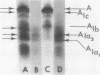
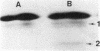
Abstract
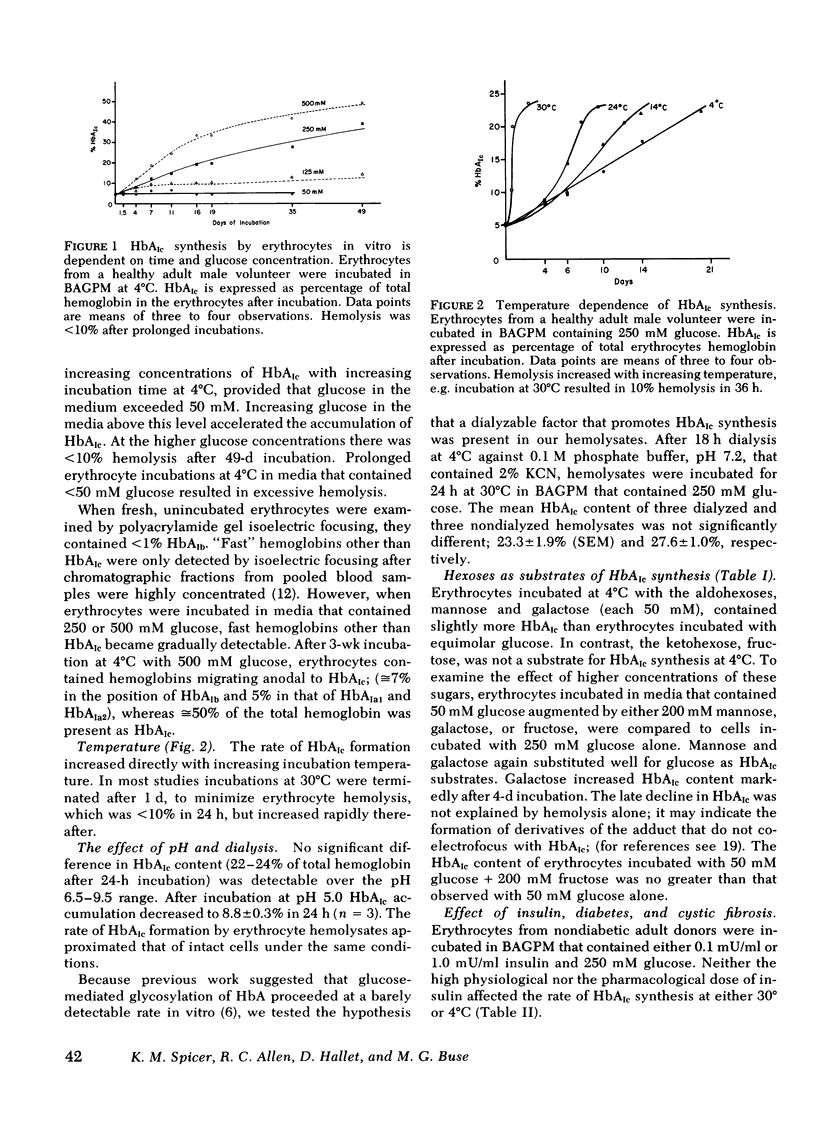
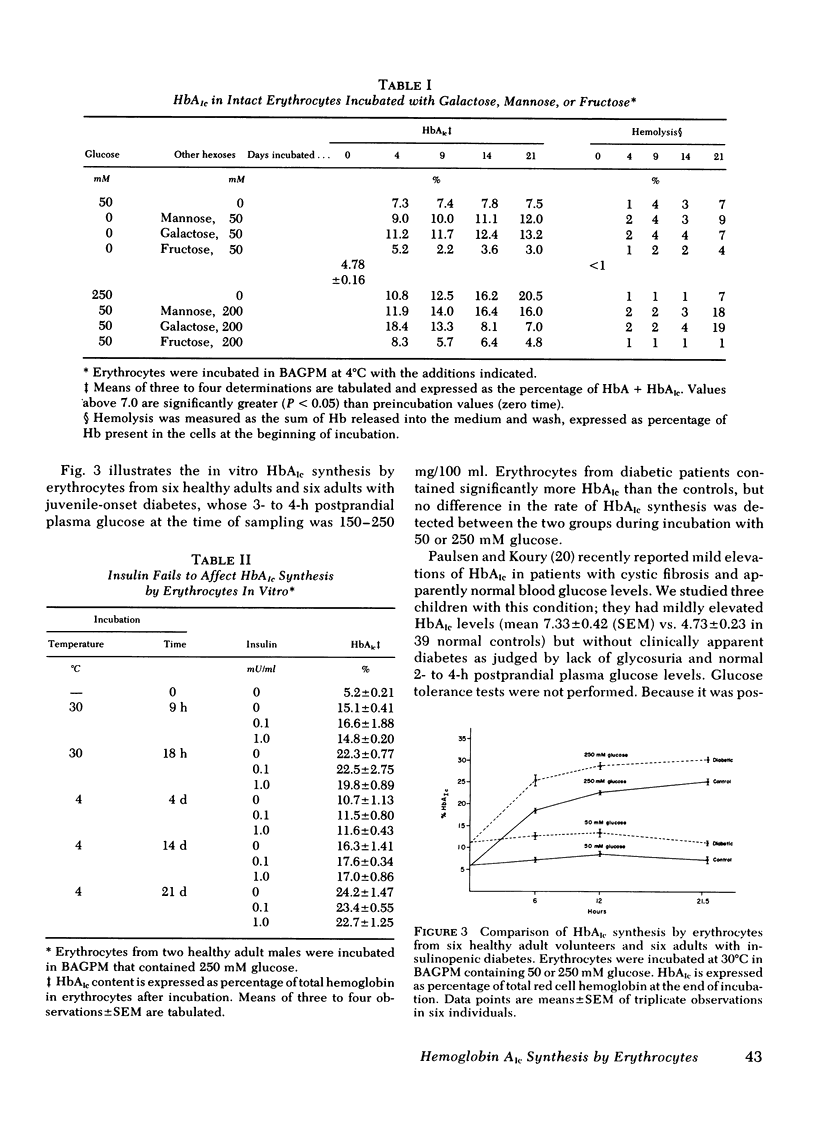
Factors that influence hemoglobin (Hb)AIc synthesis by intact erythrocytes were studied in vitro. After incubation cells were lysed, and hemoglobins were separated by isoelectric focusing on polyacrylamide slab gels and quantitated by microdensitometry. HbAIc increased with time, glucose concentrations (5-500 mM), and incubation temperature (4°-37°C). Low temperatures allowed prolonged incubations with minimal hemolysis. At 4°C HbAIc increased linearly with time for 6 wk; after incubation at the highest glucose concentration, HbAIc comprised 50% of total hemoglobin.
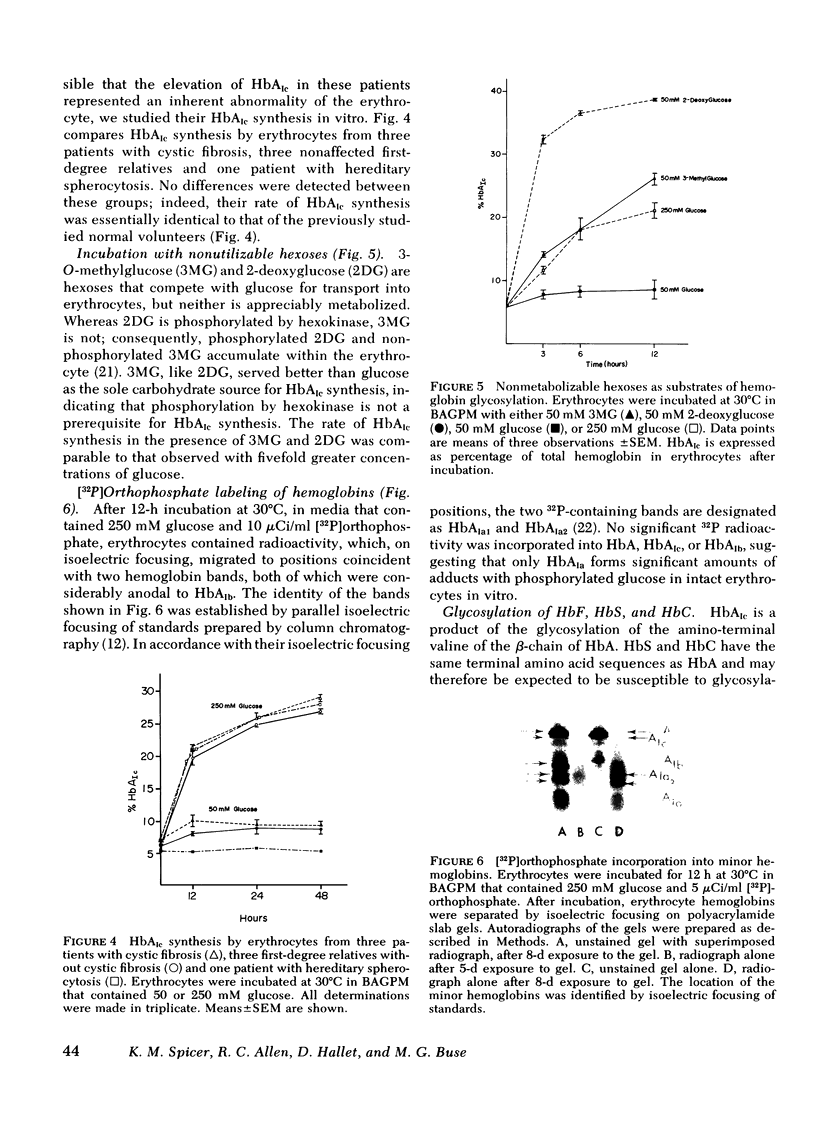
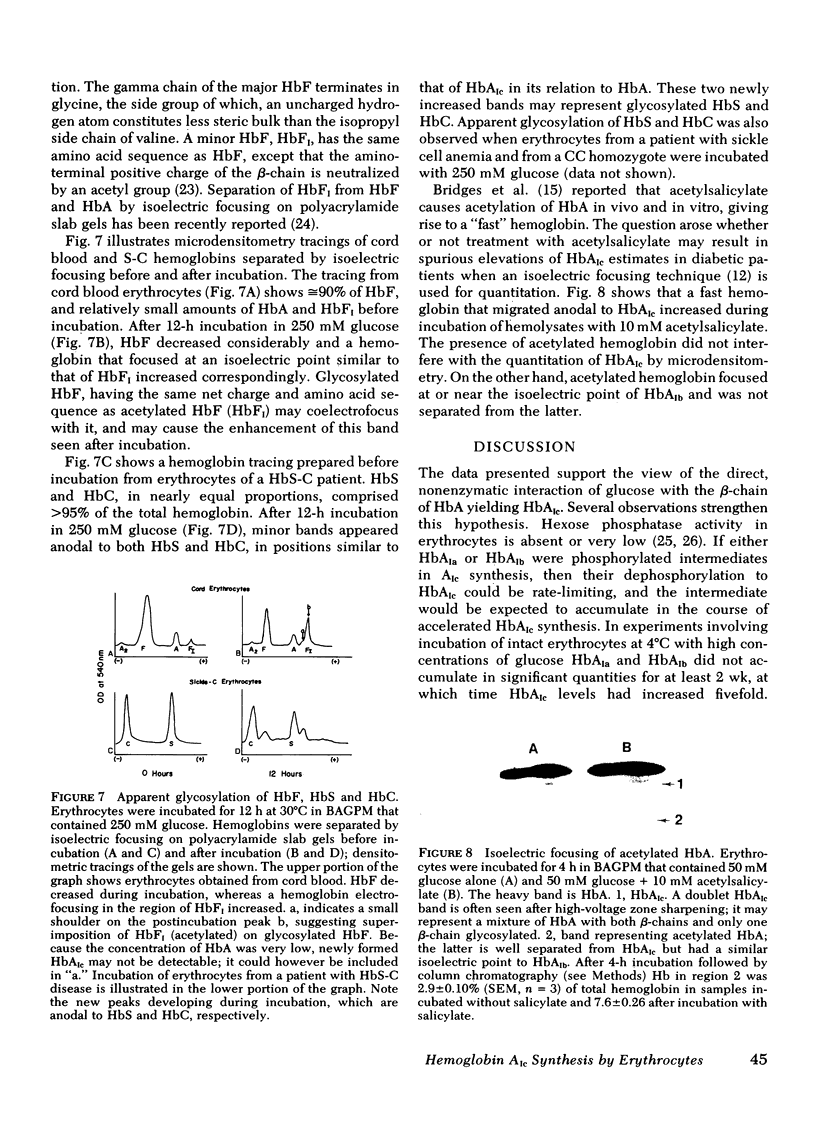
Insulin (1 and 0.1 mU/ml) did not affect HbAIc synthesis in vitro. In addition to glucose, galactose and mannose, but not fructose, served as precursors to HbAIc. A good substrate for hexokinase (2-deoxyglucose) and a poor hexokinase substrate (3-O-methylglucose), were better precursors for HbAIc synthesis than glucose, suggesting that enzymatic phosphorylation of glucose is not required for HbAIc synthesis. Autoradiography after erythrocyte incubation with 32P-phosphate showed incorporation of radioactivity into HbAIa1 and AIa2, but not HbAIb, AIc, or A. Acetylated HbA, generated during incubation with acetylsalicylate, migrated anodal to HbAIc and clearly separated from it.
Erythrocytes from patients with insulinopenic diabetes mellitus synthesized HbAIc at the same rate as controls when incubated with identical glucose concentrations. Likewise, the rate of HbAIc synthesis by erythrocytes from patients with cystic fibrosis and congenital spherocytosis paralleled controls. When erythrocytes from cord blood and from HbC and sickle cell anemia patients were incubated with elevated concentrations of glucose, fetal Hb, HbC, and sickle Hb decreased, whereas hemoglobins focusing at isoelectric points near those expected for the corresponding glycosylated derivatives appeared in proportionately increased amounts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Ritchey J. M., Tam J. W., Klotz I. M. Glycosylation of hemoglobin S by reducing sugars and its effect on gelation. Biochim Biophys Acta. 1977 Feb 22;490(2):462–470. doi: 10.1016/0005-2795(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Basset P., Beuzard Y., Garel M. C., Rosa J. Isoelectric focusing of human hemoglobin: its application to screening, to the characterization of 70 variants, and to the study of modified fractions of normal hemoglobins. Blood. 1978 May;51(5):971–982. [PubMed] [Google Scholar]
- Beutler E., Wood L. A. Preservation of red cell 2,3-DPG and viability in bicarbonate-containing medium: the effect of blood-bag permeability. J Lab Clin Med. 1972 Nov;80(5):723–728. [PubMed] [Google Scholar]
- Bridges K. R., Schmidt G. J., Jensen M., Cerami A., Bunn H. F. The acetylation of hemoglobin by aspirin. In vitro and in vivo. J Clin Invest. 1975 Jul;56(1):201–207. doi: 10.1172/JCI108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Kamin S., Gabbay K. H., Gallop P. M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976 Jun;57(6):1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. B. A reaction of glucose with peptides. Biochem J. 1972 Aug;129(1):203–208. doi: 10.1042/bj1290203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhofer R., Wieland O. H. In vitro glycosylation of hemoglobins by different sugars and sugar phosphates. FEBS Lett. 1978 Jan 1;85(1):86–90. doi: 10.1016/0014-5793(78)81254-x. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Hasty K., Breslow J. L., Ellison R. C., Bunn H. F., Gallop P. M. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977 May;44(5):859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- Gambhir K. K., Archer J. A., Bradley C. J. Characteristics of human erythrocyte insulin receptors. Diabetes. 1978 Jul;27(7):701–708. doi: 10.2337/diab.27.7.701. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A., Rochman H., Tanega S. P., Horwitz D. L. Haemoglobin A1: An indicator of the metabolic control of diabetic patients. Lancet. 1977 Oct 8;2(8041):734–737. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- Haney D. N., Bunn H. F. Glycosylation of hemoglobin in vitro: affinity labeling of hemoglobin by glucose-6-phosphate. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3534–3538. doi: 10.1073/pnas.73.10.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Jones R. L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Kilo C., Cerami A., Williamson J. R. Hemoglobin AIc as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976 Mar;25(3):230–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- McDonald M. J., Shapiro R., Bleichman M., Solway J., Bunn H. F. Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J Biol Chem. 1978 Apr 10;253(7):2327–2332. [PubMed] [Google Scholar]
- Paulsen E. P., Koury M. Hemoglobin AIc levels in insulin-dependent and -independent diabetes mellitus. Diabetes. 1976;25(2 Suppl):890–896. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Spicer K. M., Allen R. C., Buse M. G. A simplified assay of hemoglobin AIc in diabetic patients by use of isoelectric focusing and quantitative microdensitometry. Diabetes. 1978 Apr;27(4):384–388. doi: 10.2337/diab.27.4.384. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. J., Vlassara H., Abati A., Cerami A. Nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1977 May 10;252(9):2998–3002. [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Zipper H., Mawe R. C. The exchange and maximal net flux of glucose across the human erythrocyte. I. The effect of insulin, insulin derivatives and small proteins. Biochim Biophys Acta. 1972 Sep 1;282(1):311–325. doi: 10.1016/0005-2736(72)90337-9. [DOI] [PubMed] [Google Scholar]