Maintaining genomic integrity is a critical function for all cells. Damage that interrupts the efficient and accurate transcription and translation of the genetic code can lead to devastating phenotypic consequences. This problem is all the more acute in multicellular organisms where proper homeostasis is maintained by intricate mechanisms that depend on the constituent cells working coordinately with one another. This coordination depends in large measure on each cell having an identical set of ‘blueprints’ in its nucleus. Maintaining an accurate set of blueprints, therefore, puts a premium on two cellular activities – accurate DNA replication and robust mechanisms for DNA damage repair. The maintenance of genomic integrity presents a particular problem for terminally differentiated cells such as neurons. Mature nerve cells go through their final cell division during the embryonic period and must therefore maintain their genome intact for as long as eight or nine decades. Repeated DNA damage cannot be avoided during that time frame. Indeed, it has been estimated that each cell suffers on average the equivalent of 19,200 incidents of damage each day (Gorbunova et al. 2007). In response, the nerve cell has evolved numerous overlapping mechanisms to repair damaged DNA.
In keeping with the importance of DNA repair to the health and survival of neurons, it has been pointed out that mutations of DNA repair genes frequently lead to syndromes that include pronounced defects in the central nervous system (Adelman et al., 2009; Baranes et al., 2009; Jacobsen et al., 2004). Some of these strike early in development. For example knockouts of ligase IV (Barnes et al., 1998; Frank et al., 1998) or the XRCC4 (Gao et al., 1998), proteins responsible for the non-homologous end-joining form of DNA repair, produce syndromes that involve dysgenesis and death in the central nervous system followed by embryonic lethality. Note, however, that when the downstream DNA damage response and apoptosis networks are blocked, the CNS phenotypes can be largely, if not completely, rescued (Gao et al., 2000; Lee et al., 2000). One interpretation of these findings is that DNA damage on its own is probably not immediately lethal to a nerve cell. We can presume that the extent of initial DNA damage caused by the XRCC4 deficiency is the same in the presence or absence of ATM. It follows, therefore, that it is the ATM-dependent repair process that is the true neuronal assassin in this case (analogous to the situation is in stroke where most of the damage is caused not by the initial blockage of the coronary vessel, but by the reperfusion after the blockage clears). This interpretation is consistent with the idea that the nervous system places such a premium on genomic integrity that it will actively eliminate cells with damage rather than passively wait for their loss.
Of the many proteins that are involved in the DNA damage response, one of the best studied is ATM (ataxia-telangiectasia mutated). ATM is a 370 kD member of the PI3- kinase family of serine/threonine protein kinases. Its role in the DNA damage response is well studied. Upon recruitment to a break in the DNA double helix by the MRN (Mre11/Rad51/Nbs1) complex, ATM auto-activates itself by phosphorylation of the centrally placed serine 1981. This leads to the dissociation of the largely inactive dimeric form of ATM to a catalytically active monomer (Bakkenist and Kastan, 2003). Once activated, ATM targets several proteins for phosphorylation and the resulting alterations in their functions either assist in the DNA repair process itself (Brca1, Nbs1, γ-H2AX, etc.) or in the arrest of the cell cycle (p53, Chk2, etc.) to help to prevent further DNA damage (Barzilai and Yamamoto, 2004; Lavin and Kozlov, 2007; McKinnon, 2004; Shiloh, 2003).
The complexity of ATM function in the nervous system
Thus, three factors suggest that ATM must play central role in the health and survival of neurons of the adult CNS. The first is the location of ATM at a key biochemical node in the DNA damage response; the second is the special requirement for a robust DNA repair mechanism in any differentiated, non-mitotic cell such as a neuron; the third is the evidence for developmental defects in the CNS of animals with mutations in any of a wide range of DNA repair genes. Yet when we consider the CNS phenotype of the ATM-deficient brain, we are forced to conclude that the mechanism leading to the deficit is almost undoubtedly more complex than a defect in DNA repair alone. Even in the complete absence of ATM all cells, including neurons, can still repair DNA; they just do so more slowly. “Slowly” means over a time scale of minutes to hours, but not over weeks or months. This delay, though relatively short in the context of an 80-year cellular lifespan may nonetheless be enough time to put considerable stress on a cell. Yet this enhanced stress does not seem to be adequate to explain either the anatomical or temporal specificity of the neurodegeneration in ATM-deficient humans or its failure to be reproduced in Atm−/− mouse Purkinje cells with identical genetic mutations. This leads to the speculation that while a slower than normal DNA repair process undoubtedly makes a nerve cell vulnerable, there must be additional forces that expose this vulnerability at a particular time and in a particular place in the A-T brain.
One possible insight into the nature of these forces comes from considering the issue raised above, that ATM plays additional roles beyond the DNA damage response in the cells of the developing and mature CNS. Indeed, while the signaling role of ATM following DNA damage has been explored in depth, there are numerous studies that demonstrate that ATM is involved in a much wider spectrum of cellular activities. One recurring theme is the responsiveness of ATM to the state of oxidation of a cell. This activity can be seen in the induction of ATM following oxidative stress through a novel mechanism involving the integrity of several intermolecular disulfide bonds (Guo et al., 2010a). Activation of ATM by DNA breaks results in S1981 phosphorylation and dimer dissociation. By contrast, under conditions of oxidation, disulfide bonds are formed that result in covalent linkages between the subunits of the dimer. In this crosslinked configuration, kinase activity is found to be activated. This newly discovered activity is independent of the state of phosphorylation of S1981 and while H2O2-activated ATM can phosphorylate p53 and CHK2, no increase in γH2AX, a third traditional ATM product, is found.
The involvement of ATM in the response to oxidative stress can also be seen in the fact that cells that are ATM deficient are more sensitive to oxidative damage (Barlow et al., 1999; Guo et al., 2010b). The function of ATM in the oxidative response blurs the clean distinction between the process of DNA repair and other cellular functions. In part, this is because nearly all of the agents that we use to induce DNA damage will also put an oxidative stress in the treated cells, and the reverse (Barzilai and Yamamoto, 2004).
The presence of ATM in neuronal cytoplasm
In addition to its role in the oxidative response of the neuron, the ATM protein has been identified as a component of the complex insulin signaling response through its modulation of the protein translation machinery (Yang and Kastan, 2000). By far the most extensive non-nuclear function of ATM, however, can be found in its association with cellular vesicles. Shortly after its discovery, it was reported that the ATM protein binds to β-adaptin (Lim et al., 1998) and a β -adaptin related protein that is found only in the nervous system, β -NAP (Newman et al., 1995). Both of these proteins are members of the AP-2 adaptin complex that helps to coat vesicles and shepherd proteins into clathrin-coated pits for endocytosis. This suggests that ATM-deficient neurons might have abnormalities of vesicle trafficking. And, as only one example, insulin secretion is compromised in Atm−/− mice (Miles et al., 2007). Additional evidence supporting a possible participation of ATM in vesicle physiology comes from a number of different morphological studies. Combining the results from several labs, it would appear that one of the principle non-nuclear locations of the ATM protein is in association with a variety of different vesicles. Immunocytochemistry has been used to identify ATM as associated with peroxisomes (Watters et al., 1999), lysosomes (Barlow et al., 2000), endosomes (Kuljis et al., 1999), and vesicles of unspecified origin (Brown et al., 1997; Watters et al., 1997). Our own lab has expanded the significance of this apparent interaction between ATM and the synaptic vesicles of neurons (Li et al., 2009).
In the neuron, ATM can be isolated from synaptosomes where, far from the DNA of the cell nucleus, it is found in physical association with two known synaptic vesicle proteins: synapsin-I and VAMP2 (synaptobrevin). The association requires the phosphorylation of the both proteins. ATM itself serves as the synapsin-I kinase, while the VAMP2 kinase is predominantly ATR (ATM and Rad-3 related). ATR is also found in association with the two vesicle proteins and with ATM. This four-protein complex has functional importance as can be shown in several ways. In Atm−/− cerebral cortical neurons, spontaneous vesicular dye release is slowed. This same effect is observed in wild type neurons that have been transfected with a non-phosphorylatable form of VAMP2 (T25A), demonstrating that the ‘un-tethered’ VAMP2 can act as a dominant negative agent in this activity. Beyond vesicle release, the absence of ATM has profound effects on the systems-level circuitry of the brain. Long-term potentiation (LTP) in the Schaeffer collateral circuit of the hippocampus is profoundly impaired in the absence of functional ATM protein.
These results and others make it clear that the full story of ATM in the neurons is shaped in cytoplasm the as well as in the nucleus. Yet it may bear consideration that, even in the nucleus itself, the DNA damage response may be only one of many roles played by ATM. Recall that the XRCC4/ATM double mutants hint that DNA damage per se is not acutely lethal to a neuron. If ATM-deficient cells can eventually repair DNA then why do Atm−/−neurons function poorly? Part of the explanation may be impaired vesicle trafficking. Recently, however, we have uncovered a separate ATM function that directly impacts the structure of the genome, even in the absence of DNA damage. Ironically, this ATM-mediated activity is not related to the DNA itself, but instead to the histone proteins that coat the double helix and are now recognized as part of what is termed the epigenome.
The epigenetic functions of ATM and the regulation of histone deacetylase-4
The addition of acetyl groups to the various histone proteins generally leads to a more open configuration of the chromatin. Histone deacetylases (HDACs), which remove these acetyl groups, tend to result in a more compact chromatin configuration. The resulting opening and closing of regions of the genomic structure has consequences for the activity of the genes encoded therein. There are exceptions, but generally when acetyl groups are added and the chromatin opens, transcription increases. When acetyl groups are removed and the chromatin closes, transcription decreases. Thus HDACs and their counterparts, the histone acetyltransferases, exert a second level of functional control over the activity of most if not all genes.
There are as many as 18 different human HDAC proteins. Based on their structure they are divided into classes. Class I includes HDAC1–3 and HDAC8; these are related to the yeast gene, RPD3. Class II includes HDAC4–7, HDAC9 and HDAC10; these are related to the yeast Hda1 histone deacetylase. Class III comprises the sirtuins (SIRT1–7), which are related to the yeast Sir2 gene. HDAC11 has features of both Class I and II and has been suggested to define a fourth class of HDAC. We were drawn to the function of HDAC4 because its levels are high in brain and particularly so in Purkinje cells. Neuronal HDAC4 is predominantly cytoplasmic due to its tethering to the 14-3-3 protein.
The physical interaction with 14-3-3 requires that HDAC4 be phosphorylated, a function that is carried out by calcium/calmodulin kinases (Grozinger and Schreiber, 2000; Wang et al., 2000). Dephosphorylated HDAC4 tends to accumulate in the cell nucleus (Li et al., 2012b). Finally, HDAC4-deficient mice demonstrate significant cerebellar atrophy, including Purkinje cell structure and number (Majdzadeh et al., 2008). In the aggregate, these observation led us to ask whether some of the phenotypes of the Atm−/− mouse might be related to a deficit of HDAC4 function.
We found that in both A-T patient samples and in brain tissue from Atm−/− mice, HDAC4 location shifted to a predominantly nuclear location (Li et al., 2012a). HDAC5 and HDAC9, also members of Class II were little unaffected. Since ATM deficiency repositions HDAC4 to the nucleus, it follows that there must be a constitutive activity of ATM that allows HDAC4 to remain cytoplasmic. We searched the sequences of both HDAC4 itself as well as its kinases, CaMKII and CaMKIV, but could not find a favored ATM/ATR kinase site. We therefore examined the HDAC4 phosphatase, PP2A. The PP2A holoenzyme consists of a regulatory A subunit (PR65), a catalytic C subunit (pp2ca) and a tissue specificity B subunit (PR55). We found that serine 401 of the A subunit was a highly favored ATM target. When we mutated S401 to aspartic acid, PP2A could no longer be pulled down by immunoprecipitation with HDAC4 antibody suggesting that the physical interaction between the two had been disrupted. This would have the effect of blocking HDAC4 dephosphorylation. We validated that this indirect method of ATM action accurately described the in vivo situation. As one example of this proof, when we applied the PP2A inhibitor, endothall, we were able to block the nuclear accumulation of HDAC4 in Atm−/− cultured neurons.
This ATM-dependent re-localization of HDAC4 has functional consequences. At the molecular level, the global extent of histone acetylation is decreased in ATM-deficient primary neurons. We also found that once it has moved to the nucleus, HDAC4 directly suppresses MEF2- and CREB-dependent transcriptional activities. Further, HDAC4 itself associates directly with the chromatin; a number of different regions of the DNA can be isolated from HDAC4 chromatin immunoprecipitation (ChIP). This association is profoundly increased in Atm−/− neurons, consistent with the increased levels of nuclear HDAC4. Most significantly of all, at the cellular level, restoring HDAC4 to its proper nucleocytoplasmic balance reverses both the cellular and organismal phenotypes of ATM-deficiency. This can be seen at all steps of the pathway. As mentioned above, PP2A inhibition reverses the neuronal cell death phenotypes. The same effect can be achieved with over expression of a phosphomimetic (S401D) mutation, which acts as a dominant-negative in Atm−/− cells. Similar rescue is achieved by blocking HDAC4 activity either pharmacologically (Trichostatin A) or with shRNA. These treatments successfully reverse the expression of activated caspase-3 and reverse the up-regulation of cell cycle proteins – a known antecedent to neuronal death in A-T.
The predictions of the model are also borne out when tested in the whole animal. Endothal, the PP2A inhibitor, cannot be tested in vivo as it is toxic in mice at the concentrations needed to achieve PP2A inhibition. HDAC4 inhibition with Trichostatin A can be tested, however, and administration to Atm−/− mice results in a substantial improvement in their motor behavior measured either on a rota-rod or in open field behavior. We used lentiviral delivery of HDAC4 constructs by injection into the cerebellum to show that this is a location-dependent phenomenon. Delivery of viruses encoding HDAC4 mutants that are intrinsically nuclear in localization does not rescue Atm−/− animals; what’s more, it worsens the behavior of wild type mice. Significantly, HDAC4 mutants that are intrinsically cytoplasmic are sufficient to rescue the behavior of Atm−/− mice. The strong implication of these findings is that the full A-T phenotype is caused by both the destructive effects of HDAC4 in the nucleus as well as the loss of the positive effects of HDAC4 in the cytoplasm.
ATM beyond A-T
As the above examples suggest, the function of ATM is required throughout the lifetime of a neuron. Yet the condition we know of as ataxia-telangiectasia is caused by the absence of ATM protein from conception. This opens the possibility that the CNS defects that are observed in A-T may represent the loss of only the earliest neuronal functions that depend on ATM. Logically, therefore, alterations in ATM function later in life could have an important impact on neuronal health and survival. We have begun to explore this idea in the context of a late-onset neurodegenerative illness, Alzheimer’s disease (AD). Several threads of evidence make this a potentially important relationship. The first is that DNA damage, especially that caused by oxidative stress, is known to increase with age, and to increase even further in the vulnerable regions of the AD and MCI brain (Coppede and Migliore, 2009). ATM is recognized as one of the primary mediators of the response to DNA double strand breaks that arise by exposure to ionizing radiation. However, cells lacking ATM are also hypersensitive to insults other than DSBs, particularly oxidative stress. The recent study by Guo et al. showed that ATM can be directly activated by ROS, independent of the DNA damage caused by ROS (Guo et al., 2010b). As oxidation damage increases with age, it would follow that the activity of ATM is increasingly necessary to protect us against this enhanced level of damage/stress.
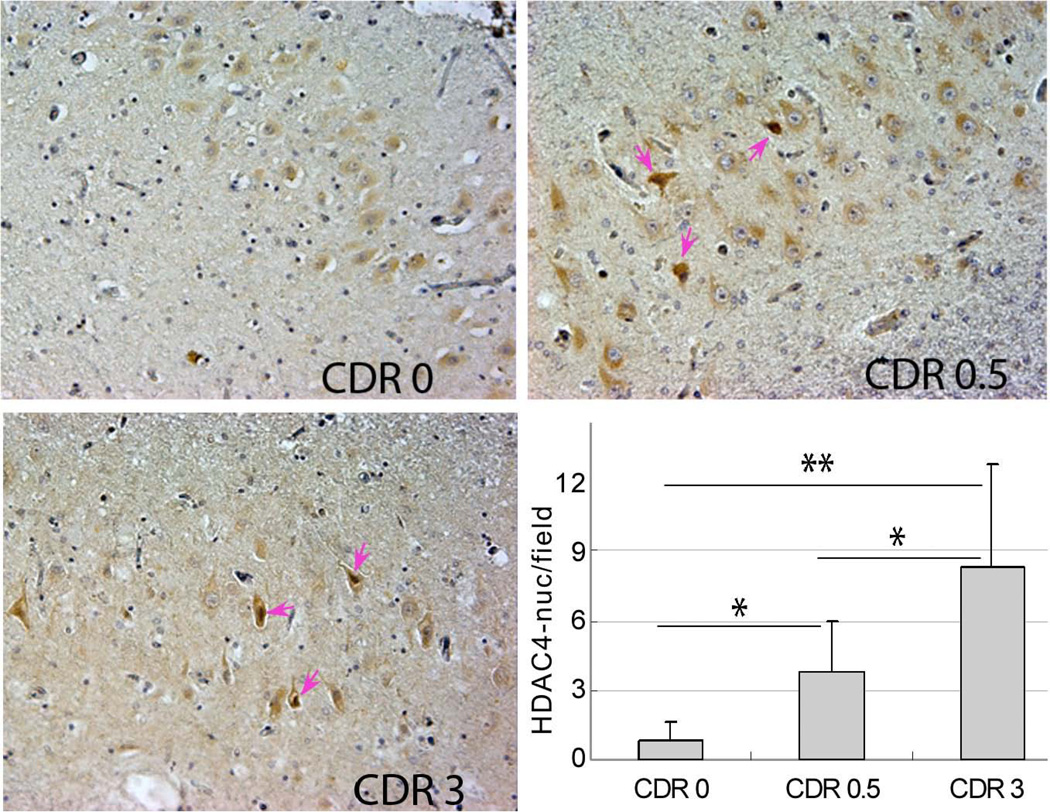
We have preliminary evidence in support of this prediction. One of the most devastating and common neurodegenerative diseases of aging is Alzheimer’s disease (AD). AD causes many cognitive and behavioral changes in its victims including the increasing inability to acquire new memories. The disease results in cell loss in many parts of the brain, but given the prominence of the memory defects, it is not surprising that early sites of neuronal loss include the entorhinal cortex and hippocampus. To determine the involvement of ATM deficiency in this cell loss we have used the location of HDAC4 as an indicator of a cell’s ATM activity. Our reasoning is that if ATM activity drops, then HDAC4 should adopt a more nuclear localization. We examined CA1 pyramidal cells from multiple individuals who died with advanced AD (a Clinical Dementia Rating, CDR, of 3), with mild cognitive impairment (CDR 0.5 – generally accepted as an early stage of AD), or with no noted mental decline (CDR 0). As can be seen in the Figure, there is a statistically significant, increase in the density of CA1 neurons with nuclear HDAC4 as AD advances in severity (Chen et al, unpublished).
Figure.
Hippocampal neurons of the CA1 field of the human hippocampus immunostained to detect HDAC4 location. In elderly individuals with no cognitive impairment (CDR 0), most neuronal HDAC4 is located in cytoplasm. In individuals with early (CDR 0.5) or late (CDR 3) stage Alzheimer’s disease, neurons with prominent nuclear HDAC4 (examples highlighted with pink arrows) become more frequent. The histogram represents the quantification of these results from 6 individuals with each condition. Different by Student’s t-test: * p ≤ 0.05; ** p ≤ 0.01.
The interaction of ATM with other features of the aging process
These responses of ATM to the altered chemistry and biology of the AD brain suggests a model of brain aging that incorporates ATM function. The idea is that as late-life neurodegenerative disease processes begin, the neurons of the CNS respond with an enhanced DNA damage response. Yet while DNA damage and oxidative stress both increase with age in the CNS, the levels of total ATM actually drop (Feng et al., 2007). One prediction of this situation would be that though ATM activation might be able to be induced normally, the cells are less and less capable of mounting a full stress response since the components of that process are more and more scarce in the cell. This perspective on brain aging has received an additional measure of support from our recent studies on the involvement of the amino acid glutamine in the health and survival of CNS neurons.
Glutamine can be synthesized by most cells through the action of glutamine synthase (GS), yet most cell types fare poorly if exogenous glutamine is not provided. Thus glutamine is known as a “conditionally essential” amino acid. There is a strong tie to the aging process as glutamine levels and glutamine synthetase activity both decline with age in the CNS. The activity of GS is vulnerable to oxidation, which as discussed previously rises exponentially with age. Oxidative stress induces region-specific loss of GS activity in the brain, and the decrease is more significant in AD patients than in agematched controls (Smith et al., 1991). For many years, clinicians have recognized the protective value of glutamine supplementation following abdominal surgery or for its effects as an anti-inflammatory agent. We have applied these lessons to the situation in AD (Chen and Herrup, 2012). We found an entire spectrum of deficiencies in the stress response of cells if they are deprived of adequate exogenous glutamine, and improvement in the same responses if glutamine levels were elevated. For example, we found that raising glutamine levels in the medium protects cultured neuronal cells against the amyloid peptide in vivo and in vitro. What is most striking, however, is that in glutamine-deprived cultures the levels of many proteins involved in the DNA damage response are reduced several fold. This includes 53BP1, ATM and ATR. Thus, in low glutamine the components of the DNA damage response are reduced. For example, in low glutamine 53BP1 levels increase after DNA damage, but both the absolute levels and the relative increase are much less than that found in cells grown in normal medium. ATM as well as ATR levels also decrease in cultures deprived of glutamine. The reduced levels of ATM have downstream consequences that magnify this effect still further. While the specialized histone – H2AX – is slightly reduced in low glutamine, after DNA damage the phosphorylated form, γH2AX, though higher than in undamaged cultures, remains far below the levels achieved in normal glutamine-containing medium. The consequences of this are straightforward and nearly predictable. Following an incident of DNA damage, the cells can begin to repair their DNA, but in low glutamine the accompanying initiation of the cell death process cannot be reversed. Thus, even though COMET assays demonstrate that cells are able to join the majority of their DNA breaks in low glutamine, there was significantly more cell death in the glutaminedeprived cultures (Chen and Herrup, 2012).
Concluding comments
These observations leave us with more a complicated model of the interplay of DNA damage and ATM function. There is little question that the integrity of the genome must be preserved if normal neuronal function is to be maintained. This is most apparent when one views the embryonic and perinatal CNS consequences of mutations in DNA repair enzymes. Yet the genetic evidence also suggests that DNA damage per se is not immediately toxic; it is the cells’ response to the damage that is the acutely lethal event. We propose that this is an adaptive response that protects the organism from having to deal with the abnormal behavior of cells with a scrambled set of “blueprints”. In a tissue in which rapid replication from a stem cell reserve can replace any cells that might be lost this response can lead to a restored and healthy function. By contrast, in a terminally differentiated population such as the neurons of the CNS, the degeneration of cells with DNA damage leads to an irreversible loss of function and eventually to severe neurological consequences. ATM plays a major role in this adaptive response, signaling to downstream effectors such as p53 whether the damage is great enough to initiate apoptosis.
Yet our work on the CNS function of ATM leads us to conclude that its participation in the DNA damage response is one of a number of roles that this large PI3-kinase family member plays. In addition to helping to monitor the genetic integrity of CNS neurons, ATM also plays other important roles in neuronal functions. It serves as a synapsin-I kinase and as a vesicle protein binding protein. Loss of this function leads to consequences for synaptic vesicle trafficking, network properties such as the establishment of LTP and a reduced dendritic arbor (Chen et al., 2003). Furthermore, even in the absence of DNA damage, ATM activity is required by adult neurons to constitutively repress the activity of the HDAC4 phosphatase, PP2A. Without this activity, HDAC4 shifts towards a more de-phosphorylated form and in so doing becomes localized more prominently in the nucleus. This is bad for neurons in two ways. The first is that, once nuclear, HDAC4 becomes a rogue HDAC and deacetylates histones H3 and H4 leading to a genome wide shift in gene expression. The second is that it deprives the neuron of some cytoplasmic activity, undefined as of now, that is supportive of the health of the cell.
All of these functions are important to a neuron during its development, but implicit in the continuing role for ATM in the adult neuron is that some of the neurodegenerative features of normal and pathological aging may reflect in part a loss of these other ATM functions. If true, then the cell biology of the neuron in an early childhood disease, A-T, may paradoxically carry lessons for the explorations of other neurodegenerative diseases.
ACKNOWLEDGEMENTS
The writing and additional support for this work was provided by Rutgers University as well as the NIH (AG029494 to KH). The authors wish to acknowledge the generosity of the the Neuropathology Core of the Alzheimer’s Disease Research Center at Washington University in St. Louis (P50 AG05681) for providing us with samples from their collection of AD and control material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adelman CA, De S, Petrini JH. Rad50 is dispensable for the maintenance and viability of postmitotic tissues. Molecular and cellular biology. 2009;29:483–492. doi: 10.1128/MCB.01525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Baranes K, Raz-Prag D, Nitzan A, Galron R, Ashery-Padan R, Rotenstreich Y, Assaf Y, Shiloh Y, Wang ZQ, Barzilai A, et al. Conditional inactivation of the NBS1 gene in the mouse central nervous system leads to neurodegeneration and disorganization of the visual system. Experimental neurology. 2009;218:24–32. doi: 10.1016/j.expneurol.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Barlow C, Dennery PA, Shigenaga MK, Smith MA, Morrow JD, Roberts LJ, 2nd, Wynshaw-Boris A, Levine RL. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci U S A. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C, Ribaut-Barassin C, Zwingman TA, Pope AJ, Brown KD, Owens JW, Larson D, Harrington EA, Haeberle AM, Mariani J, et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci U S A. 2000;97:871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004;3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Brown KD, Ziv Y, Sadanandan SN, Chessa L, Collins FS, Shiloh Y, Tagle DA. The ataxia-telangiectasia gene product, a constitutively expressed nuclear protein that is not up-regulated following genome damage. Proc Natl Acad Sci U S A. 1997;94:1840–1845. doi: 10.1073/pnas.94.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Herrup K. Glutamine acts as a neuroprotectant against DNA damage, beta-amyloid and H2O2-induced stress. PloS one. 2012;7:e33177. doi: 10.1371/journal.pone.0033177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Peng C, Luff J, Spring K, Watters D, Bottle S, Furuya S, Lavin MF. Oxidative stress is responsible for deficient survival and dendritogenesis in purkinje neurons from ataxia-telangiectasia mutated mutant mice. J Neurosci. 2003;23:11453–11460. doi: 10.1523/JNEUROSCI.23-36-11453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F, Migliore L. DNA damage and repair in Alzheimer's disease. Curr Alzheimer Res. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci U S A. 2007;104:16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL, Davidson L, Kangaloo L, Alt FW. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, Chaudhuri J, Horner J, DePinho RA, Alt FW. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Wang J, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000;97:7835–7840. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010a;9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010b;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Jacobsen E, Beach T, Shen Y, Li R, Chang Y. Deficiency of the Mre11 DNA repair complex in Alzheimer's disease brains. Brain Res Mol Brain Res. 2004;128:1–7. doi: 10.1016/j.molbrainres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Chen G, Lee EY, Aguila MC, Xu Y. ATM immunolocalization in mouse neuronal endosomes: implications for ataxia-telangiectasia. Brain Res. 1999;842:351–358. doi: 10.1016/s0006-8993(99)01813-2. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- Lee Y, Barnes DE, Lindahl T, McKinnon PJ. Defective neurogenesis resulting from DNA ligase IV deficiency requires Atm. Genes Dev. 2000;14:2576–2580. doi: 10.1101/gad.837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Ricupero C, Hart R, Schwartz M, Kusnecov A, Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia-telangiectasia. Nature Med. 2012a doi: 10.1038/nm.2709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A, Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nature medicine. 2012b;18:783–790. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han YR, Plummer MR, Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DS, Kirsch DG, Canman CE, Ahn JH, Ziv Y, Newman LS, Darnell RB, Shiloh Y, Kastan MB. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci U S A. 1998;95:10146–10151. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdzadeh N, Wang L, Morrison BE, Bassel-Duby R, Olson EN, D'Mello SR. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol. 2008;68:1076–1092. doi: 10.1002/dneu.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles PD, Treuner K, Latronica M, Olefsky JM, Barlow C. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am J Physiol Endocrinol Metab. 2007;293:E70–E74. doi: 10.1152/ajpendo.00259.2006. [DOI] [PubMed] [Google Scholar]
- Newman LS, McKeever MO, Okano HJ, Darnell RB. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AH, Kruhlak MJ, Wu J, Bertos NR, Vezmar M, Posner BI, Bazett-Jones DP, Yang XJ. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol Cell Biol. 2000;20:6904–6912. doi: 10.1128/mcb.20.18.6904-6912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters D, Kedar P, Spring K, Bjorkman J, Chen P, Gatei M, Birrell G, Garrone B, Srinivasa P, Crane DI, et al. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem. 1999;274:34277–34282. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- Watters D, Khanna KK, Beamish H, Birrell G, Spring K, Kedar P, Gatei M, Stenzel D, Hobson K, Kozlov S, et al. Cellular localisation of the ataxia-telangiectasia (ATM) gene product and discrimination between mutated and normal forms. Oncogene. 1997;14:1911–1921. doi: 10.1038/sj.onc.1201037. [DOI] [PubMed] [Google Scholar]
- Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]