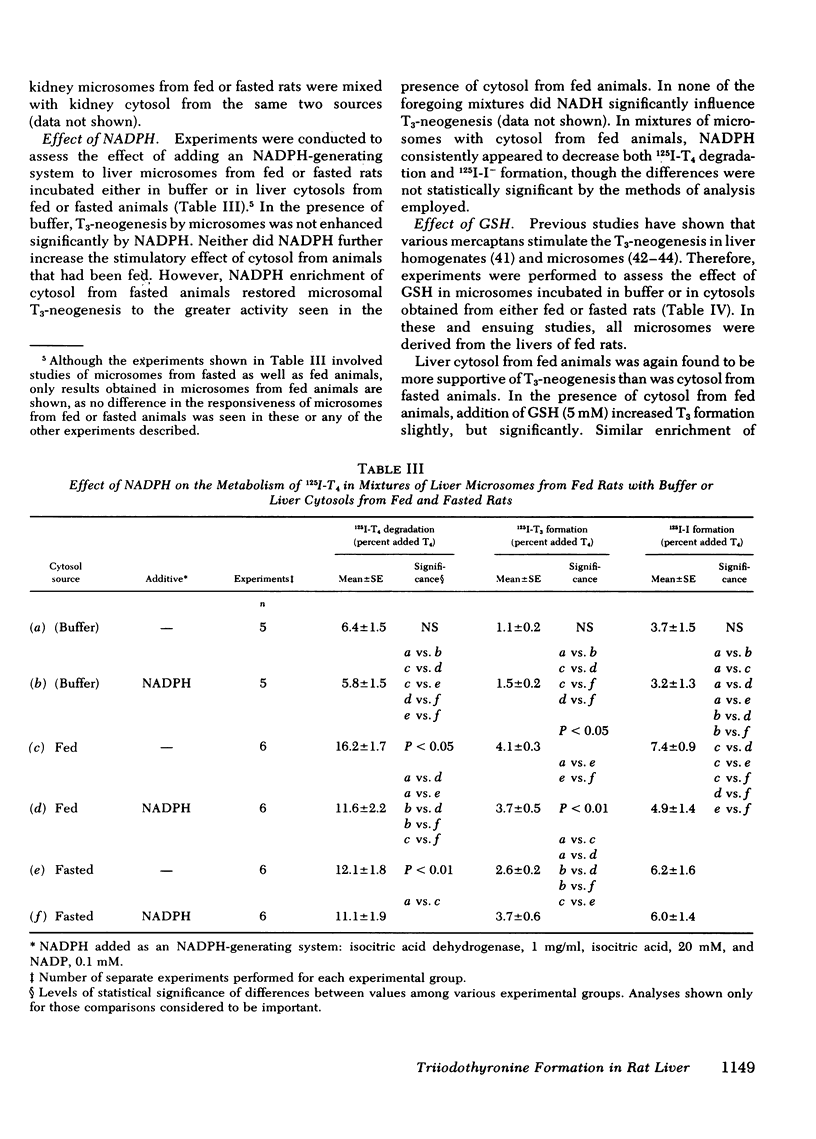
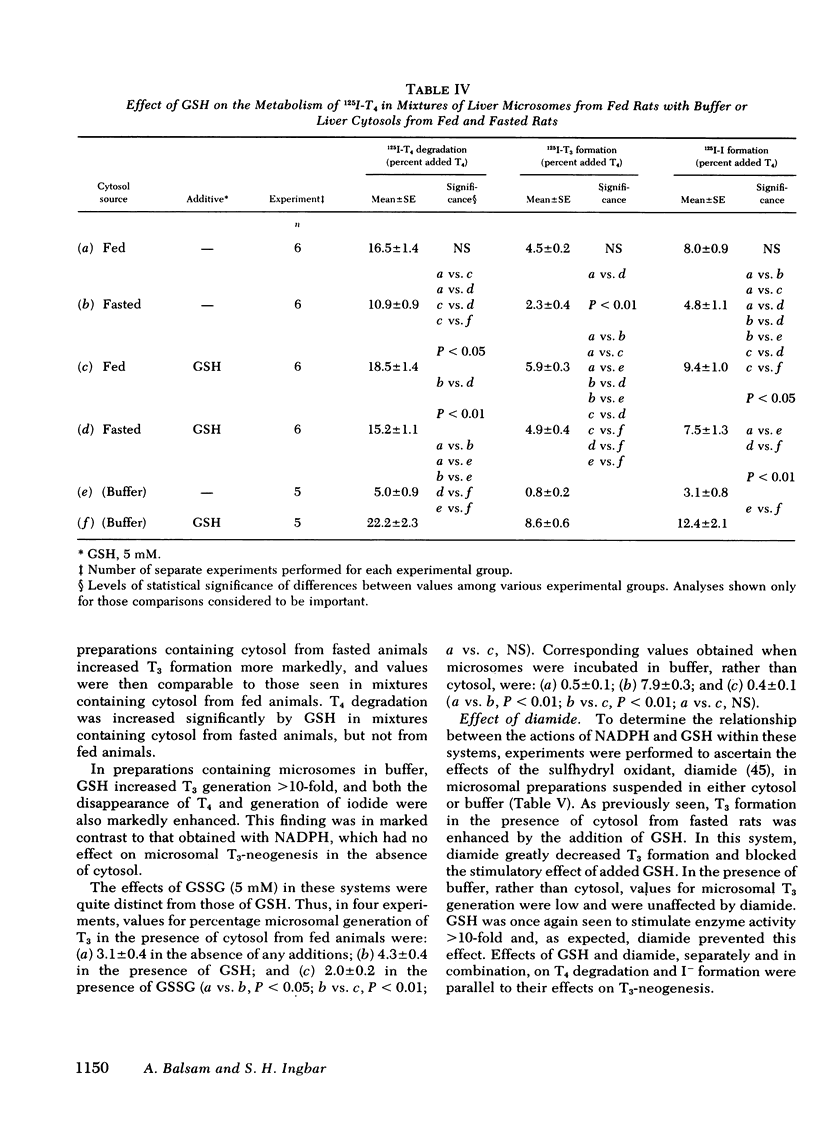
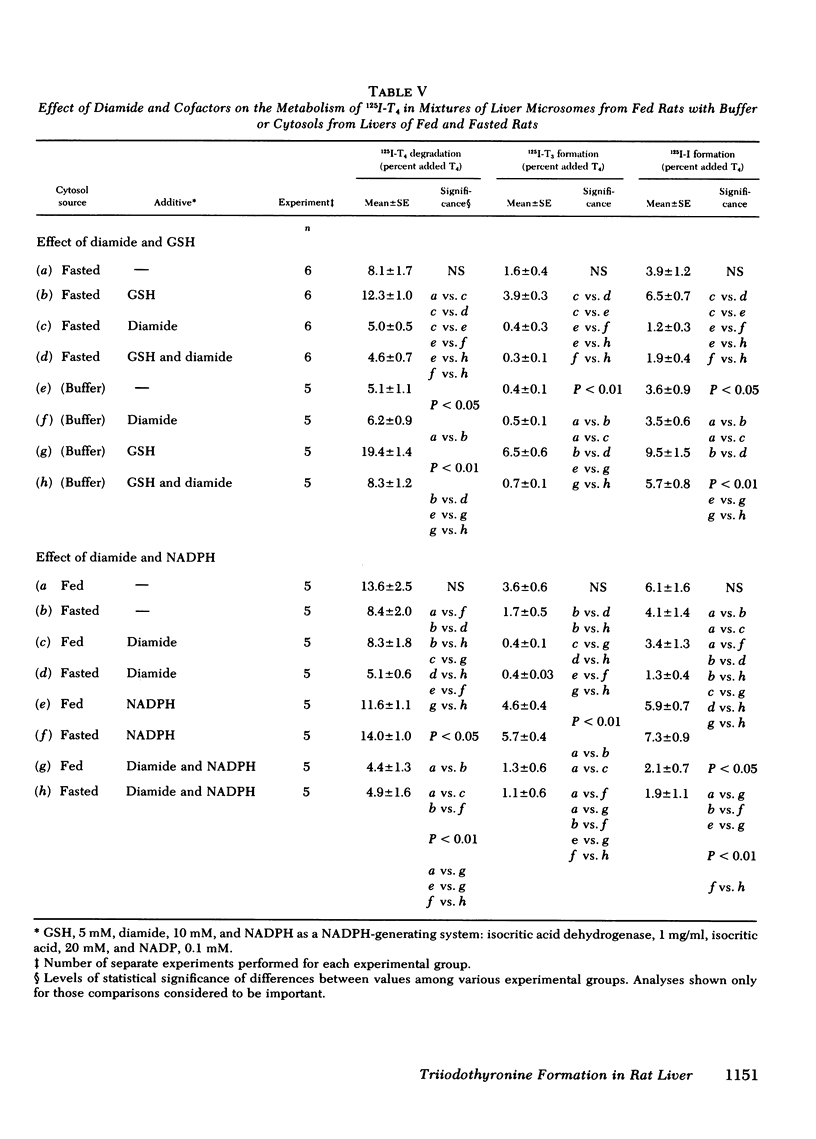
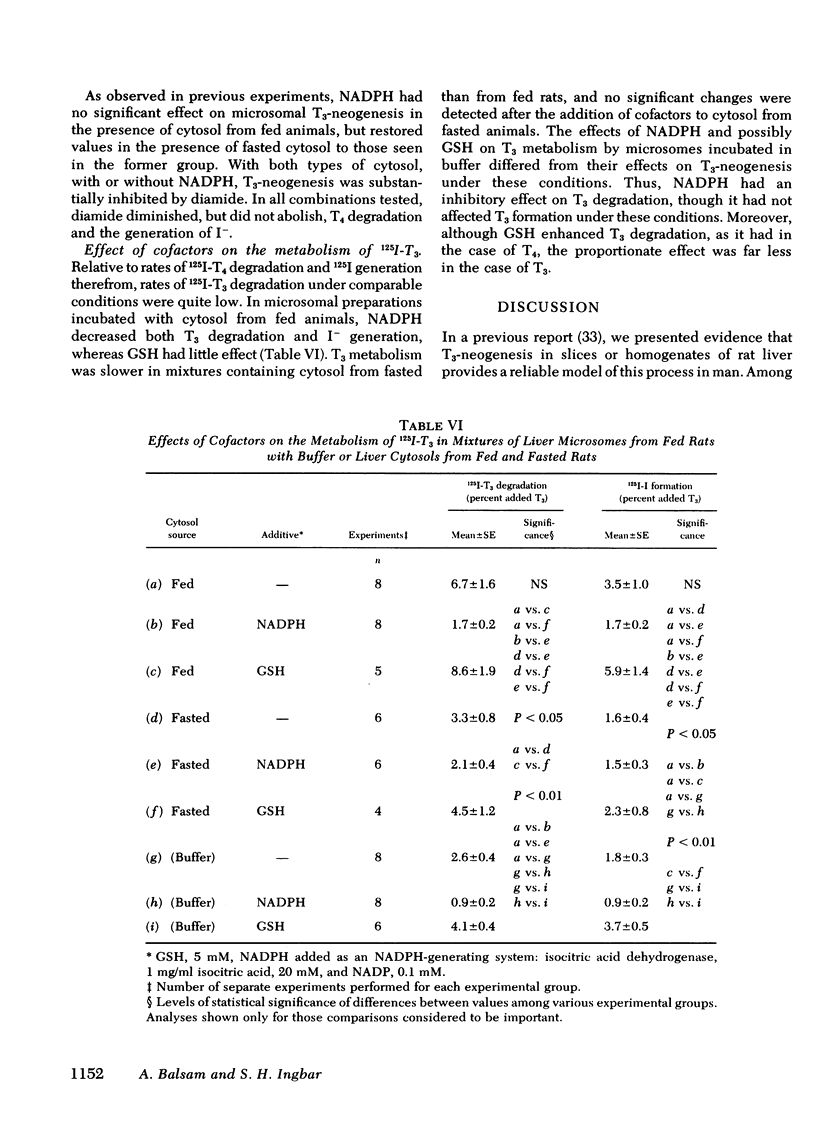
Studies were performed to explore the mechanism underlying the impaired generation of 125-I-3,5,3'-triiodothyronine (T3) from 125I-thyroxine (T4) (T3-neogenesis)) in preparations of liver from rats fasted for 48 h and the prevention of this effect by the feeding of glucose. T3-neogenesis in livers from fasted animals and those fed chow or glucose was assessed in various mixtures of crude microsomal fractions with either buffer or cytosols. T3-neogenesis was mediated by an enzyme present in the microsomal fraction whose activity was enhanced by cytosolic cofactor(s). In livers from animals fasted for 48 h, the supporting activity of cytosol was decreased, whereas the activity of the enzyme was unaffected. Administration of glucose as the sole nutritional source prevented the decrease in the supporting activity of hepatic cytosol that was regularly observed in the case of animals totally deprived of food. The diminished supporting activity for T3-neogenesis provided by liver cytosol from fasted animals was restored to normal by enrichment with either NADPH or GSH, but the two cofactors appeared to act at different loci. GSH stimulated T3-neogenesis in microsomes incubated in the absence of cytosol, i.e., in buffer, whereas NADPH did not. The stimulatory effect of both agents was blocked by the sulfhydryl oxidant, diamide, which also inhibited T3-neogenesis in mixtures of microsomes with cytosols. Taken together, these observations suggest that GSH acts directly on the enzyme in the crude microsomal fraction, whereas NADPH acts within the cytosol, possibly by increasing the concentration of GSH through the action of the enzyme glutathione reductase, for which NADPH is a cofactor. In this light, the decreased supporting activity of hepatic cytosol from starved animals appears to reflect, at least partly, a decreased concentration of one or both cofactors. The direct stimulation of enzyme activity by GSH, and the apparent lack of inhibition of unstimulated activity by diamide, suggests that the 5'-monodeiodinase for thyroxine that mediates T3-neogenesis may be a GSH transhydrogenase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER S. B., SHIMADA M., MAKIUCHI M. METABOLIC AND CARDIAC RESPONSES TO THYROXINE ANALOGS. Endocrinology. 1965 Jan;76:115–121. doi: 10.1210/endo-76-1-115. [DOI] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. The influence of fasting, diabetes, and several pharmacological agents on the pathways of thyroxine metabolism in rat liver. J Clin Invest. 1978 Aug;62(2):415–424. doi: 10.1172/JCI109143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Bermudez F., Surks M. I., Oppenheimer J. H. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975 Jul;41(1):27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- Brandt M., Kahlet H., Hansen J. M., Skovsted L. Letter: Serum triiodothyronine and surgery. Lancet. 1976 Feb 28;1(7957):491–491. doi: 10.1016/s0140-6736(76)91521-x. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Dinichert D., Nicod P., Jenny M., Lemarchand-Béraud T., Vallotton M. B. Effect of amiodarone on serum triiodothyronine, reverse triiodothyronine, thyroxin, and thyrotropin. A drug influencing peripheral metabolism of thyroid hormones. J Clin Invest. 1976 Aug;58(2):255–259. doi: 10.1172/JCI108466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgi H., Wimpfheimer C., Burger A., Zaunbauer W., Rösler H., Lemarchand-Béraud T. Changes of circulating thyroxine, triiodothyronine and reverse triiodothyronine after radiographic contrast agents. J Clin Endocrinol Metab. 1976 Dec;43(6):1203–1210. doi: 10.1210/jcem-43-6-1203. [DOI] [PubMed] [Google Scholar]
- Carter J. N., Eastman C. J., Corcoran J. M., Lazarus L. Effect of severe, chronic illness on thyroid function. Lancet. 1974 Oct 26;2(7887):971–974. doi: 10.1016/s0140-6736(74)92070-4. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Chopra U., Smith S. R., Reza M., Solomon D. H. Reciprocal changes in serum concentrations of 3,3',5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab. 1975 Dec;41(06):1043–1049. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Smith S. R. Circulating thyroid hormones and thyrotropin in adult patients with protein-calorie malnutrition. J Clin Endocrinol Metab. 1975 Feb;40(2):221–227. doi: 10.1210/jcem-40-2-221. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Chopra U., Young R. T., Chua Teco G. N. Alterations in circulating thyroid hormones and thyrotropin in hepatic cirrhosis: evidence for euthyroidism despite subnormal serum triiodothyronine. J Clin Endocrinol Metab. 1974 Sep;39(3):501–511. doi: 10.1210/jcem-39-3-501. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. Sulfhydryl groups and the monodeiodination of thyroxine to triiodothyronine. Science. 1978 Feb 24;199(4331):904–906. doi: 10.1126/science.622575. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Williams D. E., Orgiazzi J., Solomon D. H. Opposite effects of dexamethasone on serum concentrations of 3,3',5'-triiodothyronine (reverse T3) and 3,3'5-triiodothyronine (T3). J Clin Endocrinol Metab. 1975 Nov;41(5):911–920. doi: 10.1210/jcem-41-5-911. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Campbell A. C. Letter: Emigration and internal loss of pathologists. Lancet. 1976 May 15;1(7968):1071–1072. doi: 10.1016/s0140-6736(76)92238-8. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Hoye K. Dexamethasone suppression of serum T3 and T4. J Clin Endocrinol Metab. 1976 May;42(5):976–978. doi: 10.1210/jcem-42-5-976. [DOI] [PubMed] [Google Scholar]
- Duick D. S., Warren D. W., Nicoloff J. T., Otis C. L., Croxson M. S. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab. 1974 Dec;39(6):1151–1154. doi: 10.1210/jcem-39-6-1151. [DOI] [PubMed] [Google Scholar]
- Eisenstein Z., Hagg S., Vagenakis A. G., Fang S. L., Ransil B., Burger A., Balsam A., Braverman L. E., Ingbar S. H. Effect of starvation on the production and peripheral metabolism of 3,3',5'-triiodothyronine in euthyroid obese subjects. J Clin Endocrinol Metab. 1978 Oct;47(4):889–893. doi: 10.1210/jcem-47-4-889. [DOI] [PubMed] [Google Scholar]
- Geffner D. L., Azukizawa M., Hershman J. M. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975 Feb;55(2):224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken B., Ködding R., Von Zur Mühlen A., Hehrmann T., Jüppner H., Hesch R. D. Regulation of thyroid hormone metabolism in rat liver fractions. Biochim Biophys Acta. 1978 Feb 13;539(1):114–124. doi: 10.1016/0304-4165(78)90126-5. [DOI] [PubMed] [Google Scholar]
- Inada M., Kasagi K., Kurata S., Kazama Y., Takayama H., Torizuka K., Fukase M., Soma T. Estimation of thyroxine and triiodothyronine distribution and of the conversion rate of thyroxine to triiodothyronine in man. J Clin Invest. 1975 Jun;55(6):1337–1348. doi: 10.1172/JCI108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Effect of 18 hr fast and glutathione depletion on 1,1-dichloroethylene-induced hepatotoxicity and lethality in rats. Exp Mol Pathol. 1974 Apr;20(2):187–198. doi: 10.1016/0014-4800(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Subcellular alterations causing reduced hepatic thyroxine-5'-monodeiodinase activity in fasted fats. Endocrinology. 1979 Jan;104(1):58–64. doi: 10.1210/endo-104-1-58. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Thyroxine 5'-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology. 1978 Dec;103(6):2137–2144. doi: 10.1210/endo-103-6-2137. [DOI] [PubMed] [Google Scholar]
- Letter: Thyroid hormones in serious non-thyroidal illness. Lancet. 1976 May 15;1(7968):1070–1071. [PubMed] [Google Scholar]
- MONEY W. L., KUMAOKA S., RAWSON R. W., KROC R. L. Comparative effects of thyroxine analogues in experimental animals. Ann N Y Acad Sci. 1960 Apr 23;86:512–544. doi: 10.1111/j.1749-6632.1960.tb42827.x. [DOI] [PubMed] [Google Scholar]
- Maruyama E., Kojima K., Higashi T., Sakamoto Y. Effect of diet on liver glutathione and glutathione reductase. J Biochem. 1968 Mar;63(3):398–399. [PubMed] [Google Scholar]
- McGUIRE J. S., TOMKINS G. M. The multiplicity and specificity of delta 4-3-ketosteroid hydrogenases (5 alpha). Arch Biochem Biophys. 1959 Jun;82(2):476–477. doi: 10.1016/0003-9861(59)90146-8. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., TOMIZAWA H. H., WILLIAMS R. H. Sulfhydryl factors in degradation of insulin-I131 by liver extracts. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):718–721. doi: 10.3181/00379727-92-22591. [DOI] [PubMed] [Google Scholar]
- Nomura S., Pittman C. S., Chambers J. B., Jr, Buck M. W., Shimizu T. Reduced peripheral conversion of thyroxine to triiodothyronine in patients with hepatic cirrhosis. J Clin Invest. 1975 Sep;56(3):643–652. doi: 10.1172/JCI108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER V. R., ONO T. Enzyme patterns in rat liver and Morris hepatoma 5123 during metabolic transitions. Cold Spring Harb Symp Quant Biol. 1961;26:355–362. doi: 10.1101/sqb.1961.026.01.043. [DOI] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- Saberi M., Sterling F. H., Utiger R. D. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J Clin Invest. 1975 Feb;55(2):218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding S. W., Chopra I. J., Sherwin R. S., Lyall S. S. Effect of caloric restriction and dietary composition of serum T3 and reverse T3 in man. J Clin Endocrinol Metab. 1976 Jan;42(1):197–200. doi: 10.1210/jcem-42-1-197. [DOI] [PubMed] [Google Scholar]
- Suda A. K., Pittman C. S., Shimizu T., Chambers J. B., Jr The production and metabolism of 3,5,3'-triiodothyronine and 3,3',5-triiodothyronine in normal and fasting subjects. J Clin Endocrinol Metab. 1978 Dec;47(6):1311–1319. doi: 10.1210/jcem-47-6-1311. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. The hexosemonophosphate shunt and adaptive hyperlipogenesis. Diabetes. 1958 Nov-Dec;7(6):478–485. doi: 10.2337/diab.7.6.478. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA H. H. Mode of action of an insulin-degrading enzyme from beef liver. J Biol Chem. 1962 Feb;237:428–431. [PubMed] [Google Scholar]
- TOMKINS G. M. The enzymatic reduction of delta 4-3-ketosteroids. J Biol Chem. 1957 Mar;225(1):13–24. [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W. M., Touber J. L. The influence of beta-adrenoceptor blocking agents on plasma thyroxine and triiodothyronine. J Clin Endocrinol Metab. 1977 Aug;45(2):293–298. doi: 10.1210/jcem-45-2-293. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Chopra I. J., Solomon D. H., Bennett L. R. Changes in circulating iodothyronines in euthyroid and hyperthyroid subjects given ipodate (Oragrafin), an agent for oral cholecystography. J Clin Endocrinol Metab. 1978 Apr;46(4):691–697. doi: 10.1210/jcem-46-4-691. [DOI] [PubMed] [Google Scholar]


