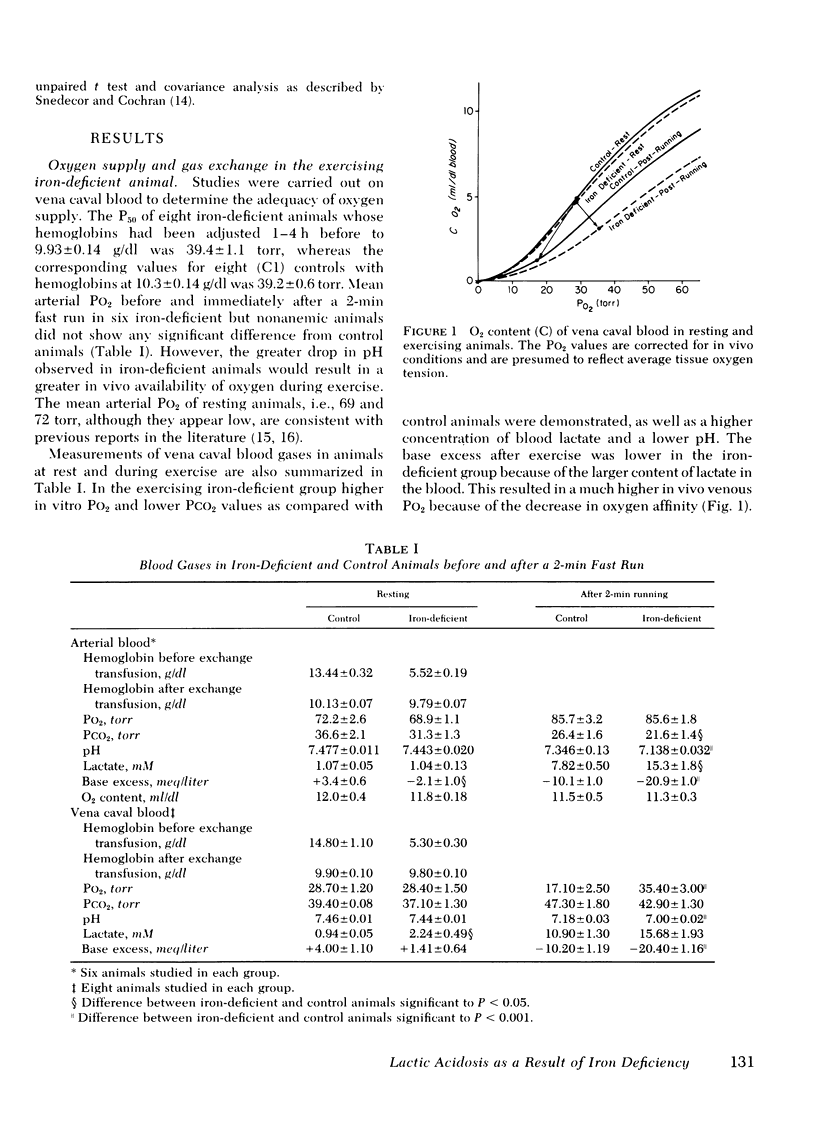
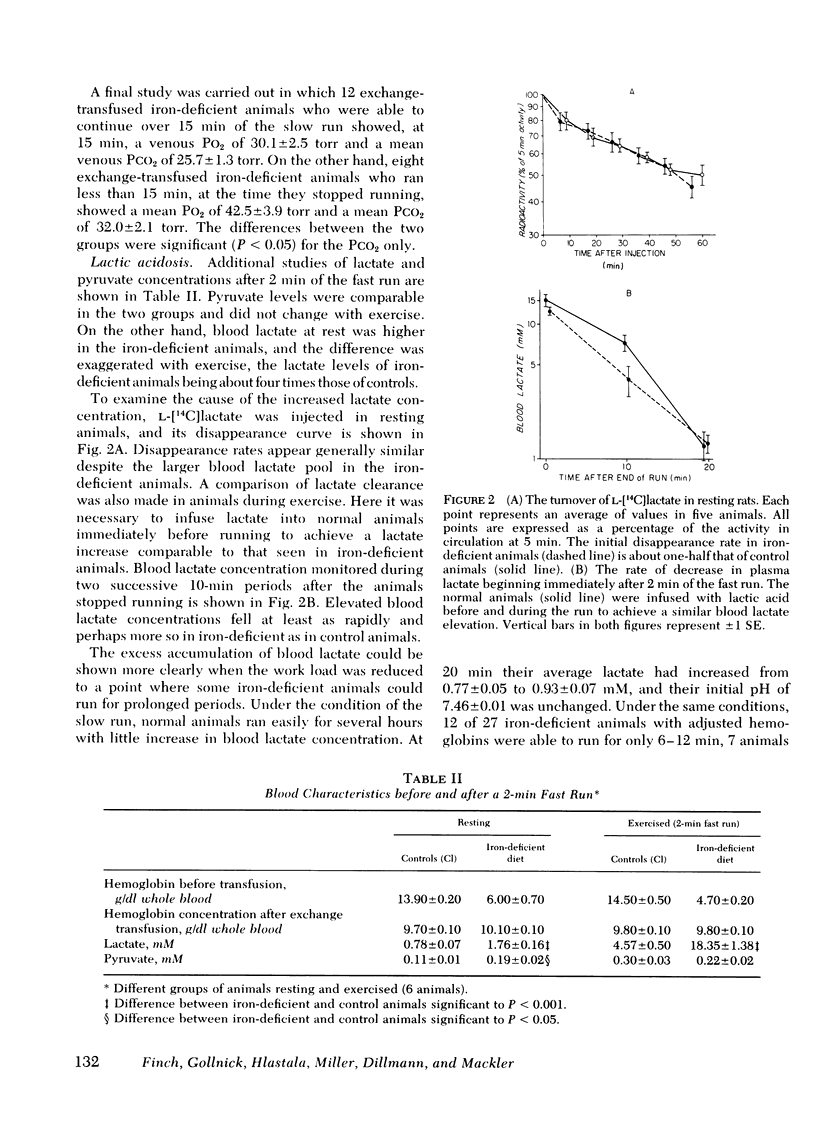
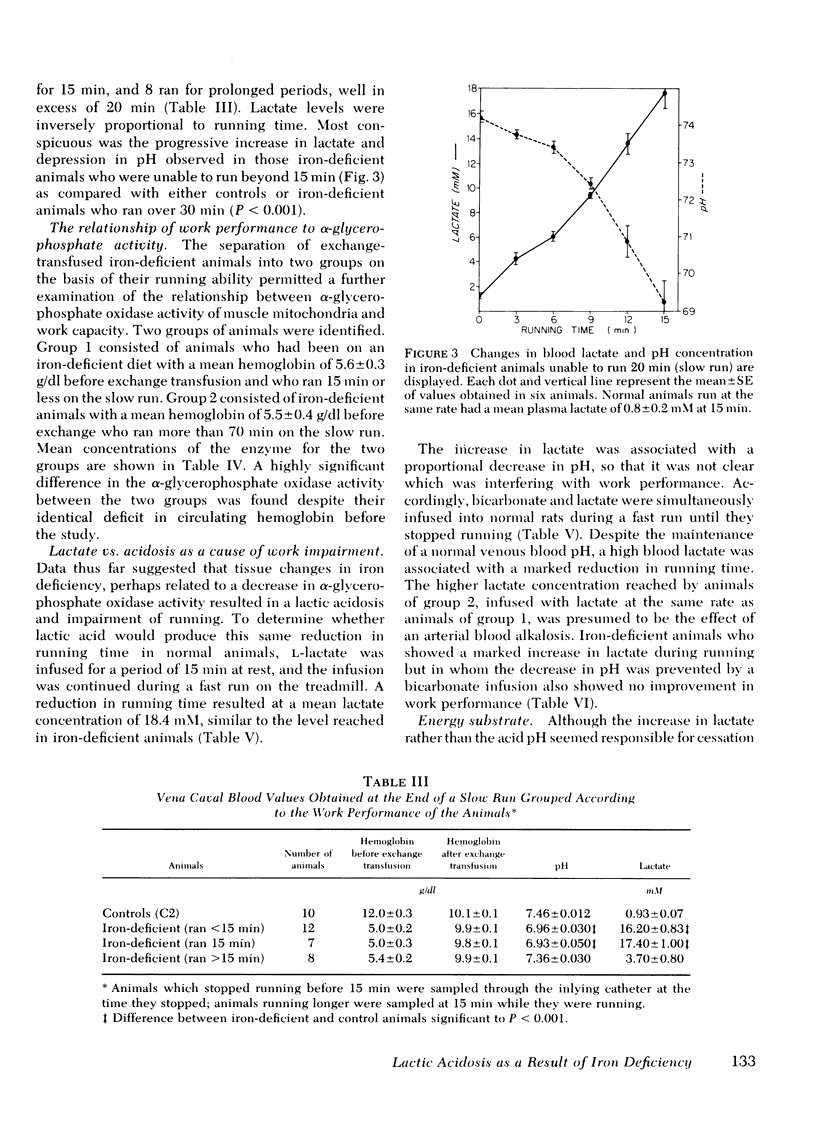
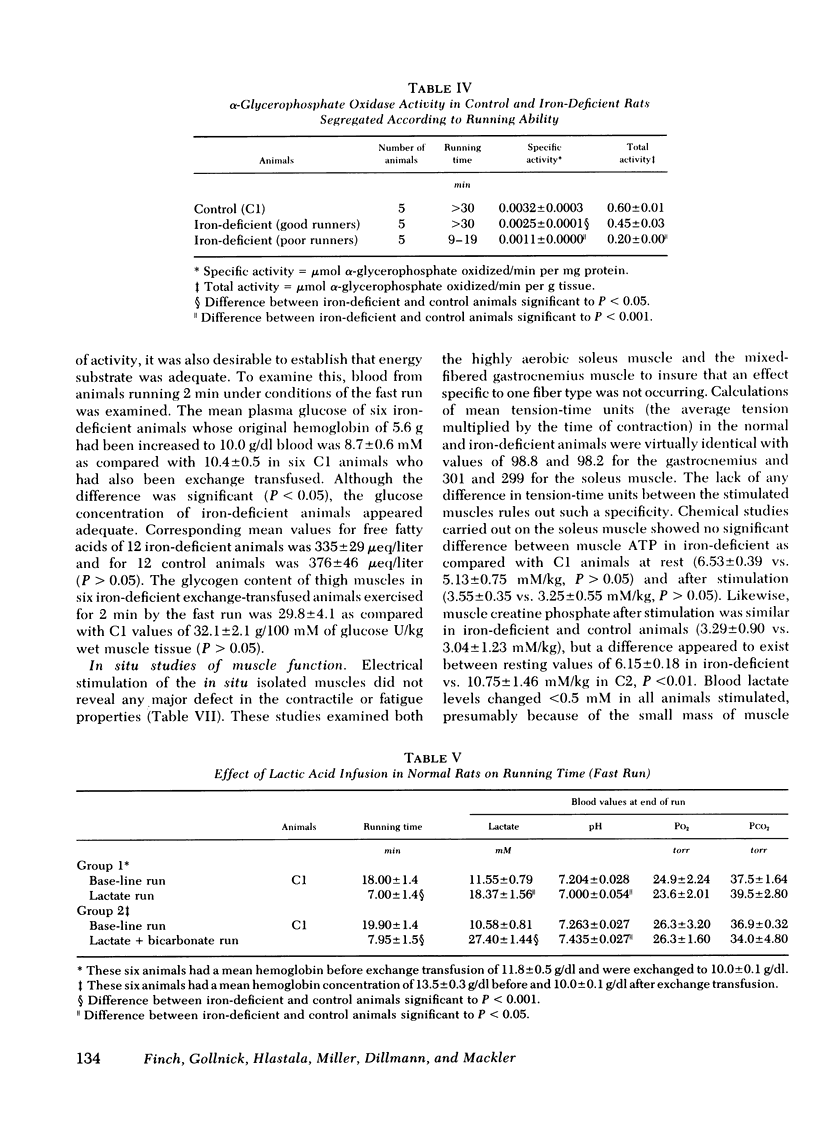
Abstract
Iron-deficient rats have an impaired work performance, even when their anemia is corrected by exchange transfusion. Muscle activity is associated with a higher blood lactate concentration than is observed in iron-replete animals. The accumulation of lactate is a result of excessive production as lactate clearance from the blood was shown to be unaffected. By adjusting the work load to a lower level, it was possible to divide iron-deficient animals into two groups, one capable of continued treadmill running and another in which animals stopped before 20 min. In the former, blood lactate concentration reached a plateau at moderate levels, whereas it continued to increase in the latter until the animal stopped running. Levels of α-glycerophosphate oxidase in skeletal muscle mitochondria were found to be much lower in the second group (P < 0.001). Lactate infusion into normal animals was shown to interfere with work performance, and maintenance of a normal pH in iron-deficient and iron-replete animals did not prevent the impairment in work associated with high blood lactate concentrations. Additional evidence was obtained that energy substrate (blood glucose and free fatty acids, muscle glycogen) was adequate in irondeficient animals. Oxygen tension in their vena caval blood was higher than in controls. Furthermore, the in situ behavior of electrically stimulated gastroenemius and soleus muscles appeared similar to that of control animals. Because the stimulation of the single muscle in the iron-deficient animal did not result in appreciable elevation of blood lactate and did not show impaired contractility further supported the hypothesis that the elevation of blood lactate caused the decreased work performance. It is concluded that iron deficiency by a depletion in the iron-containing mitochondrial enzyme, α-glycerophosphate oxidase, impairs glycolysis, resulting in excess lactate formation, which at high levels leads to cessation of physical activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altland P. D., Brubach H. F., Parker M. G., Highman B. Blood gases and acid-base values of unanesthetized rats exposed to hypoxia. Am J Physiol. 1967 Jan;212(1):142–148. doi: 10.1152/ajplegacy.1967.212.1.142. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., BLAISDELL R. K. Iron enzymes in iron deficiency. V. Succinic dehydrogenase in rat liver, kidney and heart. Blood. 1960 Jan;15:30–35. [PubMed] [Google Scholar]
- Bailey-Wood R., Blayney L. M., Muir J. R., Jacobs A. The effects of iron deficiency on rat liver enzymes. Br J Exp Pathol. 1975 Jun;56(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- Cain S. M. Appearance of excess lactate in anesthetized dogs during anemic and hypoxic hypoxia. Am J Physiol. 1965 Sep;209(3):604–610. doi: 10.1152/ajplegacy.1965.209.3.604. [DOI] [PubMed] [Google Scholar]
- Cook J. D. An evaluation of adsorption methods for measurement of plasma iron-binding capacity. J Lab Clin Med. 1970 Sep;76(3):497–506. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V. R., Bryant S. L., Gillespie C. A., Gardner G. W. Iron deficiency anemia and physical performance and activity of rats. J Nutr. 1972 Mar;102(3):381–399. doi: 10.1093/jn/102.3.381. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Miller L. R., Inamdar A. R., Person R., Seiler K., Mackler B. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest. 1976 Aug;58(2):447–453. doi: 10.1172/JCI108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles R. A., Baron P. G., Cohen R. D. Mechanism of the effect of varying PCO2 on gluconeogenesis from lactate in the perfused rat liver. Clin Sci Mol Med. 1978 Aug;55(2):183–188. doi: 10.1042/cs0550183. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Role of beta-adrenergic receptors in mobilization of energy sources in exercising dogs. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jun;44(6):869–876. doi: 10.1152/jappl.1978.44.6.869. [DOI] [PubMed] [Google Scholar]
- Koziol B. J., Ohira Y., Simpson D. R., Edgerton V. R. Biochemical skeletal muscle and hematological profiles of moderate and severely iron deficient and anemic adult rats. J Nutr. 1978 Aug;108(8):1306–1314. doi: 10.1093/jn/108.8.1306. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Ways P., Aucutt C., Cruz J. Effect of chronic hypoxic hypoxia on the O2-Hb dissociation curve and respiratory gas transport in man. Respir Physiol. 1969 Jun;7(1):7–29. doi: 10.1016/0034-5687(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Lo S., Russell J. C., Taylor A. W. Determination of glycogen in small tissue samples. J Appl Physiol. 1970 Feb;28(2):234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lauderdale V. R. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974 Aug;60(2):405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- Pepelko W. E. Interaction of chronic hypoxia and hypercapnia upon blood gases and acid base status. Proc Soc Exp Biol Med. 1972 Jun;140(2):628–632. doi: 10.3181/00379727-140-36518. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Cameron I. R. Intracellular pH and K+ of cardiac and skeletal muscle in acidosis and alkalosis. Am J Physiol. 1975 Nov;229(5):1305–1310. doi: 10.1152/ajplegacy.1975.229.5.1305. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Alvestrand A., Brandt R., Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Sep;45(3):474–480. doi: 10.1152/jappl.1978.45.3.474. [DOI] [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]
- Wranne B., Woodson R. D. A graded treadmill test for rats: maximal work performance in normal and anemic animals. J Appl Physiol. 1973 May;34(5):732–735. doi: 10.1152/jappl.1973.34.5.732. [DOI] [PubMed] [Google Scholar]


