Abstract
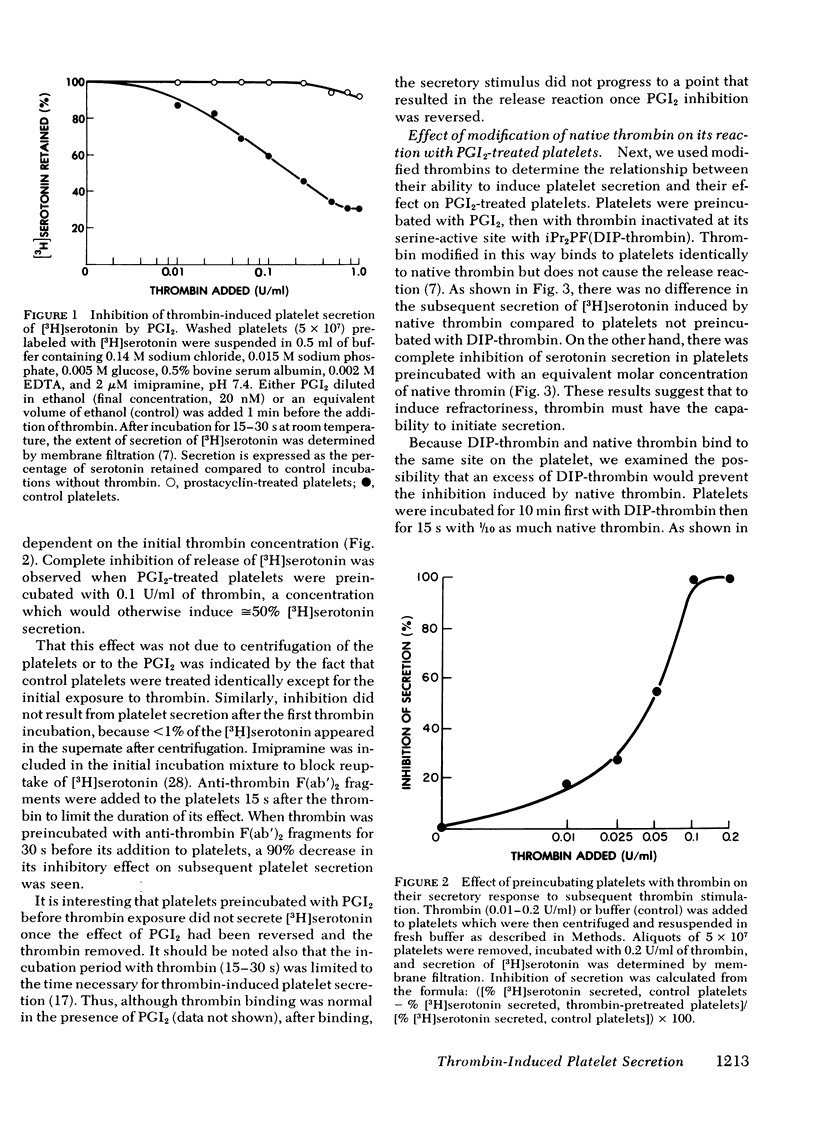
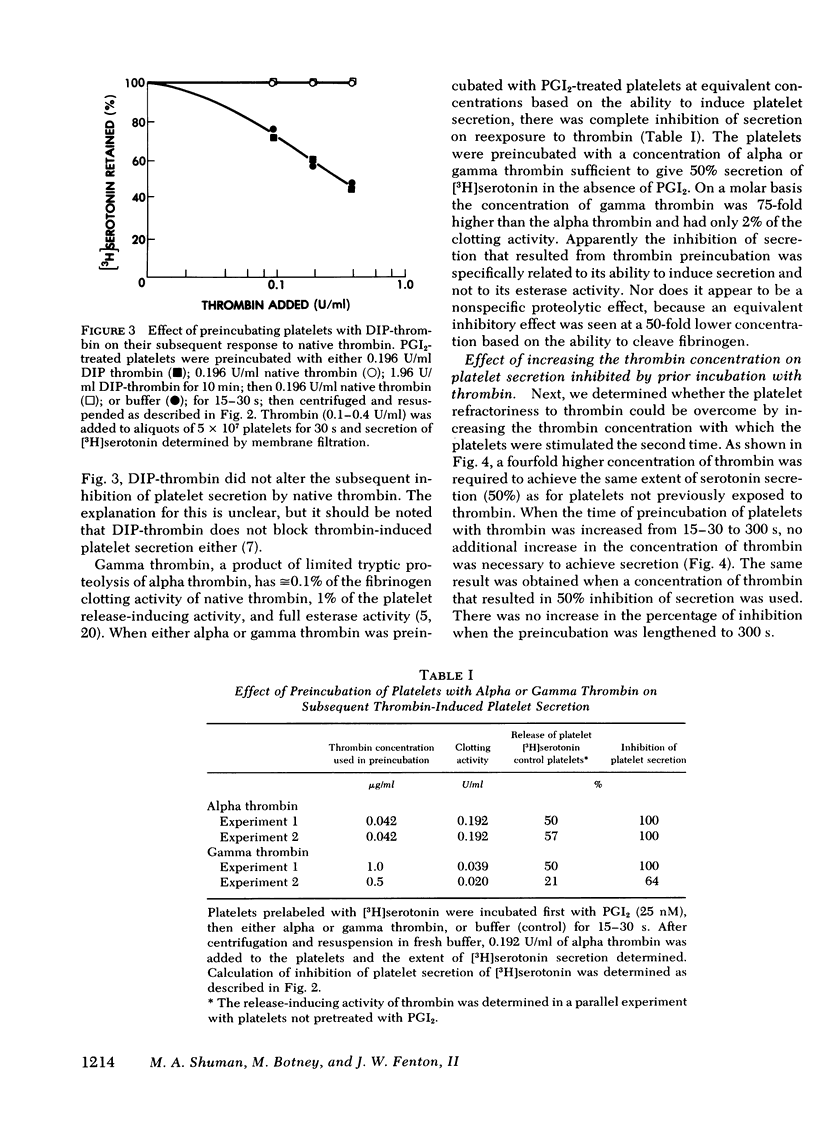
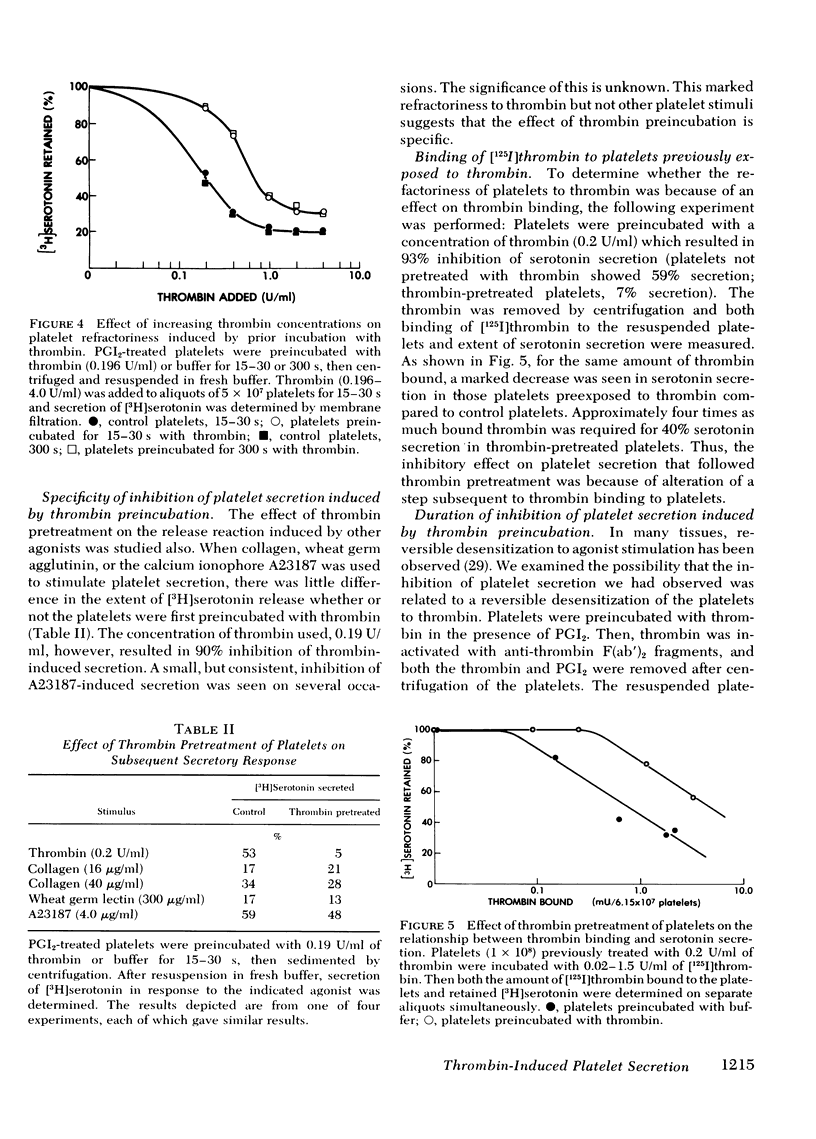
We have studied the interaction between thrombin and washed, human platelets using prostacyclin, a reversible inhibitor of platelet secretion. The effect of thrombin is limited to those reactions that are not inhibited by an increased concentration of platelet cyclic adenosine 3′,5′-monophosphate, because prostacyclin is a potent inducer of the latter. Prostacyclin-treated platelets were briefly (15-30 s) exposed to low concentrations of human thrombin (0.01-0.2 U/ml). After removal of the prostacyclin and thrombin, the platelets were incubated with fresh thrombin. Although they had not undergone the release reaction after the first thrombin incubation, these platelets had a diminished capacity to secrete [3H]serotonin when exposed to thrombin the second time. Refractoriness was concentration dependent: the higher the initial thrombin concentration, the greater the degree of inhibition of serotonin secretion on subsequent thrombin exposure. Inhibition was closely related to the ability of thrombin to induce platelet secretion and not to its esterase or fibrinogen clotting activity. Diisopropyl fluorophosphate-inactive thrombin did not induce refractoriness. Refractoriness to thrombin did not increase when the time of the initial incubation with thrombin was lengthened, nor was it reversible.
Inhibition was thrombin specific: serotonin secretion induced by collagen, wheat germ agglutinin, and the ionophore A23187 was minimally affected. For an equivalent amount of thrombin bound, a decrease was observed in serotonin secretion by thrombin-pretreated platelets compared to control platelets. Thus, there is at least one step in the secretory pathway between thrombin binding and regulation of adenylate cyclase. This step appears to transmit the signal that leads to extrusion of intracellular granular contents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRECHER G., CRONKITE E. P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950 Dec;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Brodie G. N., Baenziger N. L., Chase L. R., Majerus P. W. The effects of thrombin on adenyl cyclase activity and a membrane protein from human platelets. J Clin Invest. 1972 Jan;51(1):81–88. doi: 10.1172/JCI106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Friedman F., Detwiler T. C. Stimulus-secretion coupling in platelets. Effects of drugs on secretion on adenosine 5'-triphosphate. Biochemistry. 1975 Mar 25;14(6):1315–1320. doi: 10.1021/bi00677a033. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Bunting S., Moncada S., Flower R. J., Vane J. R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976 Nov;12(5):685–713. doi: 10.1016/0090-6980(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Harbury C. B., Schrier S. L. The effect of a partial release reaction on subsequent platelet function. J Lab Clin Med. 1974 Jun;83(6):877–886. [PubMed] [Google Scholar]
- Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J Clin Invest. 1967 Mar;46(3):409–417. doi: 10.1172/JCI105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Stanford N., Majerus P. W. Thrombin-induced protein phosphorylation in human platelets. J Clin Invest. 1975 Oct;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou K. C., Bernette W. E., Gasic B. P., Magolda R. L., Sipio W. J., Silver M. J., Smith J. B., Ingerman C. M. Rapid and easy preparation of prostacyclin. Lancet. 1977 May 14;1(8020):1058–1059. doi: 10.1016/s0140-6736(77)91297-1. [DOI] [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Pletscher A. Metabolism, transfer and storage of 5-hydroxytryptamine in blood platelets. Br J Pharmacol Chemother. 1968 Jan;32(1):1–16. doi: 10.1111/j.1476-5381.1968.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers H. J., Packham M. A., Kinlough-Rathbone R. L., Mustard J. F. Effect of repeated treatment of rabbit platelets with low concentrations of thrombin on their function, metabolism and survival. Br J Haematol. 1973 Nov;25(5):675–689. doi: 10.1111/j.1365-2141.1973.tb01780.x. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Davis J. N., Lefkowitz R. J. alpha-Adrenergic receptors in rat parotid cells. II. Desensitization of receptor binding sites and potassium release. J Biol Chem. 1977 Aug 10;252(15):5478–5482. [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Warshaw A. L., Laster L., Shulman N. R. The stimulation by thrombin of glucose oxidation in human platelets. J Clin Invest. 1966 Dec;45(12):1923–1934. doi: 10.1172/JCI105497. [DOI] [PMC free article] [PubMed] [Google Scholar]


