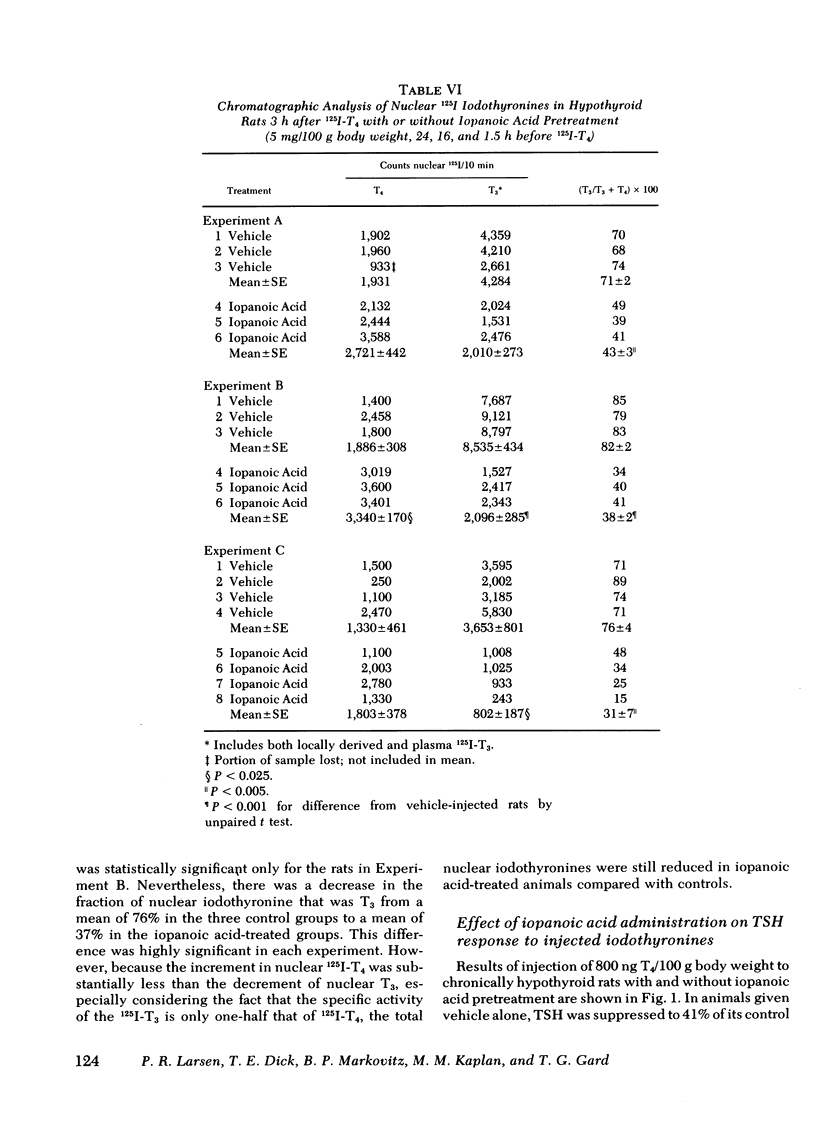
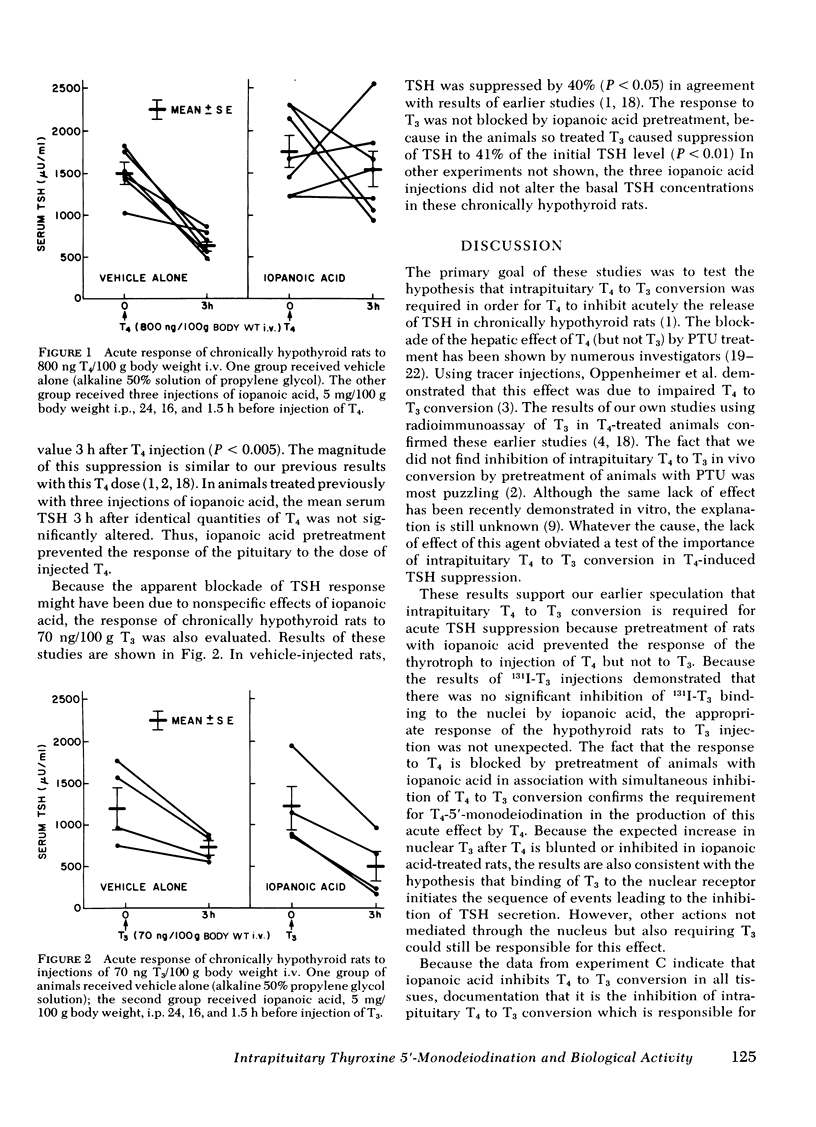
Abstract
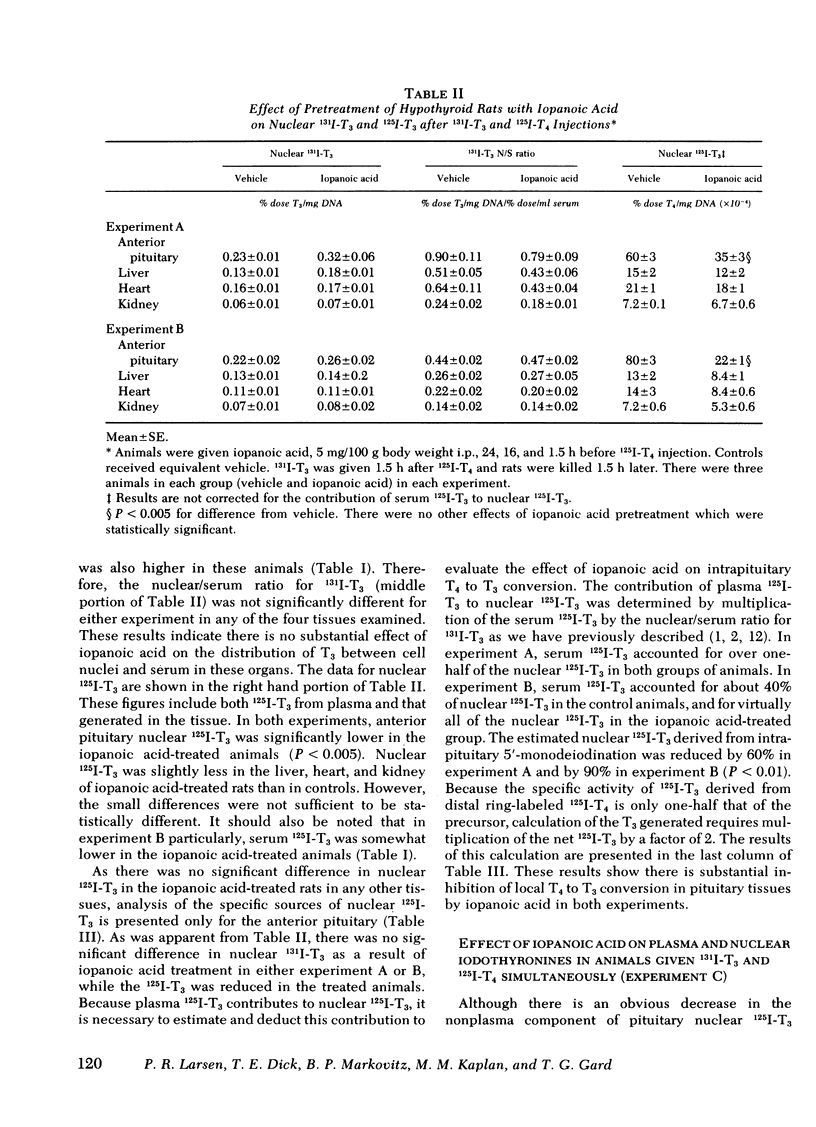
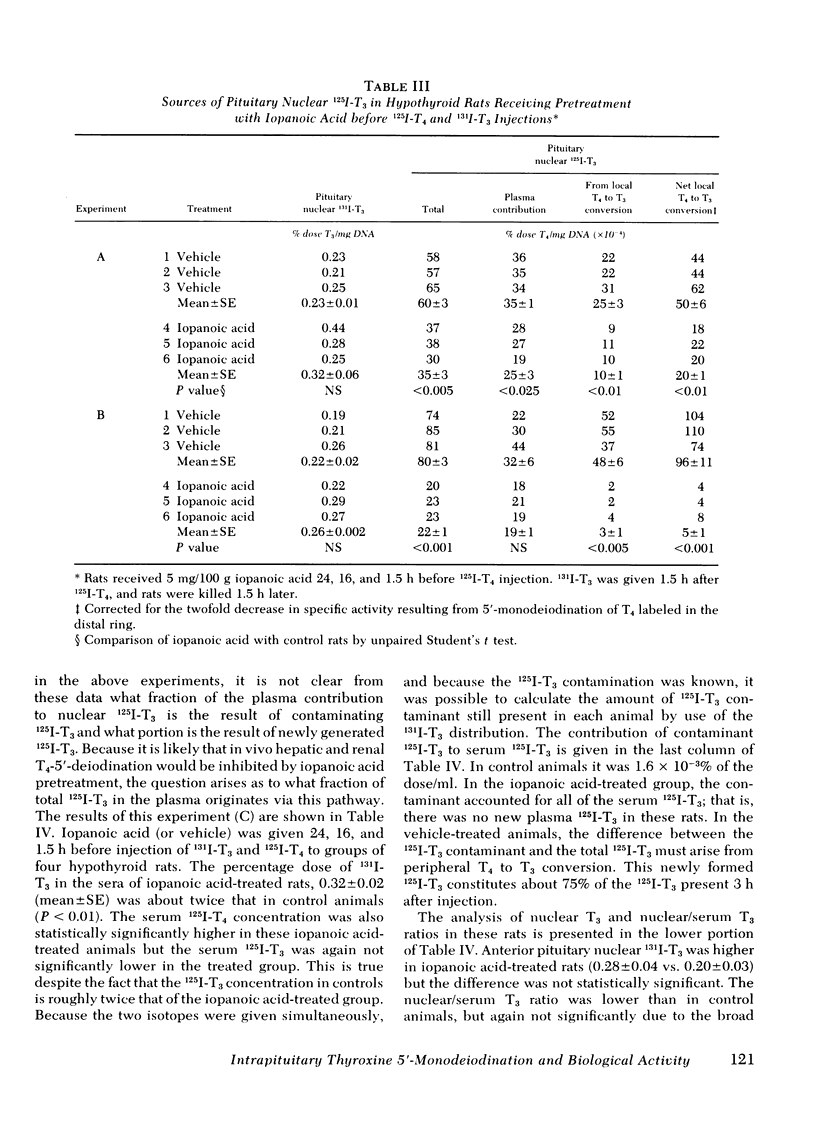
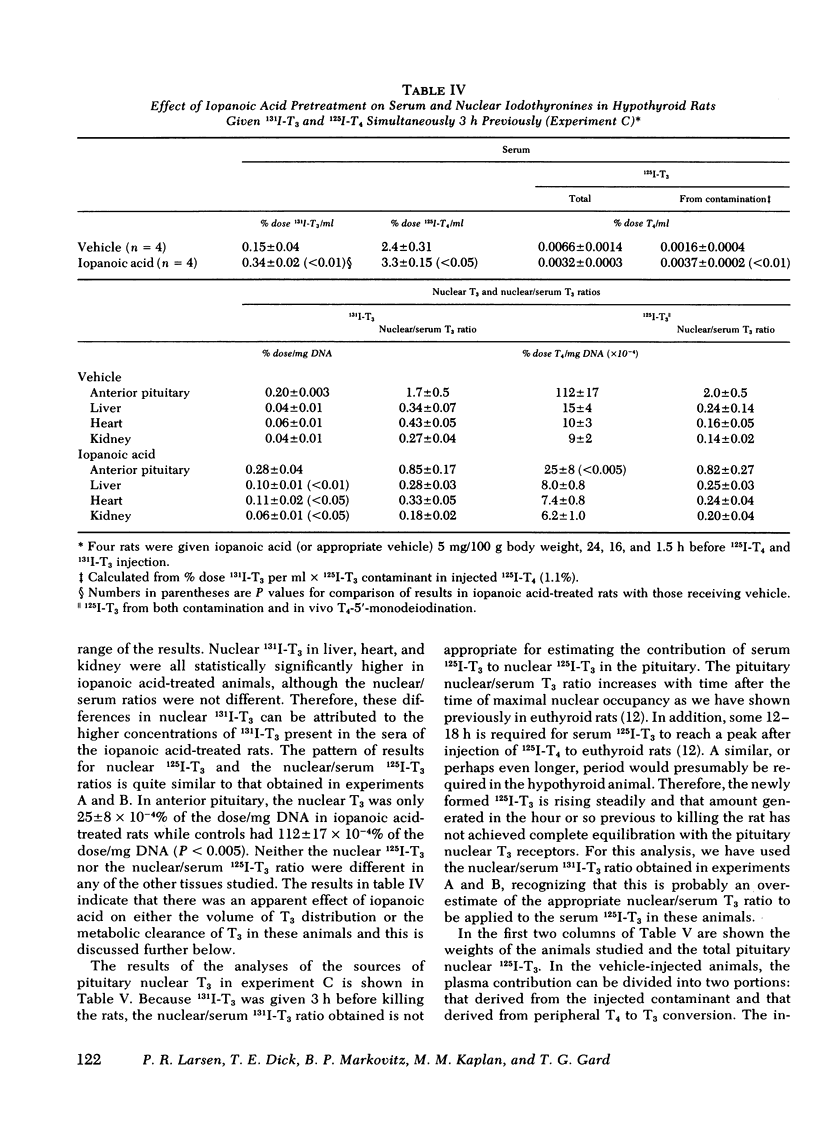
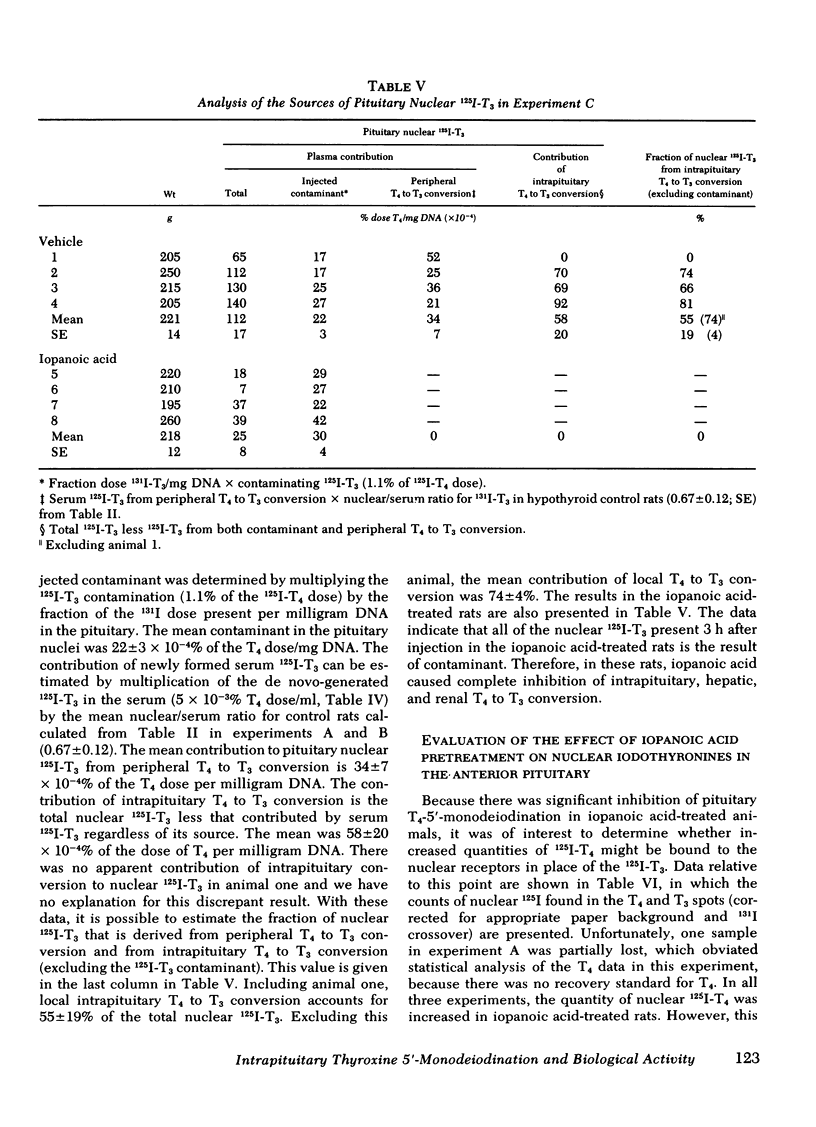
Iopanoic acid has been shown to block thyroxine (T4)-5'-monodeiodination in rat anterior pituitary in vitro. To test the hypothesis that the acute decrease in thyrotropin (TSH) after infusion of T4 into hypothyroid rats requires intrapituitary T4 to 3,5,3'-triiodothyroxine (T3) conversion, the effect of iopanoic acid treatment on the generation of nuclear T3 from intrapituitary conversion and the response to TSH were compared in control and iopanoic acid-treated animals. 5 mg/100 g body weight iopanoic acid given 24, 16, and 1.5 h before administration of 125I-T4 reduced the quantity of pituitary nuclear 125I-T3 from local (intrapituitary) T4 to T3 conversion by 60-100%. In association with inhibition of intrapituitary T4 to T3 conversion, there was an increase in the binding of 125I-T4 to the nuclear receptor of the pituitary but the total iodothyronine content of the nuclei was still less than half of the nuclear iodothyronine in control animals. Iopanoic acid did not affect the nuclear/plasma ratio of injected 131I-T3 in the same animals, but did appear to impair 131I-T3 clearance or reduce its distribution volume. Treatment with iopanoic acid did not reduce the quantity of nuclear 125I-T3 in the liver, kidney, or heart of the same animals more than expected from the changes in serum 125I-T3. In control hypo-thyroid animals pretreated with iopanoic acid, the mean TSH was not significantly decreased from the initial value by T4 injection. Iopanoic acid pretreatment did not interfere with the acute TSH response of chronically hypothyroid rats to 70 ng of T3/100 g body weight. These results establish that intrapituitary generations of T3 from T4 is required for the acute decrease in TSH which occurs after T4 infusion. The data also are consistent with the content that it is nuclear binding of the T3 generated from T4 which initiates the inhibition of TSH release.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Bürgi H., Wimpfheimer C., Burger A., Zaunbauer W., Rösler H., Lemarchand-Béraud T. Changes of circulating thyroxine, triiodothyronine and reverse triiodothyronine after radiographic contrast agents. J Clin Endocrinol Metab. 1976 Dec;43(6):1203–1210. doi: 10.1210/jcem-43-6-1203. [DOI] [PubMed] [Google Scholar]
- Frumess R. D., Larsen P. R. Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism. 1975 Apr;24(4):547–554. doi: 10.1016/0026-0495(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- JAGIELLO G. M., McKENZIE J. M. Influence of propylthiouracil on the thyroxine-thyrotropin interplay. Endocrinology. 1960 Oct;67:451–456. doi: 10.1210/endo-67-4-451. [DOI] [PubMed] [Google Scholar]
- JONES S. L., VAN MIDDLESWORTH L. Normal I-131 L-thyroxine metabolism in the presence of potassium perchlorate and interrupted by propylthiouracil. Endocrinology. 1960 Dec;67:855–861. doi: 10.1210/endo-67-6-855. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I., Koerner D., Dillmann W. H. Nuclear receptors and the initiation of thyroid hormone action. Recent Prog Horm Res. 1976;32:529–565. doi: 10.1016/b978-0-12-571132-6.50029-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- PEARSON J. D., VEALL N., VETTER H. A practical method for plasma albumin turnover studies. Strahlentherapie. 1958;107(SONDERBD):290–297. [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Peripheral metabolism of homologous thyrotropin in euthyroid and hypothyroid rats: acute effects of thyrotropin-releasing hormone, triiodothyronine, and thyroxine. Endocrinology. 1978 Jun;102(6):1783–1796. doi: 10.1210/endo-102-6-1783. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Chopra I. J., Solomon D. H., Bennett L. R. Changes in circulating iodothyronines in euthyroid and hyperthyroid subjects given ipodate (Oragrafin), an agent for oral cholecystography. J Clin Endocrinol Metab. 1978 Apr;46(4):691–697. doi: 10.1210/jcem-46-4-691. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]


