Abstract
The events controlling the transition of T cells from effector to memory remain largely undefined. Many models have been put forth to account for the origin of memory precursors, but for CD4 T cells initial studies reported that memory T cells derive from IFNγ non-producing effectors while others suggested that memory emanates from highly activated IFNγ-producing effectors. Herein, using cell proliferation, expression of activation markers, and production of IFNγ as a measure of activation, we defined two types of effector CD4 T cells and investigated memory generation. The moderately activated early effectors readily transit to memory while the highly activated late effectors, regardless of their IFNγ-production, develop minimal memory. Boosting with antigen free adjuvant, however, rescues late effectors from cell death and sustains both survival and IFNγ cytokine responses in lymphopenic hosts. The adjuvant-mediated memory transition of late effectors involves the function of toll-like receptors (TLRs) most notably TLR9. These findings uncover the mechanism by which late effector CD4 T cells are driven to transit to memory and suggest that timely boosts with adjuvant may enhance vaccine efficacy.
INTRODUCTION
Immunological memory is one of the cardinal features of the immune system and provides the fundamental basis for vaccination against microbes (1). Due to the low frequency at which memory precursors arise and the lack of memory markers, the events that control transition of effector cells into memory remain largely undefined. Despite these limitations, progress has been made and many models have been put forth to explain the origin of memory precursors (1-7). For CD4 T cells it has been shown that memory T cells derive from effectors producing low amounts of IFNγ (8). Recently, however, observations were reported indicating that effector CD4 T cells producing significant IFNγ can transit to memory (9, 10). These findings suggest that IFNγ may not be the only factor involved in the transition from effector to memory (2). An alternative hypothesis has been put forth postulating that effector CD4 T cells that surpass a minimum required activation threshold and do not fully commit to terminal differentiation have a potential to differentiate into memory (11-13). However, despite the inclusive nature of this premise, it remains difficult to explain the decreasing potential model which suggests that effector cells progressively lose their memory potential along the way to terminal differentiation (6, 14). Thus, the origin of CD4 T cell memory remains a matter of debate and further studies to bring about insight on the development of memory are justified. Herein, we used the DO11.10 T cell receptor (TCR) transgenic mouse and devised a transfer model that increases the frequency of effector T cells with potential for transition to memory (10) and investigated the development of memory in the context of the activation status of effector CD4 T cells as defined by cell division, expression of activation markers and production of effector cytokines. Accordingly, ovalbumin (OVA)-specific DO11.10 CD4 T cells were labeled with the intracellular fluorescent dye, 5(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)4 (15), transferred into BALB/c mice and the hosts were immunized with OVA323-339 peptide (OVAp) emulsified in complete Freund’s adjuvant (CFA). Three days later, expression of activation markers and intracellular cytokine production were analyzed ex vivo on CFSE-demarked effector cell divisions. These analyses revealed two levels of activation at the effector stage and the cells were classified into moderately activated early effectors and highly activated late effectors. The two types of effectors were then sorted on the basis of CFSE dilution and each cell division was transferred into naïve hosts for parking. Four months later, memory precursors from each cell division was analyzed prior to any re-challenge with OVAp and the generation of memory responses were evaluated upon challenge of the host mice with OVAp in CFA. The findings indicate that the moderately activated early effectors readily yield memory. However, the highly activated late effectors display little memory but stimulation with antigen-free adjuvant rescues the cells from apoptosis and sustains expansion, survival, and increased cytokine production that culminate in significant memory responses. Interestingly, the adjuvant effect on the late effectors is mediated through toll-like receptors (TLR), most notably TLR9. Overall, the findings explain the discrepancies concerning the ontogeny of CD4 T cell memory and advocates for timely administration of adjuvants for the development of effective vaccines.
MATERIALS AND METHODS
Mice and Antigens
DO11.10 Rag2−/− transgenic mice expressing a TCR specific for OVA323-339 peptide (OVAp) were purchased from Taconic Laboratories (Hudson, NY). BALB/c mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). Rag2-deficient BALB/c mice were obtained from Jackson laboratories (Bar Harbor, ME). All animals were used in accordance with the guidelines of the institutional animal care and use committee. Influenza virus hemagglutinin (HA) aa residues 110–120 peptide, which is immunogenic in BALB/c mice (H-2d) as OVAp, was used as a negative control (10). All peptides were purchased from EZBiolab.
CFSE labeling and Immunization
Naïve splenic DO11.10 CD4+ T cells were isolated using magnetic beads (Miltenyi). The cells (10 × 106 cells/ml) were then incubated with 10 μM CFSE at 37°C for 10 min and washed once with ice-cold DMEM-10% FCS and once with PBS. CFSE-labeled T cells (10 × 106 cells/mouse) were adoptively transferred i.v. into BALB/c mice. Two days later, the hosts were immunized subcutaneously (s.c.) with 125μg of OVAp in 200μl of PBS/CFA (1v/1v). The hosts were sacrificed 72 hours (hrs) later and their draining lymph nodes (LNs) were harvested.
Sorting of Effector T cells
LN effector T cells from BALB/c hosts were first analyzed for the number of divisions on the basis of CFSE dilution. The divisions D2 through D8 were sorted individually on the basis of CFSE and injected into BALB/c or Rag2−/− mice. Effector T cell divisions were isolated on the basis of IFNγ production by using a mouse IFNγ Secretion Assay-Cell Enrichment and Detection Kit (Miltenyi) to label IFNγ-producing cells and MoFlo XDP (Beckman Coulter) to sort the cells.
Ex vivo analysis of effector T cells
The LN cells from BALB/c hosts were stained for surface molecules without any antigen stimulation. For analysis of intracellular cytokines a brief 4hr-stimulation with OVAp (10μM) or PMA (50ng/ml) and ionomycin (500ng/ml) in the presence of 10 μg/ml Brefeldin A (BFA) was performed as described (10, 16).
Analysis of recall responses by effector T cells
Individually sorted effector divisions (D2 to D8) were injected to naive BALB/c mice (5×104 per mouse) and one day later, the hosts were immunized s.c. with a suboptimal dose of OVAp (20μg per mouse) in 200μl of PBS/CFA (1v/1v). IFNγ responses from draining LNs were analyzed at different days (4 and 6) following immunization. Briefly, LN cells (0.1, 0.3, and 0.9 × 106 cells/well) were incubated with 10μM OVAp for 24 hrs and IFNγ was detected in culture supernatants by ELISA. Immunized mice with no transfer of effector T cells were used as baseline control for IFNγ responses. HA peptide was used as a negative control for in vitro stimulation.
Analysis of memory precursor responses
Each effector division (D2 to D8) was sorted based on CFSE intensity and 5 × 104 cells were adoptively transferred i.v. into Rag2−/− hosts for long term parking. The splenic cells were harvested after 4-month parking and 0.5 × 106 cells/well were stimulated with OVAp or control HA peptide for 24 hrs. IFNγ was detected in culture supernatants by ELISA.
Analysis of memory response
The Rag2−/− hosts were immunized s.c. with a suboptimal dose (0.25μg) of OVAp/CFA (10) after 4-month parking, and the splenic and LN IFNγ responses were analyzed at day 3 post-immunization. LN (0.1 × 106 cells/well) and SP (0.5 × 106 cells/well) cells were stimulated with the indicated amounts of OVAp or control HA peptide for 24 hrs. IFNγ was detected in culture supernatants by ELISA. As memory control, DO11.10 naïve T cells were transferred into Rag2−/− mice and one day later the hosts were immunized with 125μg OVAp/CFA and their responses were analyzed 4 months later. Another memory control group was Rag2−/− mice recipient of all divisions (D2 to D8) together that were parked for 4 months.
Flow cytometry and Antibodies
Surface and intracellular staining was carried out as previously described (10) and data was acquired using Cyan ADP Analyzer (Beckman Coulter Inc.) or FACS Calibur (BD Biosciences). Fluorescence analysis was performed using Summit 5.2 software (Beckman Coulter). The antibodies used for surface staining include KJ1-26-PECy5, -FITC and -APC, anti-CD44-APC, anti-CD62L-APC, anti-CD25-PE and -APC, anti-CD127-APC, anti-CD4-PECy7 and anti-CD3-PE. For intracellular staining, we used anti-IFNγ-APC and -PE, and anti-IL-2-PE. KJ1-26 is an anti-clonotypic monoclonal antibody that binds the TCR expressed on DO11.10 T cells. To analyze cell death of CFSE-labeled effector T cells, LN cells were cultured for 42 hrs with OVAp (10μM) and stained with Annexin-V.
IFNγ measurement by ELISA
The capture (purified, R4-6A2) and detection (biotinylated, XMG1.2) rat anti-mouse IFNγ antibodies were purchased from BD Biosciences. Streptavidin peroxidase (Sigma Aldrich) was used for detection by measuring color change with hydrogen peroxide and ABTS (2,2′-azino-di-[3-ethyl-benzothiazoline-6 sulfonic acid] diammonium salt) (Sigma Aldrich) substrates. The OD405nm was measured on a SpectraMAX 340 counter (Molecular Devices) using SoftMAX Pro version 3.1.1 software. Graded amounts of recombinant mouse IFNγ (BD Pharmingen) were used to construct a standard curve.
TLR Ligands
TLR9 ligand, CpG-ODN 1886 which was synthesized on a phosphorothioate backbone was purchased from IDT. Ligands for TLR2, PGN-EK; TLR3, poly (I:C); TLR4, LPS-K12; TLR5, FLA-ST and TLR7, ssPolyU/LyoVec were purchased from Invivogen. TLR ligands were administrated i.p. into Rag2−/− mice recipient of sorted effector divisions as indicated. The TLR ligands were given at the following amounts: PGN-EK (50μg), poly(I:C) (50μg), LPS (50μg), FLA-ST (1μg), ssPolyU (5μg), CpG (50μg).
In Vitro Survival Assay
Purified T cells and APCs were cultured separately in HT-2 medium for 72 hrs in the presence or absence of CpG (50μg/ml) and live cells were counted using trypan blue exclusion. Supernatant from APCs’ culture was harvested after 12 hr incubation with CpG and transferred to T cell culture to test for indirect effect of TLRs through APCs. For these assays the naïve and memory DO11.10 Rag2−/− T cells were sorted based on KJ1-26, anti-CD3 and anti-CD4 staining while effector T cells (D2-8) were isolated on the basis of CFSE. Bulk splenic APCs were obtained from BALB/c mice by differential adherence as described (17).
Analysis of TLR expression by Real-Time PCR
RNA was extracted from naïve, effector and memory T cells with TRI reagent (Sigma Aldrich) and used as template to determine TLR expression by real-time PCR. The assay used the Power SYB Green RNA-to-CT 1-Step kit (Applied Biosystems) and run on a Step One Real-Time PCR-System (Applied Biosystems). The primers used to detect TLR mRNA expression are as follows: TLR2Forward 5′-ACCGAAACCTCAGACAAAGCGTCA-3′, TLR2Reverse 5′-AACAGCGTT TGCTGAAGAGGACTG-3′; TLR3Forward 5′-CCTTGCGTTGCGAAGTGAAGAACT-3′, TLR3Reverse 5′-AAGAGGAGGGCGAATAACTTGCCA-3′; TLR4Forward 5′-ATCATGGC ACTGTTCTTCTCCTGC-3′, TLR4Reverse 5′-GGGACTTTGCTGAGTTTCTGATCC-3′; TLR5Forward 5′-ACGCTTGGAACATATGCCAGACAC-3′, TLR5Reverse 5′-GTTGACATG CCATGATCCTGCTGA-3′; TLR7Forward 5′-TCTGTTGCCTTCTCTCTGTCTCAG-3′, TLR7Reverse 5′-GAGAAGGGAGCCAAGGACATTGAA-3′; TLR8Forward 5′-CACAATGC CAAACAACAGCACCCA-3′, TLR8Reverse 5′-TTTCAAAGACTCAGGCAACCCAGC-3′; TLR9Forward 5′-TCCTCCATCTCCCAACATGGTTCT-3′, TLR9Reverse 5′-ATGAGGCTTC AGCTCACAGGGTAG-3′; β-actinForward 5′-TACAATGAGCTGCGTGTGGC-3′, β-actinReverse 5′-AGCCTGGATGGCTACGTACA-3′; I-AβForward 5′-TGTGAGTCCTGGTG ACTGCCATTA-3′, I-AβReverse 5′-TCGCCCATGAACTGGTACACGAAA-3′
Statistics
Statistical differences between groups were calculated using Graphpad Prism 5. Unpaired t-test (Fig. 1D, Fig. 3, Fig. 7B&D) with Welch’s correction (Fig. 2C and Fig. 4B) and one-tailed paired t-test (Fig. 4C, 5, 6, 7A and 8) were used for statistical analysis.
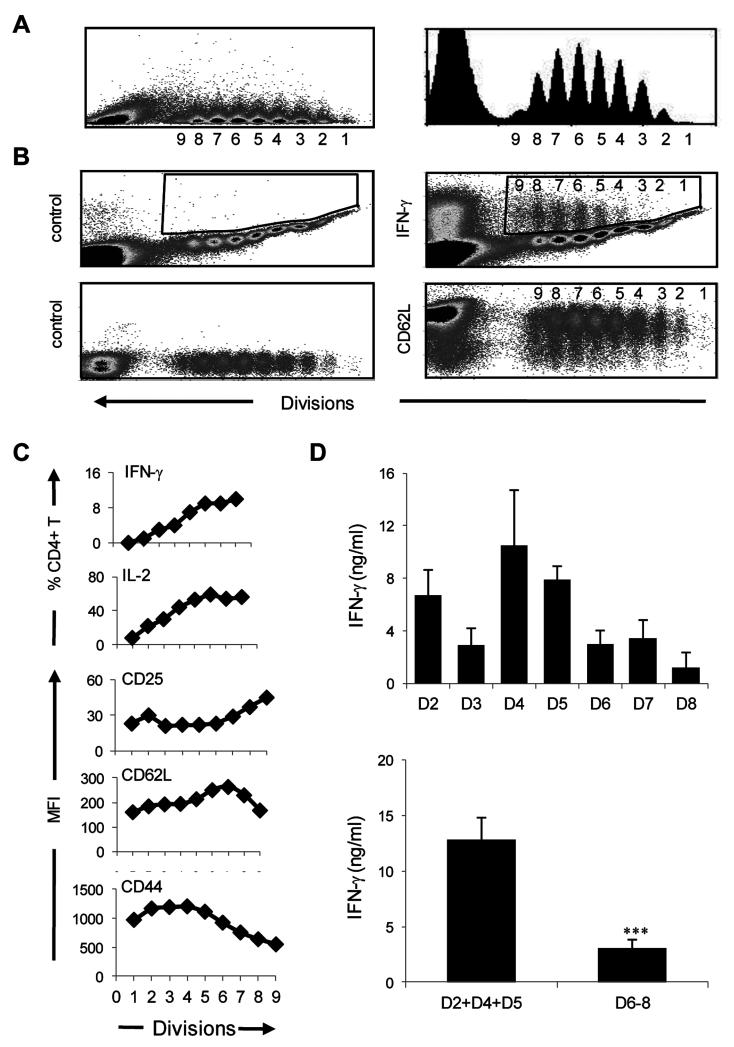
Figure 1. The effector phase includes moderately activated early and highly activated late cells that develop different recall IFNγ responses.
CFSE-labeled naïve DO11.10 T cells were transferred into BALB/c mice and the hosts were immunized with OVAp in CFA. Three days later the LN cells were harvested and the number of cell divisions, the frequency of cytokine responders and expression of activation markers were analyzed. (A) shows the effector divisions as measured by CFSE dilution and presented in dot plot and histogram format. (B) shows intracellular and surface staining of cell divisions with isotype controls (left panel) and anti-IFNγ and anti-CD62L antibodies, respectively (right panel). (C) shows percent of CD4 T cells producing cytokines and MFI for the indicated markers compared to isotype control. (D) The LN cells in each effector division were sorted, transferred into naïve BALB/c mice (5 × 104 cells/mouse) and the hosts were immunized with a suboptimal dose of OVAp in CFA. The mice were sacrificed at day 4 and 6 and the LN cells were stimulated with 10 μM OVAp and IFNγ responses were measured by ELISA. Immunized mice with no transfer of effector T cells were used as baseline control for IFNγ responses. The top plot shows the mean of IFNγ response of each division at day 4 and 6. Each point represents the mean ± SE. The bottom plot shows a summary of data comparing the mean ± SE memory IFNγ response of early (D2, D4 and D5) and late (D6, D7 and D8) divisions at the peak of their responses. ***p<0.005. The results are representative of 4 independent experiments.
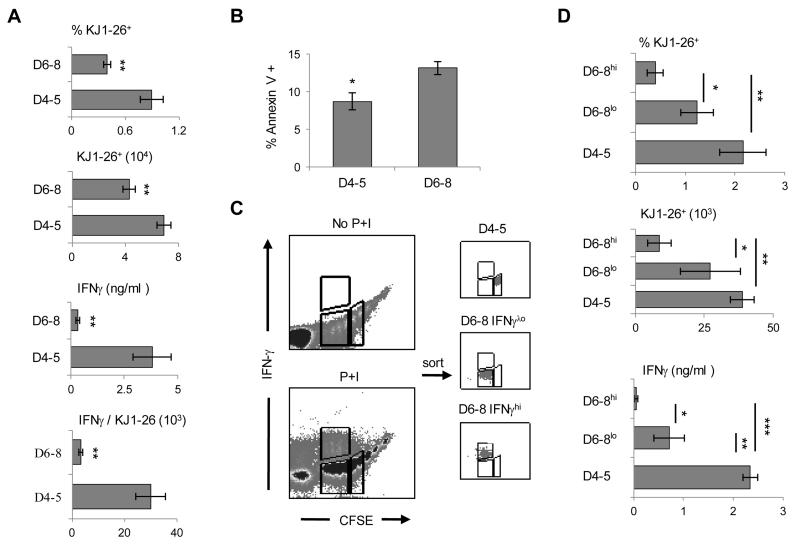
Figure 3. Late effectors display survival disadvantage relative to early effectors.
The BALB/c LN effectors were sorted from each division and the early (D4-5) and late (D6-8) effectors were transferred into Rag2−/− mice. Four months later, the SP cells were harvested and in (A) the percentage and absolute number of surviving CD4+KJ1-26+ cells were measured by flow cytometry and their IFNγ responses were evaluated by ELISA. In (B) unseparated LN cells were cultured for 42hrs in the presence of OVAp and then stained with Annexin V. The cells were then gated on CFSE and Annexin V binding was analyzed on each division. The bars show the mean percent of Annexin V+ cells for early (D4-5) and late (D6-8) effectors. (C) Prior to transfer, the CFSE-positive D2-8 effectors were sorted and stimulated with PMA and Ionomycin (P+I), labeled and analyzed for IFN-γ production (left panel) and IFNγhi and IFNγlo cells of D6-8 and D4-5 cells were separated and re-analyzed for IFNγ to confirm the purity of separation. In (D) hosts of the sorted IFNγhi and IFNγlo late effectors were sacrificed 6 weeks post transfer and the percentage, absolute number and IFNγ production by the CD4+KJ1-26+ splenic cells were measured as in (A) and compared with D4-5 early effectors. The graphs represent the mean of at least three independent experiments. * p< 0.05, **p< 0.01, ***p< 0.005.
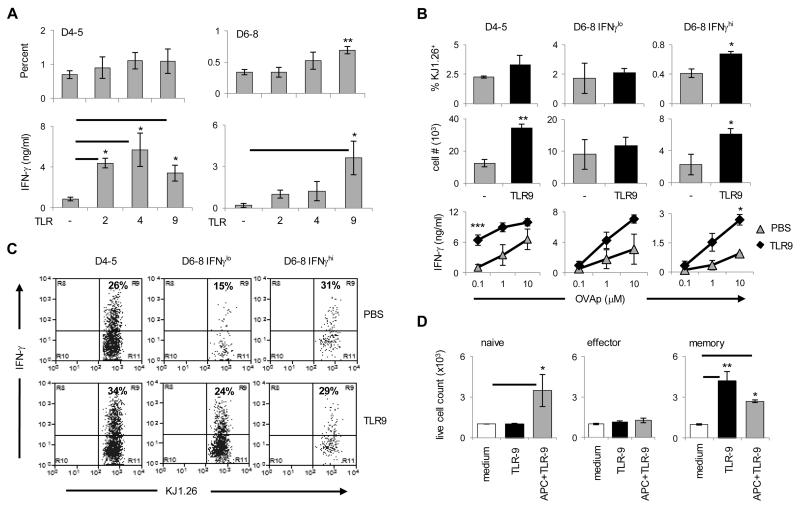
Figure 7. TLR9 ligand supports transition of late effectors into memory.
(A) Early (D4-5) and late (D6-8) effectors were isolated from BALB/c mice (as in Figure 3) and transferred into Rag2−/− hosts. On day 1, 4, 7 and 10 after cell transfer, the hosts received TLR2, TLR4 or TLR9 ligand (optimal regimen) and the percentage and IFNγ production by the CD4+KJ1-26+ cells were analyzed 3 weeks after the last TLR ligand injection. Each bar represents mean of four independent experiments. (B and C) The hosts were transferred with separated IFNγlo or IFNγhi cells from D6-8 effectors or unseparated D4-5 cells (as in Figure 3) and the hosts were given TLR9-ligand as in (A) and splenic cells were harvested 3 weeks after the last TLR injection. The graphs in (B) show the percentage, absolute number, and IFNγ production by the CD4+KJ1-26+ cells while in (C) the plots show intracellular IFNγ. Each bar represents the mean of at least three independent experiments. (D) Purified naïve, effector and memory DO11.10 T cells were cultured in the presence of media alone (open bars), media supplemented with TLR9-ligand (black bars), or media from APC culture stimulated with TLR9-ligand (gray bars). 72 hours later, live cells were counted using trypan blue exclusion. For these assays, LN effector T cells (D2-8) were isolated on the basis of CFSE while naïve T cells and memory precursors (from Rag2−/− hosts recipient of D2-8 LN effectors) were sorted based on KJ1-26, anti-CD3 and anti-CD4 staining. Purified splenic APCs were cultured simultaneously but separately from T cells in the presence of TLR9-ligand (CpG-ODN 1886; 50 μg/ml). 12 hours after the initiation of culture, APC supernatant was transferred to T cell culture. Each bar represents the mean of at least four independent experiments. *p< 0.05, ** p< 0.01. The cell numbers were relative to culture without TLR ligands (set at 1 × 103).
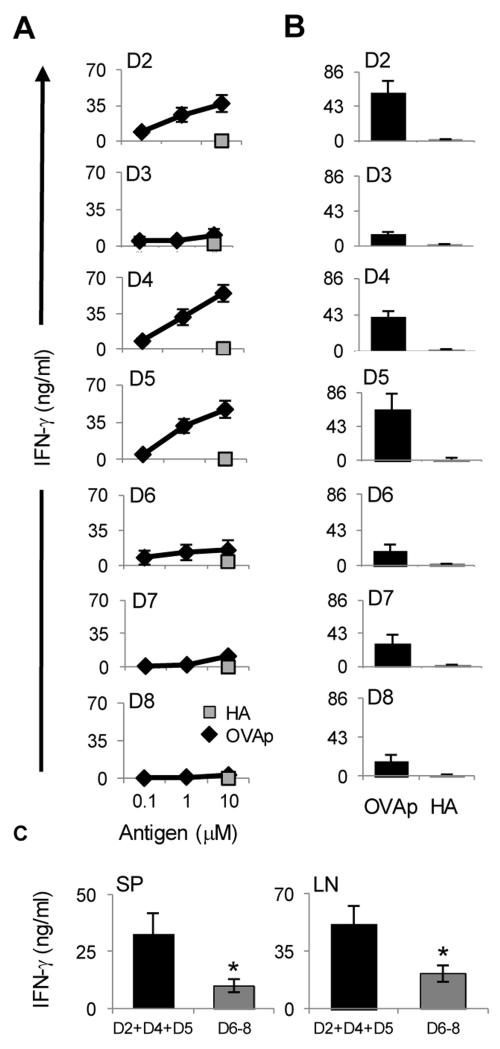
Figure 2. Transition to memory is more effective with early compared to late effectors.
The BALB/c LN effectors described in Figure 1 were sorted from each division and transferred into Rag2−/− mice for parking. Four months later, the hosts were divided into two groups, one of which was sacrificed for analysis of splenic memory precursor responses (A) and the other group was immunized with OVAp/CFA and three days after immunization the SP and LN cells were isolated and their IFNγ responses were analyzed (B and C). (A) The precursor cells were stimulated with graded amounts of OVAp (diamond) and IFNγ responses were measured by ELISA. Stimulation with HA peptide (10μM) was included in all experiments for control purposes (squares). (B) LN cells were stimulated with 10μM OVAp or HA peptide and IFNγ responses were measured by ELISA. (C) shows summary bar graph of data for SP and LN comparing the mean memory IFNγ response of early (D2, D4 and D5) and late (D6, D7 and D8) effector at 10 μM. (A-C) Each point represents the mean of at least three independent experiments. *p< 0.05.
Figure 4. Continuous contact with CFA sustains superior memory.
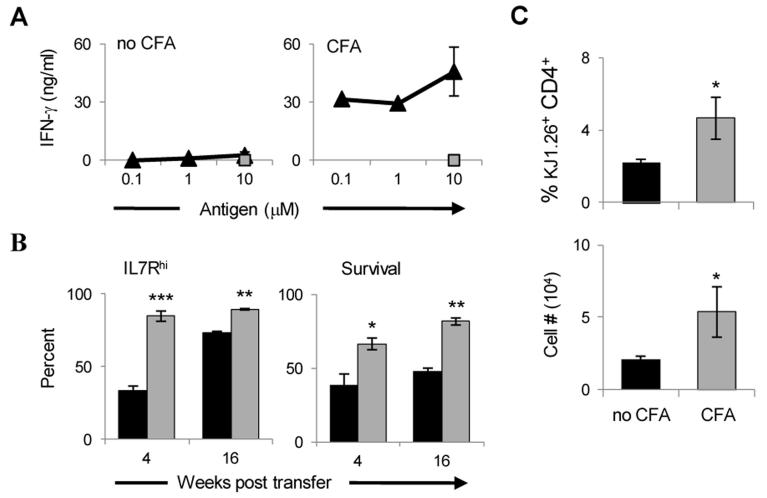
(A) Naïve CFSE-labeled DO11.10 T cells were transferred into Rag2−/− and BALB/c mice and the hosts were immunized with 125μg OVA/CFA. Three days later the mice were divided into two groups, the Rag2−/− mice remained as is (the T cells continue contact with CFA) and the BALB/c mice were used to harvest the LN effectors which were re-transferred into Rag2−/− mice for parking (no CFA). In this group the T cells are no longer exposed to CFA. Four months later, the splenic cells from both groups were harvested, stimulated with graded concentrations of OVA peptide (triangle) or 10 μM HA peptide (rectangles) as control, and IFNγ responses were measured by ELISA. (B) The LN from the CFA (gray bars) and no CFA (black bars) groups were harvested after 4 or 16 weeks parking and the cells were analyzed for IL-7R expression and apoptosis by 7-AAD using flow cytometry. (C) Naïve CFSE-labeled DO11.10 T cells were transferred into BALB/c mice and the hosts were immunized with 125μg OVA/CFA. Three days later the LN effectors were isolated and re-transferred into Rag2−/− hosts. One day later, one group of mice was given CFA and the other was left without CFA. After 120 days of parking the spleen cells were harvested and the percent KJ1-26+ CD4+ T cells were estimated among total splenic cells. The graph represents the mean of four independent experiments. *p< 0.05, **p< 0.01, ***p< 0.005.
Figure 5. Late effector T cells require stimulation with adjuvant for survival and generation of effective memory.
(A) Early (D4-5) and late (D6-8) effectors were isolated from BALB/c mice (as in Figure 1) and transferred into Rag2−/− hosts. D2 and D3 were also sorted and transferred to Rag2−/− mice to serve for control purposes. One day later, the mice were given antigen-free CFA and parked for 3 months. The percentage, absolute number and IFNγ production by the CD4+KJ1-26+ splenic cells were then measured by flow cytometry and ELISA, respectively. Each bar represents the mean of four independent experiments. *p< 0.05, **p< 0.01. (B) shows the summary bar graph of data in (A) comparing the mean percent of CD4+KJ1-26+ splenic cells and the mean memory IFNγ response of early (D4 and D5) and late (D6, D7 and D8) effectors.
Figure 6. TLR-4 and -9 ligands improve the IFNγ memory response of effector T cells.
CFSE-positive total (D2-8) lymph node effector T cells were sorted and re-transferred into Rag2−/− hosts. On day 1, 5, and 8 after cell transfer, TLR ligands were administered i.p. The spleen cells were harvested 5 weeks after the last TLR injection and the percentage and the cell number as well as IFNγ production by the surviving CD4+KJ1-26+ cells were measured by flow cytometry and ELISA, respectively. The cell number was relative to PBS (set at 1 ± 0.1 × 104). Each bar represents the mean of nine independent experiments. *p< 0.05, **p< 0.01.
Figure 8. TLR9 mRNA is expressed by naïve and memory DO11.10 CD4+ T cells.
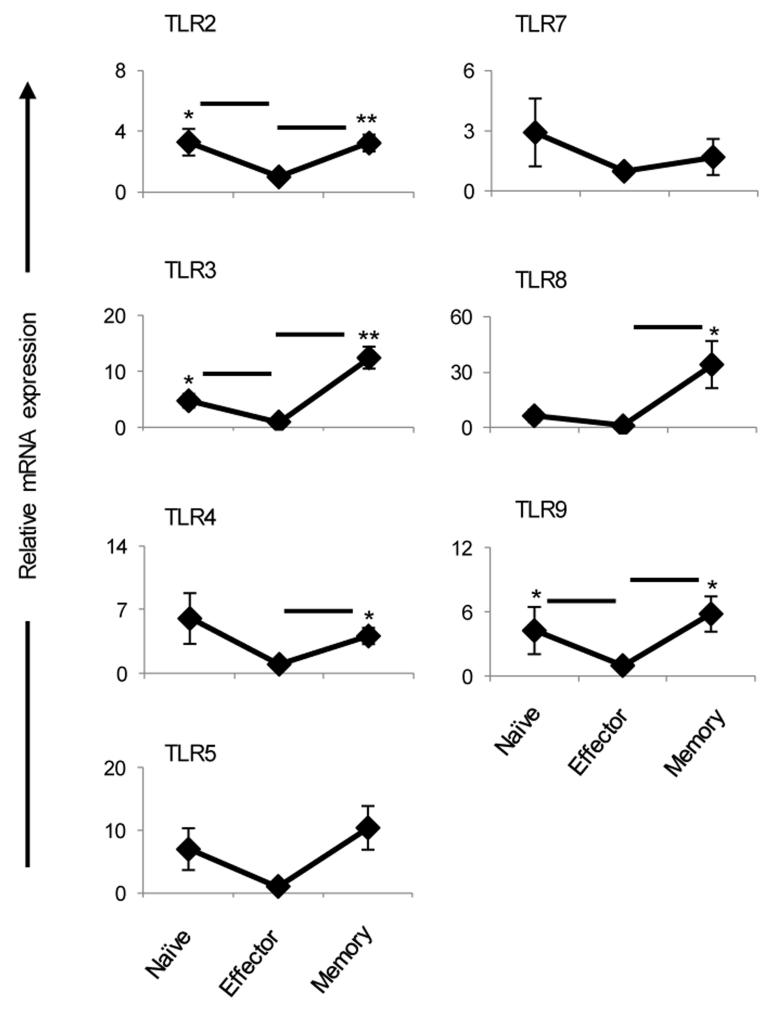
Naïve, effector and memory DO11.10 T cells were obtained as described in Figure 7D and RNA was isolated by Trizol. TLR expression was measured by real time-PCR as described in Methods. Fold changes were relative to the effector group (set at 1). Each point represents the mean of five independent experiments with ± SEM. *p < 0.05, **p< 0.01.
RESULTS
Highly activated late effectors develop diminished IFNγ recall responses relative to moderately activated early effectors
The study of the initial events controlling transition from effector to memory is hampered by the low frequency at which memory precursors arise and the lack of specific memory markers. Recently, TCR transgenic T cell transfer models were used to increase the frequency of effector T cells with potential for transition to memory and proved suitable for tracking the initial events underlying the development of memory (8, 10). These and other investigations, however, yielded discrepancies as to the origin of memory when IFNγ was used to gauge the activation status of effector T cells (8-10). Herein we sought to define the activation status of effector T cells on the basis of the number of cell divisions the effector T cells undergo upon exposure to antigen, the expression of activation markers and the production of IFNγ cytokine, and to determine transition to memory. Accordingly, T cell receptor (TCR) transgenic CD4 T cells specific for OVAp were purified from the spleen of naïve DO11.10 mice, labeled with the cell division marker CFSE, transferred to naïve BALB/c mice, and the hosts were immunized s.c. with OVAp in CFA as we previously described (10). The draining LN CD4 T cells were then analyzed ex vivo for cell division, expression of activation markers and cytokine production. As illustrated in Figure 1 the cells have undergone 8 divisions with peak 1 as undivided cells and peak 9 as cells that divided 8 times (Fig. 1A). We refer to peak 1 through 9 as division (D) 1 through (D) 9 and since D1 and D9 have very few cells they will not be considered for investigation of transition to memory. This limitation is related to the number of cells to transfer into the BALB/c hosts and further increase in cell number might require higher dose of Ag for priming which could interfere with memory differentiation (18, 19). Moreover, when the LN cells were stained ex vivo for intracellular IFNγ and production of the cytokine was analyzed per cell division there was an increase in IFNγ production as the cells progressed in cell division (Fig. 1B, right panel). The isotype control did not show any significant IFNγ production (Fig. 1B, left panel). As shall be seen below, the cells not only divided and produced IFNγ but also expressed activation markers, indicating a loss of naïve phenotype and acquisition of effector status. The results for IL-2 production and expression of activation markers are presented in a graph format alongside IFNγ (Fig. 1C). The findings indicate that the effector phase can be divided into moderately activated early divisions (D2-5) and highly activated late divisions (D6-8). The early division T cells had lower levels of CD25 and CD62L with a small percentage of cells had IFNγ and IL-2 cytokines (Fig. 1B and 1C). The late division T cells had an overall higher CD25 expression, and a significant percentage produced IFNγ and IL-2. CD62L expression varied among the divisions possibly reflecting TCR signal strength (20). CD44 expression was higher on the early divisions but decreased in the late effectors, which could be related to survival and T cell memory development (21). Overall, the effector phase can be divided into moderately activated early effectors (D2-5) and highly activated late effectors (D6-8).
To further characterize the activation status of the effector cells each division was sorted based on CFSE dilution, transferred into naïve BALB/c mice and the hosts were challenged with a suboptimal dose of OVAp in CFA. The LN cells were then harvested, stimulated with OVAp and recall IFNγ responses were measured. Surprisingly, the early effector T cells (D2, D4, and D5) produced significant amounts of IFNγ when recalled with OVAp in vivo while the late effectors (D6, D7, and D8) secreted minimal IFNγ (Fig. 1D). D3 displayed a low recall response pattern as was previously observed (10). When the mean IFNγ level among early (D2+D4+D5) and late (D6+D7+D8) divisions was compared, there was a superior IFNγ recall response from the early versus late effectors. Thus, recall with OVAp stimulates moderately activated early effectors to secrete IFNγ but leads to unresponsiveness of highly activated late effectors perhaps due to apoptosis and/or restoration of a resting state which could impact transition to memory (1).
Transition to memory is more effective for early compared to late effectors
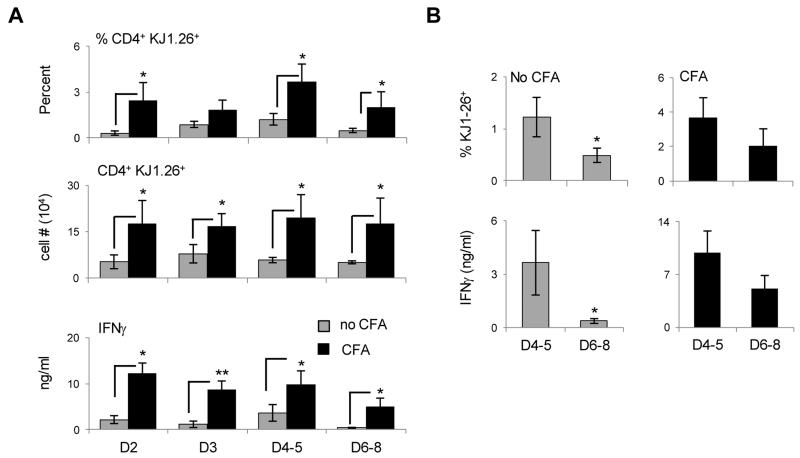
The fact that the late effectors could not develop significant in vivo recall responses could translate into defective transition to memory (22). To test this premise the early and late effector divisions were individually transferred to Rag2−/− hosts and subjected to a long-term (4 months) parking without antigen. The rationale for parking the effector cells in a Rag2−/− host stems from the following reasoning: The lymphopenic environment should sustain survival of memory precursors which arise at a very low frequency (23-27), thereby making the readout of memory responses feasible. Second, the effector cells of all divisions are subjected to identical survival mechanisms in the absence of Ag, and any difference in the memory responses will solely reflect how each cell division affects memory development. The validation of this model was shown in our earlier work where memory precursors were shown to give rise to rapid and robust IFNγ responses as compared to control naïve T cells (10). The Rag2−/− hosts were then used to test the effectors for generation of memory precursors prior to immunization with antigen and the development of memory responses upon re-challenge with suboptimal dose of OVAp. The findings show that prior to any re-immunization, the early effectors (D2, D4 and D5) gave rise to significant IFNγ-producing memory precursors while the late effectors (D6-8) had a much lower precursor response (Fig. 2A). Note D3 had a low precursor response as was previously defined (10). Again, while these findings parallel with the in vivo recall responses (Fig. 1D) they are surprising as highly activated effectors yielded limited memory precursors. Upon challenge with OVAp/CFA, the LN responses analyzed on day 3 post immunization were reflective of the precursor pattern as the early effectors yielded higher memory responses than late effectors (Fig. 2B). After 4 days, though, memory responses began to develop for late effectors (not shown). When the response of combined early effector divisions (D2,4-5) was compared with the response of late effector divisions (D6-8) there was a significantly higher memory IFNγ response by early versus late LN effectors (Fig. 2C). A similar trend was observed with splenic memory responses (Fig. 2C). Also, highly activated cells that have undergone more than 8 divisions did not yield significant IFNγ memory responses (Fig. S1). Overall, the early effectors are more efficient than the late effectors in transitioning to memory as they develop rapid and robust cytokine responses to a suboptimal dose of Ag, which is the hallmark of memory.
Late effectors display survival disadvantage relative to early effectors
Late effectors developed poor IFNγ responses upon recall with antigen and were unable to generate significant memory responses. It is thus logical to envision that the late effectors may manifest a survival disadvantage and yield limited memory precursors. To test for survival, the D6-8 effectors were isolated, transferred into Rag2−/− hosts and after 4 month parking, the percentage and absolute number of KJ-126+CD3+CD4+ T cells in the spleen were evaluated by flow cytometry and compared to D4-5 early effectors which unlike D2 or D3 generate unconditional memory responses (10). The results indicate that the late effectors persisted at a much lower frequency than the early effectors as the percentage among total splenic cells was 0.4 ± 0.04 for D6-8 as compared to 0.9 ± 0.13 for D4-5 and the absolute number of KJ-1-26+CD3+CD4+ cells per spleen was 43,000 ± 5,000 for D6-8 vs 69,000 ± 5,000 for D4-5 (Fig. 3A). Moreover, when the splenic cells were stimulated with OVAp the precursors generated from the late effectors produced significantly lower amounts of IFNγ (0.3 ± 0.09 ng/ml for D6-8 versus 3.8 ± 0.9 ng/ml for D4-5) as tested by ELISA (Fig. 3A). When the amount of IFNγ was divided by the percent of T cells (Fig. 3A, top panel) there was less cytokine per cell in the late versus early effectors (3.2 ± 0.7 ng/ml for D6-8 versus 29.8 ± 5.6 ng/ml for D4-5). Thus, the late effectors yielded lower numbers of long-lived memory precursors producing low levels of IFNγ. This survival disadvantage is likely related to increased apoptosis among late effectors because analysis of cell death indicated that D6-8 effectors had a slightly higher rate (13.2 ± 0.9%) of apoptosis relative to D4-5 early effectors (8.8 ± 1.1%) (Fig. 3B). It was previously shown that IFNγ-producing effector cells do not persist because they undergo cell death in an IFNγ-dependent paracrine manner (28, 29). Since within the late effectors some cells produce more IFNγ than others, it is possible that late effectors producing high levels of IFNγ undergo apoptosis at a significantly higher level than late effectors producing low levels of IFNγ. To explore this possibility, sorted D2-8 LN effector T cells from BALB/c hosts recipient of CFSE-labeled naïve DO11.10 T cells and immunized with OVA/CFA were shortly stimulated with PMA and ionomycin and the late effector T cells producing high (D6-8 IFNγhi) and low (D6-8 IFNγlo) IFNγ were sorted on the basis of CFSE dilution and IFNγ intensity (see boxes in Fig. 3C, left panels). Low IFNγ-producing D4-5 early effectors were also sorted for control purposes and the purity of the effectors was assessed (Fig. 3C, right panels) and the cells were transferred into Rag2−/− hosts for parking. Subsequently, the percentage, absolute number and IFNγ response of the surviving KJ1-26+ T cells were determined. As indicated in Fig. 3D, the high IFNγ-producing late effectors (D6-8hi) were very low in percentage (0.4 ± 0.16% for D6-8hi versus 2.2 ± 0.5% for D4-5) and absolute cell number (8.7 ± 4.3 × 103 cells for D6-8hi versus 38.8 ± 4.3 × 103 cells for D4-5) and did not produce detectable memory IFNγ cytokine by ELISA. The low IFNγ-producing late effectors (D6-8lo), while yielding significant cell percentage and number relative to D6-8hi effectors (1.2 ± 0.3% and 27.2 ± 10.9 × 103 cells for D6-8lo), remained inefficient at producing memory IFNγ relative to memory T cells arising from D4-5 early effectors (0.7 ± 0.3 ng/ml for D6-8lo versus 2.3 ± 0.1 ng/ml for D4-5). The frequency and absolute number of KJ1-26+ cells in blood, bone marrow (BM), LNs and lungs showed the same trend (data not shown). These findings suggest that high IFNγ-producing late effectors have a survival disadvantage relative to both low-IFNγ-producing late effectors and early effector cells. Overall, late effectors are not effective in the generation of memory because the IFNγ-producing cells are short-lived and possibly dividing less,while the long-lived counterparts remain poor IFNγ producers compared to early effectors.
Late effectors require continuous contact with adjuvant for survival and generation of effective memory
To begin investigation of the mechanism underlying the differential survival among early and late effectors we sought to determine whether CFA plays a role in transition of the effectors into memory cells. The rationale for this stems from the observation that effector T cells that remain parked in the immune host recipient of OVAp/CFA regimen develop a strong memory response (10). To this end, naïve DO11.10 T cells were transferred into naïve Rag2−/− and BALB/c mice and the hosts were immunized with OVAp/CFA. Rag2−/− mice were used for a 4-month rest (parking of the effector cells without isolation or transfer into a new host) and BALB/c mice were used to separate effector T cells that were then transferred into Rag2−/− hosts for parking.
After 4 months, the splenic cells from both groups were harvested, stimulated with graded concentrations of OVAp or control HA peptide, and IFNγ precursor responses were measured. The results show that the effector cells that remained in the host developed a strong memory response while the effectors that were transferred into a new host had minimal IFNγ response (Fig. 4A). Moreover, when the LNs from both hosts were harvested after 4 or 16 weeks parking and the cells were analyzed for IL-7R expression and apoptosis, the effectors that remained in the immune host displayed significantly higher IL-7R expression (84.7 ± 3.5% versus 33.7 ± 2.8% on week 4 and 90 ± 1.2% versus 73 ± 0.5% on week 16) and survival rate (66.5 ± 3.9% versus 38.5 ± 7.6% on week 4 and 81.7 ± 2.4% versus 48 ± 2.1% on week 16) which is indicative of a better transition into memory (Fig. 4B). This could be due to more efficient IL-2 signaling during priming leading to earlier re-expression of IL-7R on memory precursors (Fig. S2). These observations suggested that continuous contact with CFA perhaps influences transition to memory and prompted us to test Ag free adjuvant for such a premise. Accordingly, when LN DO11.10 CD4 T effectors were transferred from BALB/c mice into Rag2−/− hosts that were given CFA, a high percentage of KJ1-26+CD4+ cells among total splenic cells was found after 4 months parking (Fig. 4C). This percentage and the absolute number were significantly higher relative to Rag2−/− hosts that were not given CFA (4.7 ± 1.2% and 5.4 ± 1.7 × 104 cells for CFA versus 2.2 ± 0.16% and 2.1 ± 0.2 × 104 cells for no CFA). Similar results were obtained when the effector T cells were generated in Rag2−/− mice and transferred into new Rag2−/− hosts for parking and exposure to CFA (Fig. S3). Overall, parking of effector T cells in CFA-containing environment increases survival of the cells and transition to memory.
To determine the specific type of effectors that respond to CFA adjuvanticity to generate effective memory, we sorted the early and late effectors, transferred them into Rag2−/− mice and the hosts were given antigen-free CFA. Subsequently, the frequency and absolute number of memory cells and their ability to produce IFNγ were evaluated. The results presented in Figure 5 show that the percentage and absolute number of both early and late effector-derived memory splenic cells increased significantly when the hosts were given CFA. The IFNγ response, which was minimal for D6-8-derived memory precursors, also increased for both types of effectors. Even the non-responsive D3 and the LN-conditioned D2 divisions (10) had increased memory precursors and IFNγ memory responses (Fig. 5A). More important, the otherwise short-lived unresponsive late effectors (D6-8) which yielded minimal long-lived cells relative to D4-5 effectors in the absence of CFA (0.5 ± 0.14% for D6-8 versus 1.2 ± 0.38% for D4-5) have became responsive when the mice were given CFA boost and developed long-lived memory cells like the D4-5 early effectors (2.02 ± 1.0% for D6-8 versus 3.7 ± 1.2% for D4-5) (Fig. 5B). Similar results were observed at the level of IFNγ production as the amount of cytokine increased from 0.37 ± 0.14 ng/ml with no CFA to 5.1 ± 1.8 ng/ml with CFA for late effectors. Although this is half the amount observed with the early effectors, the index of IFNγ increase when CFA is given was much higher for late effectors (ratio of CFA/no CFA 15.6 ± 9.5 for late effectors versus 5.4 ± 2.2 for early effectors). Overall, late effectors acquire the ability to develop long-lived cells that produce significant IFNγ when the hosts are given CFA.
Adjuvant effects on transition to memory involves toll-like receptors most notably TLR9
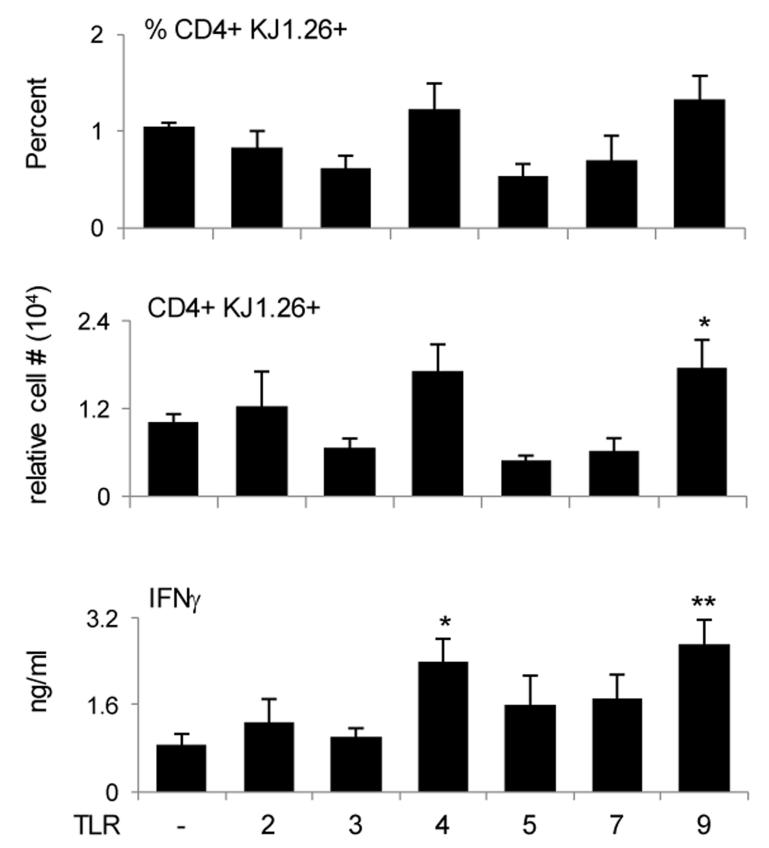
CFA adjuvanticity likely works through Toll like receptors (30). The improved survival and IFNγ-production attributes may have resulted from TLR-mediated adjuvanticity. Indeed, when the CFSE+ LN effector T cells (D2-D8) were parked in naïve Rag2−/− hosts and exposed to different TLR ligands, the survival and IFNγ responses increased with some but not all TLR ligands tested (Fig. 6). Specifically, while the percent of KJ1-26+ CD4+ T cells among total splenic cells was not significantly affected by any of the TLR ligands, the number of CD4 T cells increased significantly with TLR9-ligand (1 ± 0.1 × 104 cells for PBS versus 1.8 ± 0.4 × 104 cells for TLR9) and IFNγ responses were significantly higher with TLR4 and TLR9 ligands (0.86 ± 0.2 ng/ml for PBS versus 2.4 ± 0.44 ng/ml for TLR4 and 2.7 ± 0.45 ng/ml for TLR9). Thus, the restoration of transition to memory by CFA is likely due to TLR-mediated stimulation.
Since both TLR4 and TLR9 ligands increased IFNγ memory responses of effectors we sought to determine whether such an effect operates at the level of cell survival or amplification of IFNγ secretion. To test this, D4-5 early and D6-8 late effectors were sorted, transferred into Rag2−/− mice and the hosts were given TLR4 and TLR9 ligands. TLR2 ligand was included as a control since it had no significant effect on the percent or IFNγ response of memory precursors (Fig. 6). As indicated in Fig. 7A, while all of the TLR ligands increased the IFNγ memory responses of D4-5 early effectors (0.9 ± 0.2 ng/ml for PBS versus 5.7 ± 1.6 ng/ml for TLR4 and 3.4 ± 0.8 ng/ml for TLR9), only TLR9 ligand augmented both the survival (0.3 ± 0.04% for PBS versus 0.7 ± 0.06% for TLR9) and IFNγ memory responses of late effectors (0.18 ± 0.14 ng/ml for PBS versus 3.6 ± 1.2 ng/ml for TLR9). In addition, the TLR9 effects manifest in both IFNγhi and IFNγlo late effectors (Fig. 7 B and C). Indeed TLR9-ligand increased the survival of IFNγhi late effectors leading to amplification of IFNγ secretion (0.4 ± 0.06% and 2.3 ± 1.3 × 103 cells for PBS versus 0.7 ± 0.03% and 6.1 ± 0.7 × 103 cells for TLR9). However, TLR9-ligand did not increase the percent or number of memory CD4 T cells but augmented the frequency of IFNγ-producing cells among IFNγlo late effectors (Fig. 7 B and C). It was previously shown that both T cells and APCs can express TLR9 and TLR9-ligand can improve the survival of activated T cells through MyD88 pathway in T cells (31). To determine whether TLR9 ligand exercises its effect directly on rested memory T cell precursors or through APCs, KJ1-26+ CD4+ CD3+ T cells were sorted from naïve DO11.10 mice (naïve), LN of OVAp/CFA immunized BALB/c mice that include CFSE-labeled effector cells (effector) and from SP of Rag2−/− hosts that contain long-term parked memory DO11.10 T cells (memory). The sorted cells were then cultured for a short-term with or without TLR9 ligand to test its effect on the survival of T cells. The results show that TLR9 ligand can exert its effect directly on the memory T cells because incubation of sorted T cells with TLR9 ligand significantly increased their survival relative to incubation without TLR9 ligand (1 ± 0.05 × 103 cells for medium alone versus 4.2 ± 0.7 × 103 cells for TLR9) (Fig. 7D). Supernatant from TLR9-stimulated APCs also had an effect on T cell survival. TLR9-ligand had no direct effect on naïve or effector T cells (Fig. 7D) despite the fact that both memory and naïve T cells express TLR9 (Fig. 8). Interestingly, effector T cells were unresponsive to TLR9 ligand under any culture condition. This could be explained by temporary down-regulation of TLR9 on effector T cells (Fig. 8). Such down-regulation was more prominent on late effector CD4 T cells (data not shown). Moreover, the results in Figure 8 indicate that this temporary regulation of toll-like receptor gene expression is not unique to TLR9 but also observed with other receptors. These results suggest that memory precursors are programmed to respond to TLR ligands directly as well as through factors secreted by APCs to promote better survival and provide advantage over newly primed naïve cells. In vivo exposure of D6-8 late effectors to CpG increases the frequency of IL-2 producing IFNγ-positive memory T cells (Fig. S4). Thus, it is possible that IL-2 increases resistance to apoptosis (32) by diminishing withdrawal of growth/survival factors and increasing anti-apoptotic molecules such bcl-xL, c-FLIP and bcl-2, as was suggested previously (33).
DISCUSSION
The study presented in this report puts forth plausible evidence as to the events controlling the transition of T cells from effector to memory. Indeed, much of our current understanding of memory development stands on two disparate observations, one of which claims that moderation in T cell activation favors transition to memory while the second argues that development of memory ensues when the effector cells are undergoing heightened activation (8, 9). Herein, we provide evidence that both early and late effectors, which display moderate and robust activation, respectively, are endowed with potential for the generation of memory. However, the latter require sustained adjuvant function to transit to memory. Indeed, D4-5 early effectors, which display a lower level of activation, readily generate memory as shown in other studies (34, 35) and bodes well with the postulate that a threshold level of activation is required for transition to memory (11, 36). Similarly, the results concur with earlier findings suggesting that transition to memory is more prominent with low IFNγ producing effectors (8). However, differently from this model, data shown here imply that highly activated late effectors, regardless of their IFNγ production, could not efficiently transit to memory unless a boost with antigen-free CFA was made. The underlying mechanism for this phenomenon is that the late effectors are susceptible to apoptosis, and CFA, through TLR-activation, diminishes cell death and possibly increases cell division. In fact, we demonstrate that TLR9 is most prominent in this model for acquisition of survival and proliferation attributes by high IFNγ producers as well as amplification of cytokine production among low IFNγ producing late effectors. Interestingly, this effect can occur either by direct interaction of TLR9-ligand with the T cells as was shown previously (37, 38) or through induction of soluble factors by APCs. These observations support the findings indicating that high IFNγ producers transit to memory (9) when transferred into infected mice as the T cells could be responding to TLR-mediated viral adjuvanticity (9, 39). Timely administration of antigen-free adjuvant may be critical as the effector T cells may need to terminate antigen-dependent activation and restore a resting state in order for adjuvant to sustain transition to memory. Preliminary experiments indicated that adjuvant does not induce re-expression of IL-7R (not shown), suggesting that other yet to be defined pathways are involved in the contraction and maintenance of memory precursors. It is possible that TLR-9 operates through the apoptotic/cell cycle pathways and involves genes such as Bmi-1, mcl-1, c-flip, Noxa, CD40L, ICOS, OX-40, PD-1, CD95 and other TNFR family members that were suggested to be taking part in maintenance of memory cells (32, 40-42). Essentially, the findings bode well with reports suggesting that transition to memory is intrinsically programmed early during priming of naïve T cells (43), and that adjuvant assists effectors to reach their full potential for transition to memory. Finally, given that both TCR signaling strength and duration of contact between T cells and APCs control the level of expansion, differentiation and survival of effectors (40, 44), the interplay between antigen-free adjuvant and these parameters remains to be defined.
Overall, the findings support a model in which both moderately activated early effectors and highly activated late effectors have potential to transit to memory. However, the highly activated late effectors, except perhaps the terminally differentiated cells (45), will require antigen-free adjuvant stimulation through TLRs to transit to memory. While these observations resolve the discrepancies among high and low IFNγ producers and moderate versus hyperactive effectors as progenitors for memory development, there is an added implication for the role of TLR-ligands in sustaining better memory responses. It is thus logical to envision vaccination strategies incorporating antigen-free adjuvant boosts for the development of effective memory. In addition, different microbes may require different combinations of TLR ligands and TLR-independent pathways for the development of strong memory responses (46-51).
Supplementary Material
Acknowledgments
Funding. This work was supported by grants RO1AI048541 (to H.Z.) from the National Institutes of Health. J.A.C. was supported by Life Sciences Fellowships from the University of Missouri, Columbia. J.S.E. and C.M.H. were supported by a training grant GM008396 from NIGMS.
Footnotes
Abbreviations: BFA, Brefeldin A; CFSE, 5(and 6)-carboxyfluorescein diacetate succinimidyl ester; HA, hemagglutinin; LN, lymph node; OVAp, ovalbumin peptide; SP, spleen.
REFERENCES
- 1.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 3.Bell EB, Westermann J. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends in immunology. 2008;29:405–411. doi: 10.1016/j.it.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunology and cell biology. 2008;86:343–352. doi: 10.1038/icb.2008.13. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 7.Lefrancois L, Masopust D. The road not taken: memory T cell fate ‘decisions’. Nat Immunol. 2009;10:369–370. doi: 10.1038/ni0409-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 9.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 10.Bell JJ, Ellis JS, Guloglu FB, Tartar DM, Lee HH, Divekar RD, Jain R, Yu P, Hoeman CM, Zaghouani H. Early effector T cells producing significant IFN-gamma develop into memory. J Immunol. 2008;180:179–187. doi: 10.4049/jimmunol.180.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lees JR, Farber DL. Generation, persistence and plasticity of CD4 T-cell memories. Immunology. 2010;130:463–470. doi: 10.1111/j.1365-2567.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 15.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 17.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 18.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao CC, Jensen R, Dailey MO. Mechanisms of L-selectin regulation by activated T cells. J Immunol. 1997;159:1686–1694. [PubMed] [Google Scholar]
- 21.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. The Journal of experimental medicine. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosco N, Agenes F, Ceredig R. Effects of increasing IL-7 availability on lymphocytes during and after lymphopenia-induced proliferation. J Immunol. 2005;175:162–170. doi: 10.4049/jimmunol.175.1.162. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 25.Lee SK, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev. 2005;208:169–180. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 26.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. The Journal of experimental medicine. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, Kim SY, Na R, Hennighausen L, Kurtulus S, Erman B, Matzinger P, Merchant MS, Mackall CL. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foulds KE, Rotte MJ, Paley MA, Singh B, Douek DC, Hill BJ, O’Shea JJ, Watford WT, Seder RA, Wu CY. IFN-gamma mediates the death of Th1 cells in a paracrine manner. J Immunol. 2008;180:842–849. doi: 10.4049/jimmunol.180.2.842. [DOI] [PubMed] [Google Scholar]
- 29.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. The Journal of experimental medicine. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieg AM. Toll-free vaccines? Nat Biotechnol. 2007;25:303–305. doi: 10.1038/nbt0307-303. [DOI] [PubMed] [Google Scholar]
- 31.Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45:25–36. doi: 10.1007/s12026-009-8113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol Rev. 2010;236:110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davila E, Velez MG, Heppelmann CJ, Celis E. Creating space: an antigen-independent, CpG-induced peripheral expansion of naive and memory T lymphocytes in a full T-cell compartment. Blood. 2002;100:2537–2545. doi: 10.1182/blood-2002-02-0401. [DOI] [PubMed] [Google Scholar]
- 34.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. The Journal of experimental medicine. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. The Journal of experimental medicine. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaRosa DF, Stumhofer JS, Gelman AE, Rahman AH, Taylor DK, Hunter CA, Turka LA. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci U S A. 2008;105:3855–3860. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Current opinion in immunology. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 42.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends in immunology. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, Williams MA. Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology. 2010;131:310–317. doi: 10.1111/j.1365-2567.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. The Journal of experimental medicine. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. The Journal of experimental medicine. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu D, Komai-Koma M, Liew FY. Expression and function of Toll-like receptor on T cells. Cell Immunol. 2005;233:85–89. doi: 10.1016/j.cellimm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 50.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 51.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.