Abstract
BACKGROUND
LuCaP serially transplantable xenografts derived from primary and metastatic human prostate cancer encompass the molecular and cellular heterogeneity of the disease and are an invaluable resource for in vivo preclinical studies. A limitation of this model, however, has been the inability to establish and passage cell cultures derived from the xenografts. Here, we describe a novel spheroid culture system that supports long-term growth of LuCaP cells in vitro.
METHODS
Xenografts were minced and digested with collagenase. Tissue dissociation was terminated while the majority of cells remained as clusters rather than single cells. The cell clusters were suspended in StemPro medium supplemented with R1881 and Y-27632, a Rho kinase inhibitor, and placed in ultralow attachment dishes for spheroid culture. Serial passage was achieved by partial digestion to small clusters with trypsin/EDTA in the presence of Y-27632. Cell viability, growth and phenotype were monitored with LIVE-DEAD®, MTS, qRT-PCR and immunocytochemical assays.
RESULTS
Cells from six LuCaP xenografts formed proliferating spheroids that were serially passaged a minimum of 3 times and cryopreserved. Two of the cell lines, LuCaP 136 and LuCaP 147, were further passaged and characterized. Both expressed biomarkers characteristic of the xenografts of origin, were determined to be of independent origin by STR fingerprinting, and were free of mycoplasma. LuCaP 147 formed tumors similar to the original xenograft when injected into mice.
CONCLUSIONS
The ability to culture LuCaP cells affords new opportunities for fast, cheap, and efficient preclinical studies and extends the value of the LuCaP xenograft models.
Keywords: prostate cancer, preclinical model, spheroids
INTRODUCTION
One of the principle obstacles in prostate cancer research is the scarcity of realistic preclinical models of advanced disease (1). In vitro cell culture models represent a cost-efficient and reproducible method for preclinical investigation of tumorigenesis and treatment. Yet, current in vitro models of prostate cancer are limited in their representation of the disease. For example, none of the three most commonly used prostate cancer cell lines, DU 145, PC-3, and LNCaP, express a wild-type androgen receptor (AR), a key player in the natural progression of prostate cancer and a primary target of most prostate cancer therapeutics (2). In addition, prostate cancer is well-known for its heterogeneity. Recent evidence suggesting that successful treatment of prostate cancer may depend on identifying individual tumor susceptibility through multiple distinct molecular characteristics, including the existence of an ETS gene fusion, PTEN loss, or AR variants, showcases the need for models that can recapitulate this diversity (3–6). More realistic in vitro models that are both reproducible and cost-effective would greatly aid in both the elucidation of these complex pathways of prostate cancer progression and the search for novel therapeutics to combat them.
Multiple hurdles have prevented the robust generation of accurate in vitro models of both primary and metastatic prostate cancer. First, more aggressive screening of prostate cancer has led to a reduction in the number of high volume and/or high grade prostate cancer cases that present in the clinic. Second, metastatic prostate cancer is rarely removed surgically, and therefore rarely available for culture. Third, primary cells derived from cancer and cultured by traditional methods are difficult to maintain in the lab and do not accurately reflect many properties of in situ prostate cancer. One way to bypass such problems is to grow prostate cancer tissue directly in murine models after harvesting. When successful, this technique allows for even small amounts of prostate cancer tissue to give rise to serially transplantable xenografts.
One such collection of xenografts, the LuCaP series, was initiated over 15 years ago and now contains dozens of serially transplantable xenografts (7). Importantly, the LuCaP xenografts reflect the diverse stages and properties of prostate cancer, as some are derived from primary tumors and others from various metastatic sites, including lymph node and bone. These xenografts encompass both androgen-dependent and castration-resistant tumors and sublines, modeling the transition to castration-resistant prostate cancer (CRPC). Finally, these xenografts express many of the various aberrant pathways commonly researched in the field, including the TMPRSS2-ERG fusion, the epithelial-mesenchymal transition (EMT), and altered miRNA profiles (8–10).
Despite previous attempts, it has not been possible to maintain cells derived from LuCaP xenografts in culture for longer than a few weeks (11–13). In order to generate new in vitro models of prostate cancer, we systematically tested various cell culture methods with the goal of achieving long-term culture of LuCaP cells that recapitulate the properties of the original xenograft. Cells from six LuCaP xenografts have been successfully cultured and passaged using a method that maintains cell-cell contact between LuCaP cells at all points of the culture process. As a result, cultured LuCaP cells are viable, proliferative, and retain many characteristics of their xenografts of origin, including the ability to form tumors when re-established in vivo. This novel culture method has the potential to greatly expand the number of preclinical models available for the prostate cancer research community.
METHODS
LuCaP xenograft digestion and spheroid cell culture
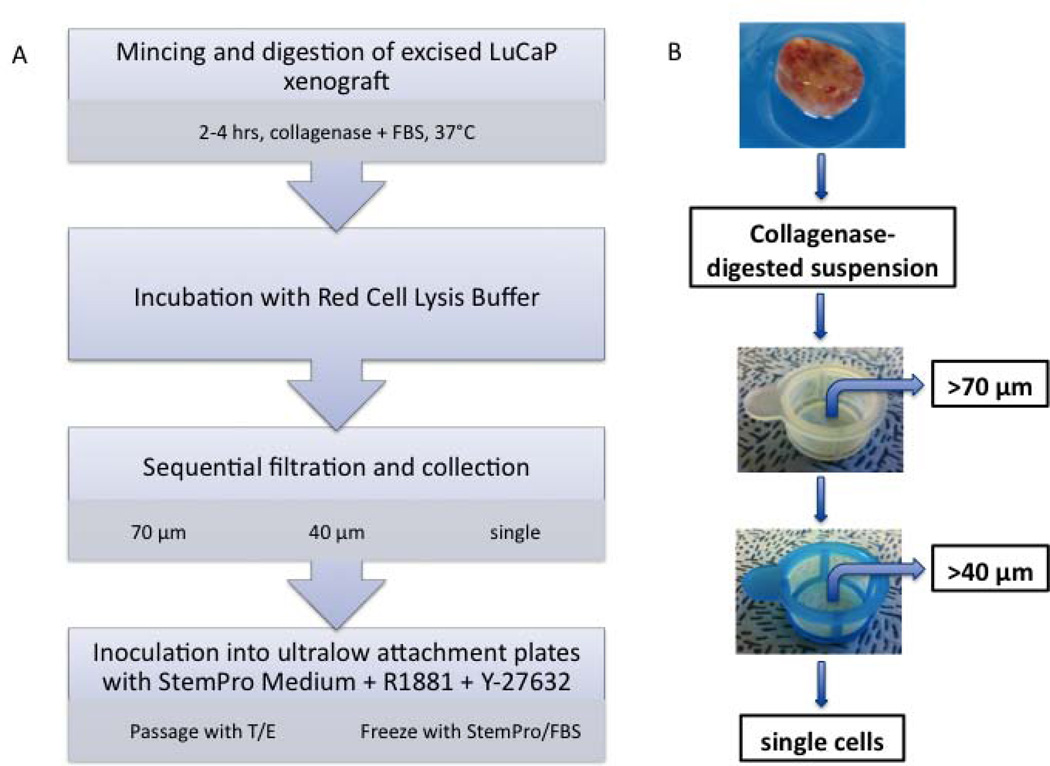
A scheme of the procedure developed for processing and culturing LuCaP xenografts is shown in Fig. 1. Xenografts were excised at the University of Washington and shipped to Stanford by overnight express, submerged in medium and on ice. Xenografts were washed multiple times with HEPES-buffered saline (HBS), minced, and then digested in medium PFMR-4A (14) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and 200 units/ml of type I collagenase (Sigma-Aldrich, St. Louis, MO) at 37°C over a period of 2–4 hours. The tissue was pipetted every 30 minutes to aid dissociation. Digestion was periodically assessed under the microscope and was stopped once small clusters or clumps of cells were released from the xenograft and before complete digestion to single cells occurred. The digested tissue was incubated with Red Cell Lysis Buffer (eBioscience, San Diego, CA) for 3 minutes followed by passage through 70-µm and 40-µm cell strainers (BD Biosciences, Bedford, MA) to isolate each size fraction separately. The final flow-through, consisting mainly of single cells, was also saved. Each fraction was centrifuged, resuspended in StemPro hESC SFM (Invitrogen, Grand Island, NY) supplemented with 10 nM R1881 (Sigma-Aldrich) and 2 µM Y-27632 (Sigma-Aldrich), and placed in 6-well ultralow attachment plates (Corning, Lowell, MA). Cells were fed twice per week by centrifuging at 200g, removing spent medium, and gently resuspending in fresh StemPro medium supplemented with 10 nM R1881.
Fig. 1.
(A) Flowchart depicting digestion and culture of LuCaP xenograft-derived cells. (B) Flowchart depicting sequential filtration and collection of cells from digested LuCaP xenograft.
Serial Passage
Spheroids were assessed under the microscope and passaged when reaching ~200 µm in diameter. Cell cultures were collected and passed through a 40-µm filter in order to eliminate single cells. Remaining spheroids and cell clusters were centrifuged and then digested in HBS containing 0.2% trypsin/0.2% EDTA and 2 µM Y-27632 at 37°C for 2–3 minutes or until larger spheroids were reduced to small clusters of a few or more cells. Trypsin was inhibited by adding medium containing 10% FBS. Cell clusters were centrifuged, resuspended in StemPro medium supplemented with R1881 and Y-27632, and split 1:2 into ultralow attachment plates.
LIVE/DEAD® Cell Viability Assay
The LIVE/DEAD® viability assay (Invitrogen) was performed according to the manufacturer’s instructions. Briefly, LuCaP cells were centrifuged and incubated in phosphate-buffered saline (PBS) supplemented with 0.1 µM calcein AM and 1 µM Ethidium homodimer-1 (EthD-1) for 45 minutes in the dark at 37°C. Cells were visualized under the fluorescence microscope at 517 nm for calcein AM and 617 nm for EthD-1.
MTS Assay
Intact cell clumps or spheroids were first isolated by filtering through a 40-µm cell strainer and then centrifuged. Cells were then digested as described above for serial passage, counted, and inoculated at a density of 2×104 cells per well in a 96-well ultralow attachment plate (Corning). Cell proliferation was assayed one day and seven days after inoculation using the CellTiter AQueous One Solution (Promega, Madison, WI) according to the manufacturer’s instructions. Absorbance was read at a wavelength of 490 nm with a reference wavelength of 670 nm.
Cryopreservation and Thawing
Intact spheroids or cell clusters were cryopreserved one day after feeding. Cells from one well of a 6-well dish were centrifuged and resuspended in one ml of either StemPro medium supplemented with 10% FBS and 10% DMSO, or 90% embryonic stem cell qualified FBS (Invitrogen) and 10% DMSO. Cells were frozen overnight at −70°C in a cryopreservation freezing container (Nalgene, Rochester, NY) before transfer to liquid nitrogen.
To thaw cells, one ampule containing frozen cells in 1 ml of cryopreservation medium was thawed in a water bath at 37°C and then added to nine ml of StemPro medium supplemented with R1881 and Y-27632. Cells were then centrifuged, resuspended in StemPro medium supplemented with R1881 and Y-27632 and placed in an ultralow attachment dish. Y-27632 was removed 48–72 hours later by feeding with StemPro medium without the drug.
Immunocytochemistry
Intact cell clumps or spheroids were first isolated by filtering through a 40-µm cell strainer and then centrifuged. Either intact spheres or spheres that were digested to single cells with trypsin/EDTA were applied to glass slides by spinning for 2 minutes in a StatSpin Cytofuge (Iris Sample Processing, Westwood, MA). Cells were fixed with 2% paraformaldehyde for 15 minutes at room temperature, then permeabilized with 0.25% Triton X-100 for 10 minutes. Non-specific binding was blocked by incubation in 10% horse serum for 10 minutes. Cells were then incubated for 30 minutes at room temperature with primary antibodies (Table I). After rinsing, slides were incubated for 30 minutes with the secondary antibody, goat anti-mouse AlexaFluor 488 (Invitrogen) (1:250). Slides were coverslipped using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and sealed with rubber cement.
Table I.
Primary Antibodies used for Immunocytochemistry
| Antibody | Dilution | Source |
|---|---|---|
| ms anti-keratin 5 | 1:50 | Imgenex (San Diego, CA) |
| ms anti-p63 | 1:50 | Abcam (Cambridge, MA) |
| ms anti-CD44 | 1:100 | Abcam |
| ms anti-keratin 18 | 1:100 | Santa Cruz Biotechnology (Santa Cruz, CA) |
| ms anti-AR | 1:100 | BD Biosciences (Bedford, MA) |
| ms anti-EpCAM | 1:100 | Millipore (Billerica, MA) |
| ms anti-PCNA | 1:100 | Santa Cruz Biotechnology |
| ms anti-HuNu | 1:100 | Millipore |
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from LuCaP spheroids using Trizol (Invitrogen) according to the manufacturer’s instructions. RNA was then reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen). qRT-PCR was performed using SYBR GreenER qPCR Super Mix (Invitrogen) on a Mx3005 QPCR System (Stratagene, La Jolla, CA). Transcript levels in each sample were determined in triplicate and normalized to the transcript level of TATA box binding protein (TBP). Primer sequences for PSA were 5’-GCAGCATTGAACCAGAGGAG-3’ (forward) and 5’-CACCATTACAGACAAGTGGGC-3’ (reverse). Primer sequences for TBP were 5’-TGCTGAGAAGAGTGTGCTGGAG-3’ (forward) and 5’-TCTGAATAGGCTGTGGGGTC-3’ (reverse).
Short Tandem Repeat (STR) Analysis
STR analysis was performed by the Johns Hopkins University Fragment Analysis Facility (Baltimore, MD) using the StemElite ID System (Promega). STR profiles were compared to reference profiles in the ATCC, DSMZ, and JCRB databases.
Tumor-forming Capability of Cultured LuCaP cells
All animal studies were done in compliance with the regulations at Stanford University. Intact LuCaP 147 spheroids were collected from one, two, or three wells of 6-well dishes, centrifuged, suspended in cold Matrigel (BD Biosciences) diluted 1:3 in HBS, and injected subcutaneously (100 µl per site) into each of three 6- to 8-week-old male CB.17 SCID mice (Charles River Laboratories International, Inc., Wilmington, MA), respectively. A 25-mg testosterone pellet with a release rate of 0.2 mg/day was inserted into a small incision made under the skin between the shoulder blades to aid tumor engraftment. Tumors were measured twice a week with a caliper and the volume was calculated using the standard ellipsoid formula (L × W × H × π/6) (15). Tumor doubling time was calculated by best-fit regression analysis using GraphPad prism 5 software. After sacrifice, tumors were resected and fixed in 10% buffered formalin. After embedding in paraffin, 5-µm slices were cut and stained with hematoxylin and eosin.
RESULTS
Initial Attempts to Process and Culture LuCaP Xenografts
The origins and properties of the six LuCaP xenografts used in this study are listed in Table II. We began our attempts to culture LuCaP cells by testing traditional 2-dimensional monolayer cell culture methods. Xenografts were dissociated into single cells with several different types of enzymes. Numerous media that are typically used to culture prostate or other epithelial cell lines (MCDB 105, PFMR-4A, CNT-12, BRFF-HPC1, medium 199, DME, KSFM, MCaP medium, hESC medium) as well as media conditioned by fibroblastic feeder cells or other prostate cancer cell lines were evaluated. These media were supplemented with serum (0–20%) or other additives (SCF, LIF, bFGF, NAC, 3-MA, glucose, glutamine) in addition to the components of the standard formulations of each. Likewise, various culture substrates (collagens, Matrigel, poly-D-lysine, laminin, fibronectin, poly-L-ornithine/laminin, and extracellular matrices derived from fibroblastic or epithelial cell cultures) were tested. Although cells from all of the xenografts attached in one culture condition or another and often remained viable for months, there was little actual proliferation and serial passage could not be sustained. Additionally, LuCaP cells were often overgrown with mouse cells from the xenografts. Attempts to enrich the cell cultures with the LuCaP epithelial cells by sorting with an EpCAM antibody using either FACS or MACS led to poor attachment and growth of the cells once in culture.
Table II.
Derivation and In Vivo Characteristics of Original LuCaP Xenografts Cultured to date In Vitro
| LuCaP xenograft |
Source | Tissue Type |
Histology | Response to CX1 |
Response to DOC2 |
AR3 | PSA4 | Doubling Time (days) |
|---|---|---|---|---|---|---|---|---|
| 58 | OR5 | LN6 | adenocarcinoma | NR7 | HR8 | WT9 | Low | 15 |
| 92 | Autopsy | LN | adenocarcinoma | Pending | Pending | WT | High | 20 |
| 93 | OR | TURP10 | Small cell carcinoma | NR | IR11 | NA12 | NA | 18 |
| 96 | OR | TURP | adenocarcinoma | HR | NR | WT | High | 20 |
| 136 | OR | Ascites | adenocarcinoma | IR | IR | WT | Low | 20 |
| 147 | Autopsy | Liver | adenocarcinoma | NR | NR | Mut13 | Low | 25 |
Castration
Docetaxel
Androgen receptor
Prostate-specific antigen
Operating room
Lymph node
No response
Highly responsive
Wild-type
Transurethral resection of the prostate
Intermediate response
Not applicable
Mutated
Interestingly, many of the LuCaP-derived adherent cultures that survived the first passage exhibited a similar morphology (Fig. 2A). As previously reported by others (11), LuCaP cells quickly formed tight clumps within a few days following digestion to single cells. These clumps were difficult to maintain as adherent cultures, despite the addition of small molecules such as Thiazovivin and Y-27632 reported to aid in recovery of cell-cell interactions during passaging (16). These cell clumps were strikingly similar in appearance to monolayer cultures of the VCaP prostate cancer cell line. Much like adherent LuCaP cultures, VCaP cells form tight cell clusters, are loosely adherent, and proliferate slowly when cultured by traditional methods (17). Additionally, VCaP cells thrive when cultured at high densities, allowing for the cells to rapidly re-associate in clumps following dissociation. Given these similarities, as well as the ability of VCaP cells to rapidly form spheres when placed in suspension culture, we speculated that the maintenance of cell-cell contact might be critical for the survival and proliferation of LuCaP-derived cells.
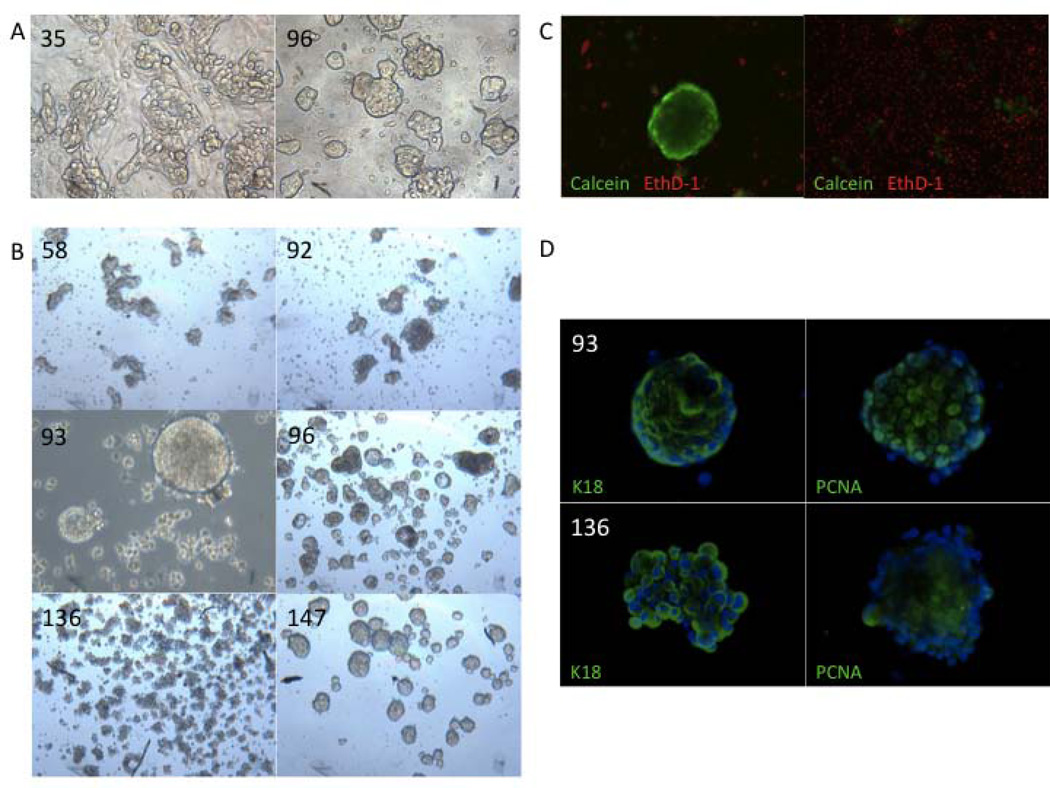
Fig. 2.
Clusters of LuCaP cells survive in suspension culture. (A) Previous attempts to culture adherent LuCaP 35 and 96 cells on 3T3 feeder layers revealed a morphology of tight cell clusters (40X); (B) Morphology of various LuCaP-derived suspension cultures (40X); (C) LIVE/DEAD® assay of LuCaP 147 culture showing intact live (green) clusters compared to dead (red) single cells (100X); (D) Immunofluorescent staining of LuCaP 93 and 136 spheroids for keratin 18 (K18) and PCNA (100X).
Suspension Culture of LuCaP Cells
A recent report investigating primary culture of colorectal cancer cells similarly suggested that conservation of cell-cell contact throughout suspension culture was key to recovering viable cancer cells for sustained in vitro culture (18). Furthermore, the described methods of dissociation and spheroid culture resulted in isolation of pure epithelial cell cultures, selecting against contaminating stromal cells. With this in mind, we hypothesized that maintaining cell-cell contact of LuCaP cells grown in suspension might facilitate their long-term growth in culture.
In order to sustain cell-cell contact, our tissue digestion protocol was modified to promote recovery of small, intact cell clusters from LuCaP xenografts as opposed to single cells (Fig. 1). Xenografts were minced into ~1-mm3 pieces and then digested with collagenase aided by intermittent pipetting over a period of two to four hours at 37°C. The digestion process was monitored closely and terminated once intact “clumps” of cells started to release from the tissue but before these cell clusters were reduced completely to single cells. The tissue digest was then passed sequentially through 70-µm and 40-µm cell strainers in order to separate any single cells from intact clumps of cells. Each cell fraction was resuspended in StemPro, a serum-free medium used in hESC culture, supplemented with a synthetic androgen (R1881) as well as Y-27632, a Rho kinase inhibitor, to promote cell-cell adhesion. Cell fractions were then placed separately in ultralow attachment plates. Immediately following this digestion, flow-through material that passed through the cell strainers consisted mostly of single cells while material caught by the cell strainers consisted of varying sizes of cell clusters.
LuCaP Cells Form Viable Spheroids in Suspension Culture
Following digestion, isolated clumps of cells retained their cell-cell contact in suspension over the following weeks. Some of the single cells also exhibited an ability to reform small cell clumps within one day of the initial digestion. During the first week of in vitro culture, cell clumps derived from each specific LuCaP xenograft began to exhibit consistent and unique morphology (Fig. 2B). While some grew as well-organized spheroids with defined edges (LuCaPs 93 and 96), others grew in disordered clumps of irregular size (LuCaPs 58, 92 and 136). One exception was the long-term culture of LuCaP 147. While LuCaP 147 initially formed tight and organized spheres in vitro, its shape became more disordered with serial passage. This observation may reflect the “hypermutator phenotype” associated with in vivo passage of LuCaP 147 (19).
Next, the viability of the cells within the LuCaP-derived clumps was determined as well as the viability of the single cells that were unable to quickly reform clumps following digestion (Fig. 2C). LIVE/DEAD® assays showed that multicellular clusters or spheroids consisted mostly of live cells with a minority of dead cells. In contrast, the majority of single cells were dead. These data demonstrate that the modified digestion and culture protocol resulted in viable suspension cultures of LuCaP-derived cells. In addition, these results imply that retention or rapid re-establishment of cell-to-cell contact between LuCaP cells is critical to their survival in vitro. Immunochemical detection of the proliferation antigen, PCNA, as well as a classic prostate epithelial cell-type marker, keratin 18, in the spheroids also signified epithelial survival and proliferation (Fig. 2D).
Subculture and Cryopreservation
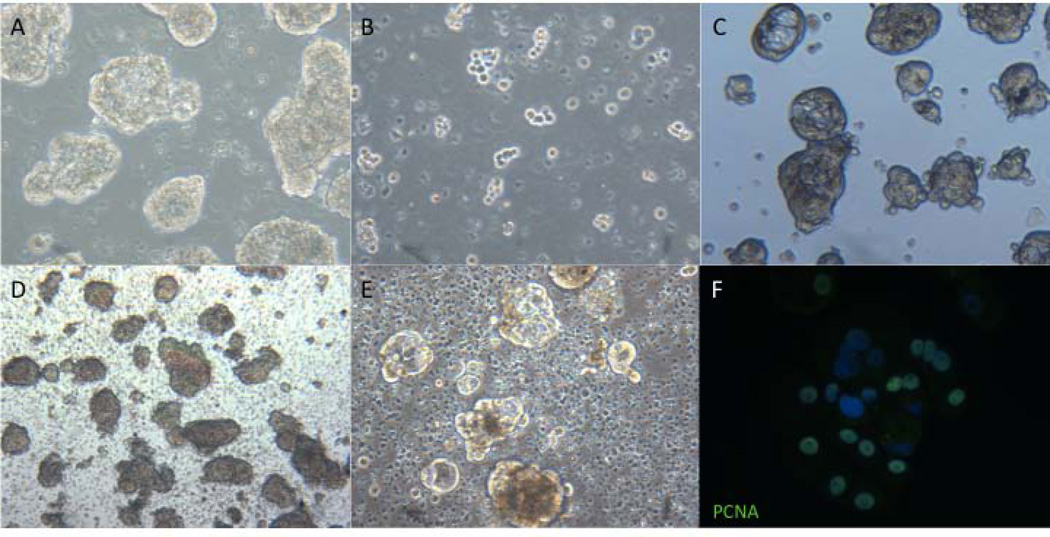
A method for passaging spheroids was optimized in order to promote cell expansion and continued survival of LuCaP cells in culture (Fig. 3A–C). Because the modified culture protocol suggested the importance of sustained cell-cell contact, we postulated that a gentle passaging protocol would be required in order to digest each large LuCaP cluster or spheroid into multiple smaller cell clusters as opposed to single cells. Multiple digestion agents were tested, and it was found that a very brief digestion with trypsin/EDTA resulted in many small clumps of cells. Single cells, which were previously typically found to be dead, were removed with a 40-µm cell strainer before each digestion. Additionally, Y-27632 was added at all steps of the passaging process in order to promote recovery of cell-cell contact. Within three to four days after a 1:2 split, the cells gradually reformed larger clusters or spheroids with a similar morphology to their original cultures. Using this method, LuCaP cells from each of six xenografts have been passaged at least 3 times to this point, with LuCaP 147 now beyond passage 12.
Fig. 3.
Subculture, cryopreservation, and proliferation of LuCaP cultured cells. Images of LuCaP 96 spheroids before (A), immediately following (B), and four days after digestion with trypsin/EDTA (C). Images of LuCaP 96 immediately following (D) and four days after thawing (E) as well as immunofluorescent detection of nuclear PCNA, indicating active proliferation (F). All images 100X.
For cryopreservation (Fig. 3D–E), spheroids were collected intact after filtration through a 40-µm cell strainer and suspended either in StemPro medium supplemented with 10% FBS and 10% DMSO or 90% embryonic stem cell qualified FBS with 10% DMSO. After freezing in a Nalgene cryopreservation chamber, the cells were stored in liquid nitrogen. The spheroids retained their morphology upon thawing from either cryopreservation medium and resumed proliferation, as shown by the expression of PCNA (Fig. 3F).
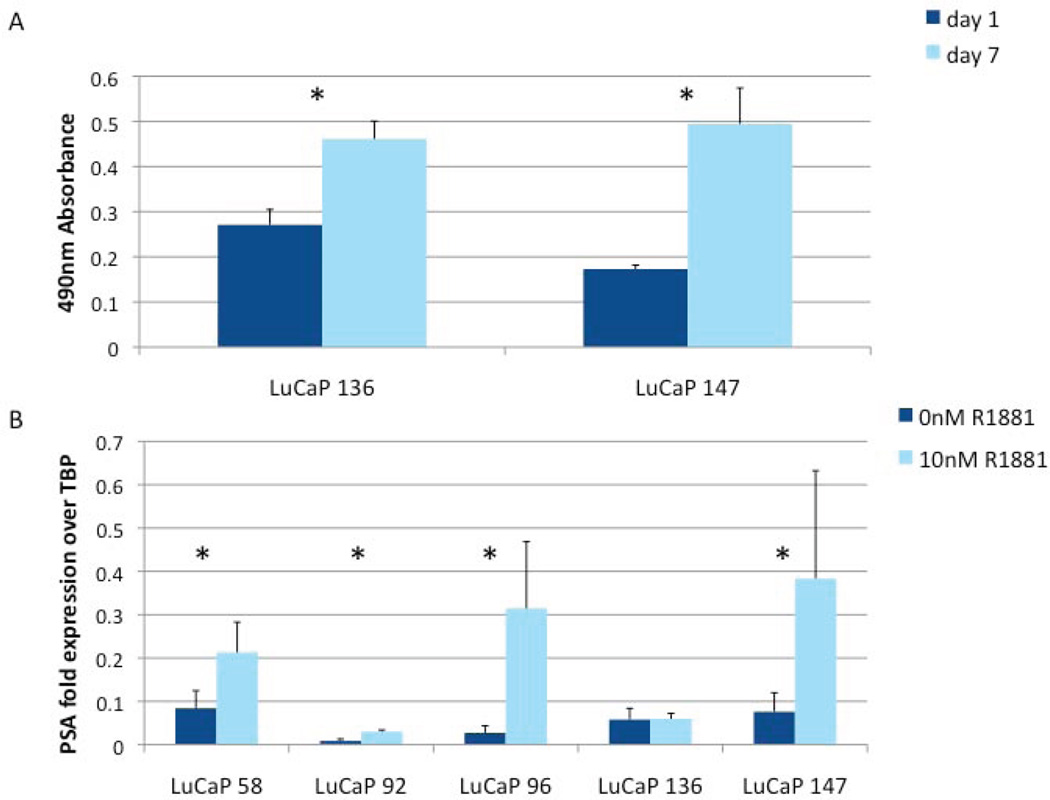
Proliferation of LuCaP Spheroids
After testing multiple assays for quantifying growth of cells in suspension, the MTS assay (20) was chosen for giving most consistent results with the LuCaP cultures, likely because the formazan product is soluble in culture medium and therefore the assay requires very little cell manipulation. After one week of growth, both LuCaP 136 and 147 exhibited a statistically significant increase in viable cells (Fig. 4A). These results, in conjunction with immunocytochemical detection of the proliferation marker PCNA in the majority of cells in spheroids (Figs. 2D and 3F), indicate that LuCaP-derived cells actively proliferate in suspension culture.
Fig. 4.
In vitro growth of LuCaP cultured cells. (A) LuCaP cells proliferated in vitro as shown by MTS assay of cultured LuCaP 136 and LuCaP 147 cells over seven days (*p < 0.005). (B) Response to androgen (+/− 10 nM R1881 for 48 hours) of various cultured LuCaP-derived cells as assayed by qRT-PCR for PSA expression (*p < 0.005).
LuCaP Spheroids Retain Expression of Differentiation and Tumor Markers
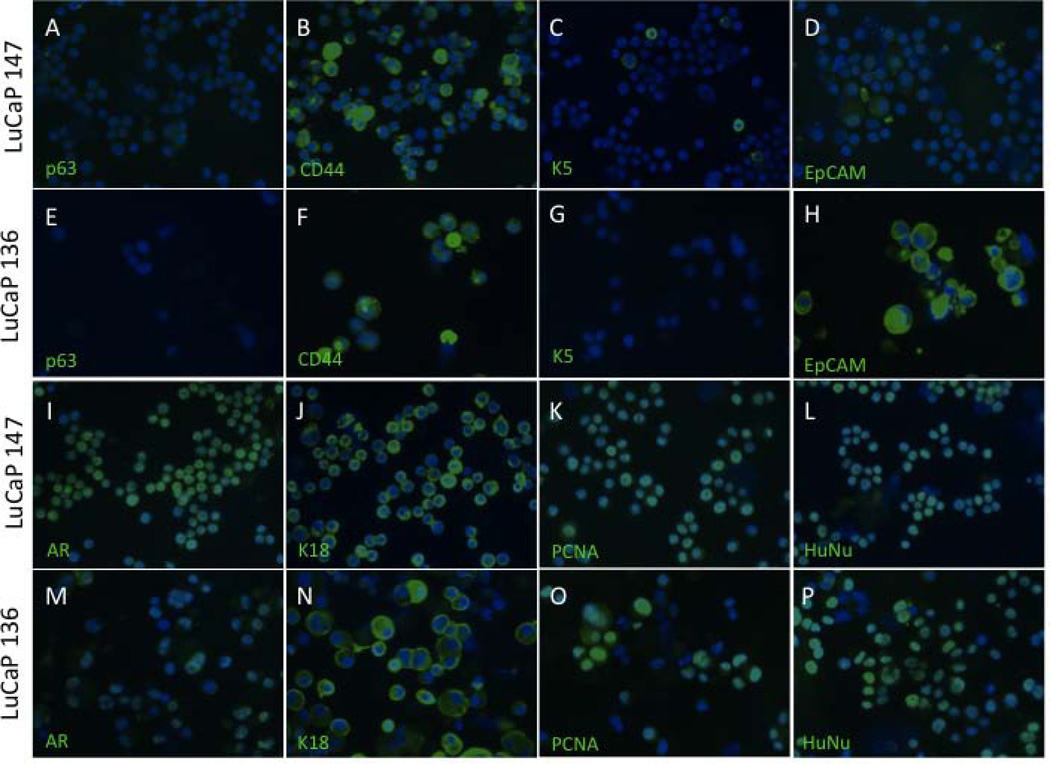
The phenotypic fidelity of cultured LuCaP cells compared to their original xenografts was determined by immunofluorescent staining of LuCaP 136 and 147 with various markers of prostate differentiation and tumorigenesis (Fig. 5). Staining was performed by passing cultures through a cell strainer, discarding the single cells and retaining intact clumps or spheroids. These cell clumps and spheroids were then digested to single cells and immediately applied to slides and fixed for immunofluorescence. Both cell lines exhibited a luminal cell phenotype characteristic of prostate cancer, with the majority of cells expressing androgen receptor (AR) and keratin 18. Additionally, both lacked expression of the basal cell markers, p63 and keratin 5, with the exception of rare keratin 5-positive cells in the LuCaP 147 population. Both showed heterogeneous expression of CD44, a marker of basal cells in normal prostate as well as a putative prostate cancer stem cell marker. Interestingly, EpCAM, the cell-surface antigen that is used for clinical detection of circulating prostate cancer cells, is prominently displayed by LuCaP 136 cells but is rare or absent on LuCaP 147 cells. Finally, positive staining for PCNA in both LuCaP 136 and 147 cells further confirmed the previous findings regarding active proliferation, and human origin of in vitro cultures of LuCaP-derived cells was indicated by expression of the human-specific antigen HuNu.
Fig. 5.
Immunofluorescence of prostate cell markers in cells from cultured LuCaP 136 and 147 spheroids. Cells lack expression of basal cell markers p63 (A and E) and K5 (C and G) but show heterogeneous staining for CD44 (B and F) and EpCAM (D and H). Cells are strongly positive for luminal cell markers AR (I and M) and K18 (J and N) as well as the proliferative marker PCNA (K and O) and HuNu (L and P), a marker of human nuclei.
LuCaP Spheroids Respond to Androgen
One of the most valuable characteristics of the LuCaP xenografts is their ability to recapitulate the complexity of androgen responsiveness that is observed in prostate tumorigenesis and progression to CRPC. Since we determined that expression of AR was retained by LuCaP cell lines derived from AR-expressing xenografts, we investigated whether expression of a classic AR target gene, prostate-specific antigen (PSA), was regulated by androgen in these cells. Spheroid cultures were grown for 2 days +/− 10 nM R1881, RNA was isolated, and PSA mRNA levels were measured by qRT-PCR. As shown in Fig. 4B, with the exception of LuCaP 136, all showed statistically significant increases in PSA expression in response to R1881.
LuCaP-derived Cultures are Unique and of Human Origin
Given that LuCaP tumors are often infiltrated by mouse stroma in vivo (11,13,21), and recent evidence suggests that continuous in vitro culture can lead to cell line contamination (22,23), LuCaP 136 and LuCaP 147 cell cultures were tested by STR analysis to confirm their identity (24). Analysis showed that each culture is of human male origin and is uncontaminated by mouse cells after growth in vitro. Additionally, the STR profiles of each culture did not match that of any cell line found in the ATCC, DSMZ, or JCRB databases (Table III). These results indicate that in vitro cultures derived from LuCaP xenografts consist entirely of human prostate cancer cells. Testing for mycoplasma was performed by the same facility and the cultures were found to be free of mycoplasma contamination.
Table III.
STR Profiles of Cultured LuCaPs 136 and 147
| Locus ID | LuCaP 136 | LuCaP 147 |
|---|---|---|
| AMEL | X,Y | X,Y |
| CSF1PO | 10,11 | 10,11 |
| D13S317 | 11 | 8,12 |
| D16S539 | 9 | 10,13 |
| D21S11 | 30,31.2 | 30 |
| D5S818 | 9,12 | 12,13 |
| D7S820 | 7,12 | 8.3,10 |
| Mus | ||
| TH01 | 9 | 7,9.3 |
| TPOX | 8 | 7,11 |
| vWA | 14,17 | 17,20 |
Cultured LuCaP Cells are Tumorigenic In Vivo
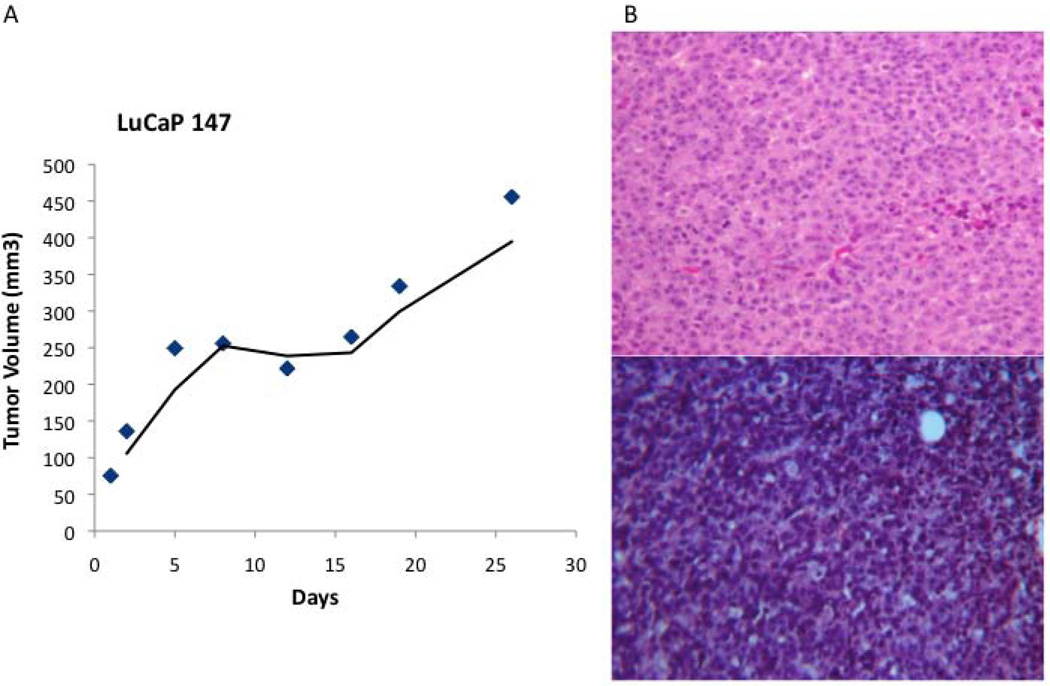
Intact LuCaP 147 spheroids at passage 9 were collected, suspended in Matrigel, and injected subcutaneously into male SCID mice. It was estimated that ~2 million, 4 million and 6 million cells were injected into each of three mice. Tumor formation occurred in all of the mice within 4 weeks of injection. The growth curve of the tumor in the mouse injected with ~4 million cells is shown in Fig. 6A. Doubling time was estimated to be ~15 days, somewhat faster than the doubling time (25 days) of the original xenograft (Table II). The histology of the resultant tumors from the cell cultures was similar to that of the original xenograft (Fig. 6B).
Fig. 6.
In vivo growth of cultured LuCaP 147 cells. (A) LuCaP 147 in vivo growth curve. (B) Comparison of LuCaP 147 tumors by H&E in the original xenograft (top) and the cell culture-derived xenograft (bottom) (100X).
DISCUSSION
For decades, efforts to establish new prostate cancer cell lines have met with only sporadic success. Attempting ourselves to establish monolayer cultures of cells from LuCaP xenografts, we tested multiple methods of tissue disaggregation, diverse culture media, numerous supplemental growth factors and hormones, and a wide variety of attachment substrata, to no avail. The best we could achieve was attachment of LuCaP cells in primary culture, with some limited potential for serial passage. However, throughout establishment and passage, there was little actual proliferation, and monolayer cultures could not be sustained.
Our breakthrough came after reading a publication on culturing human colon cancer cells by Kondo et al. (18). Like prostate cancer, colon cancer has traditionally been difficult to establish in cell culture. The key to successful culture of colon cancer cells was retaining cell-cell contact throughout all stages of culture. Colon tumors were dissociated into cell clusters, which rapidly formed spheroids in suspension culture in serum-free medium. These investigators demonstrated that sustained E-cadherin-mediated cell-cell interaction, accompanied by AKT activation, was the critical element that allowed cancer cells to survive, while single cells rapidly underwent apoptosis.
Our first attempts to culture LuCaP cells by the methods described by Kondo et al. were immediately gratifying; spheroids readily formed from partially disaggregated LuCaP tumors, and these spheroids enlarged over time and could be serially passaged. While spheroid culture methodology has previously been reported for prostate cancer cell lines or prostate stem/progenitor cells (25), the methodology described by Kondo et al. is entirely different in that the spheroids aren’t derived from single cells, as are those typically grown from cell lines or stem cells. Instead, the spheroids are formed by clusters of cells that are never permitted to exist as single cells. Whether these LuCaP spheroids contain stem cells or “cancer initiating cells” (CICs) that maintain cell renewal during serial passage will be investigated in the future; indeed, whether the LuCaP spheroid cultures are in fact immortal remains to be determined over time. Chen et al. reported that prostaspheres derived from single cells obtained from primary adenocarcinomas of the prostate do not contain CICs and therefore exhibit only limited self-renewal capacity in vitro (26). It would be interesting to investigate whether using the spheroid culture methodology described here for LuCaP cells would permit the survival of CICs from primary prostate cancers and perhaps enable extended culture.
To the cell culture platform derived from that for colon cancer cells, we added another element taken from stem cell culture methodology. As described for colon cancer cells, survival and self-renewal of pluripotent stem cells is also dependent on cell-cell adhesion. For example, human embryonic stem cells (hESCs) are very vulnerable to single cell dissociation, and massive cell death occurs in single cells. Using a high-throughput chemical screen, Xu et al. identified two small molecules that enhanced the survival of hESCs (16). These compounds regulated E-cadherin-mediated cell-cell interactions, and the cause of death in single hESCs was disruption of E-cadherin signaling. The molecular targets of these small molecules were identified as Rho-associated kinase (ROCK). Concurrently, others showed that Y-27632, a selective ROCK inhibitor, promoted survival of hESCs. Given the implication of E-cadherin-mediated signaling in survival of colon cancer cells, we added Y-27632 to our formulation for LuCaP cell culture. Of note, a recently described cell culture system that reportedly enables cells from many tissues to proliferate indefinitely in vitro includes the addition of Y-27632 to the medium (27). We did not find, however, that this culture system supported the growth and passage of LuCaP cells (unpublished data).
Altogether, our novel approach to the culture of LuCaP cells promises to overcome the barriers that have prevented the generation of adequate preclinical models of prostate cancer. The LuCaP serially transplantable xenografts, developed at the University of Washington from metastatic and primary tissues under a “rapid autopsy” program or from surgeries, respectively, are among the most realistic models of prostate cancer. Although widely used for in vivo studies, applications of LuCaPs have been limited by the inability of researchers to establish in vitro cell cultures from these xenografts. This technological failure is not specific to LuCaPs but evinces a manifest incapability of reliably growing cells from prostate cancer metastases or even from primary prostate cancer (14). Using novel spheroid culture methodology, we have demonstrated that LuCaP cells can be established in primary culture and serially passaged for extended periods of time. Further development of this culture system and extension to additional LuCaP xenografts, as well as to patient-derived tissues or other xenografts, will result in the addition of a multitude of new cell lines to the prostate cancer research community.
The availability of LuCaP cell cultures presents many new opportunities. Development of clinically efficacious drugs depends on realistic and predictive preclinical models. Advanced prostate cancer has proven extremely difficult to treat, and the heterogeneity of metastases is part of the challenge. The availability of cell lines that span the molecular and cellular heterogeneity of the disease could have an enormous impact on developing better treatment strategies. While LuCaP xenografts themselves provide this diversity, they are not amenable to high-throughput assays as are cell lines. Literally thousands of novel and promising therapeutics could be tested in LuCaP cell lines. The fact that LuCaP cells do not grow readily in monolayer culture but grow instead as spheroids may actually be fortuitous. Three-dimensional cultures are considered to more accurately mimic the in vivo situation of solid tumors than two-dimensional cultures (28,29), and therefore spheroid cultures of LuCaP cells may better predict response to therapeutics when translated to clinical application.
CONCLUSIONS
For years, researchers have tried with little success to establish cell lines from prostate cancer metastases and/or transplantable xenografts. From hundreds of attempts, a handful of cell lines have resulted. Maintaining cell-cell contact at all times throughout dissociation, cryopreservation, thawing and spheroid culture has permitted the establishment and serial passage of cell lines from six LuCaP xenografts, and may permit the establishment of many more lines from additional LuCaP xenografts, other xenografts, and even patient-derived tissues.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of the following entities in generating the LuCaP xenografts: the Richard M. Lucas Foundation, the Prostate Cancer Foundation, NIH P01 CA85859 (PI R.l. Vessella), and NIH Pacific Northwest Prostate Cancer SPORE, P50 CA097186 (PI P. Nelson, Biospecimen Core Director E/L. Vessella).
Grant sponsor: Prostate Cancer Foundation Challenge Award (RLV and DMP) and Ferdinand Eisenberger Grant of the German Society of Urology ID SaM1/FE-11 (MS)
Footnotes
Disclosure statement: The authors declare that they have no affiliations with any organization that may have a direct interest in the research described, or a real or perceived conflict of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation on the manuscript.
REFERENCES
- 1.Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM, Hahn WC, Isaacs JT, Navone NM, Peehl DM, Simons JW, Solit DB, Soule HR, VanDyke TA, Weber MJ, Wu L, Vessella RL. The current state of preclinical prostate cancer animal models. Prostate. 2008;68(6):629–639. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J Urol. 2005;173(2):342–359. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 3.Ateeq B, Tomlins SA, Laxman B, Asangani IA, Cao Q, Cao X, Li Y, Wang X, Feng FY, Pienta KJ, Varambally S, Chinnaiyan AM. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med. 2011;3(72):72ra17. doi: 10.1126/scitranslmed.3001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, Patel S, Wang X, Liang H, Yu J, Palanisamy N, Siddiqui J, Yan W, Cao X, Mehra R, Sabolch A, Basrur V, Lonigro RJ, Yang J, Tomlins SA, Maher CA, Elenitoba-Johnson KS, Hussain M, Navone NM, Pienta KJ, Varambally S, Feng FY, Chinnaiyan AM. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19(5):664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Weerden WM, Bangma C, de Wit R. Human xenograft models as useful tools to assess the potential of novel therapeutics in prostate cancer. Br J Cancer. 2009;100(1):13–18. doi: 10.1038/sj.bjc.6604822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, Tchinda J, Tomlins SA, Hofer MD, Pienta KG, Kuefer R, Vessella R, Sun XW, Meyerson M, Lee C, Sellers WR, Chinnaiyan AM, Rubin MA. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66(17):8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 9.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67(13):6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72(2):527–536. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 11.Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55(4):239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 12.Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin Cancer Res. 1996;2(6):1039–1048. [PubMed] [Google Scholar]
- 13.True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, Lange P, Vessella R. A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49. Am J Pathol. 2002;161(2):705–715. doi: 10.1016/S0002-9440(10)64226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12(1):19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- 15.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107(18):8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korenchuk S, Lehr JE, L MC, Lee YG, Whitney S, Vessella R, Lin DL, Pienta KJ. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15(2):163–168. [PubMed] [Google Scholar]
- 18.Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, Ohue M, Inoue M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A. 2011;108(15):6235–6240. doi: 10.1073/pnas.1015938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, Coleman I, Ng SB, Salipante SJ, Rieder MJ, Nickerson DA, Corey E, Lange PH, Morrissey C, Vessella RL, Nelson PS, Shendure J. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011;108(41):17087–17092. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3(7):207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 21.Morrissey C, Dowell A, Koreckij TD, Nguyen H, Lakely B, Fanslow WC, True LD, Corey E, Vessella RL. Inhibition of angiopoietin-2 in LuCaP 23.1 prostate cancer tumors decreases tumor growth and viability. Prostate. 2010;70(16):1799–1808. doi: 10.1002/pros.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee R. Cell biology. Cases of mistaken identity. Science. 2007;315(5814):928–931. doi: 10.1126/science.315.5814.928. [DOI] [PubMed] [Google Scholar]
- 23.(NIH) NIoH. NOT-OD-08-017: Notice regarding authentication of cultured cell lines. 2007
- 24.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, Kelland LR, Harrison M, Virmani A, Ward TH, Ayres KL, Debenham PG. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98(14):8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein AS, Drake JM, Burnes DL, Finley DS, Zhang H, Reiter RE, Huang J, Witte ON. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat Protoc. 2011;6(5):656–667. doi: 10.1038/nprot.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Principessa L, Isaacs JT. Human prostate cancer initiating cells isolated directly from localized cancer do not form prostaspheres in primary culture. Prostate. 2012;72(13):1478–1489. doi: 10.1002/pros.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 180(2):599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9(4):273–285. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- 29.Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 136(3):473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]