Abstract
These studies were undertaken to investigate (a) the permeability properties of the blood-brain barrier (BBB) to the major gonadal and adrenal steroid hormones, and (b) the role of the binding proteins of plasma (albumin and specific globulins) in the regulation of BBB steroid hormone transport.
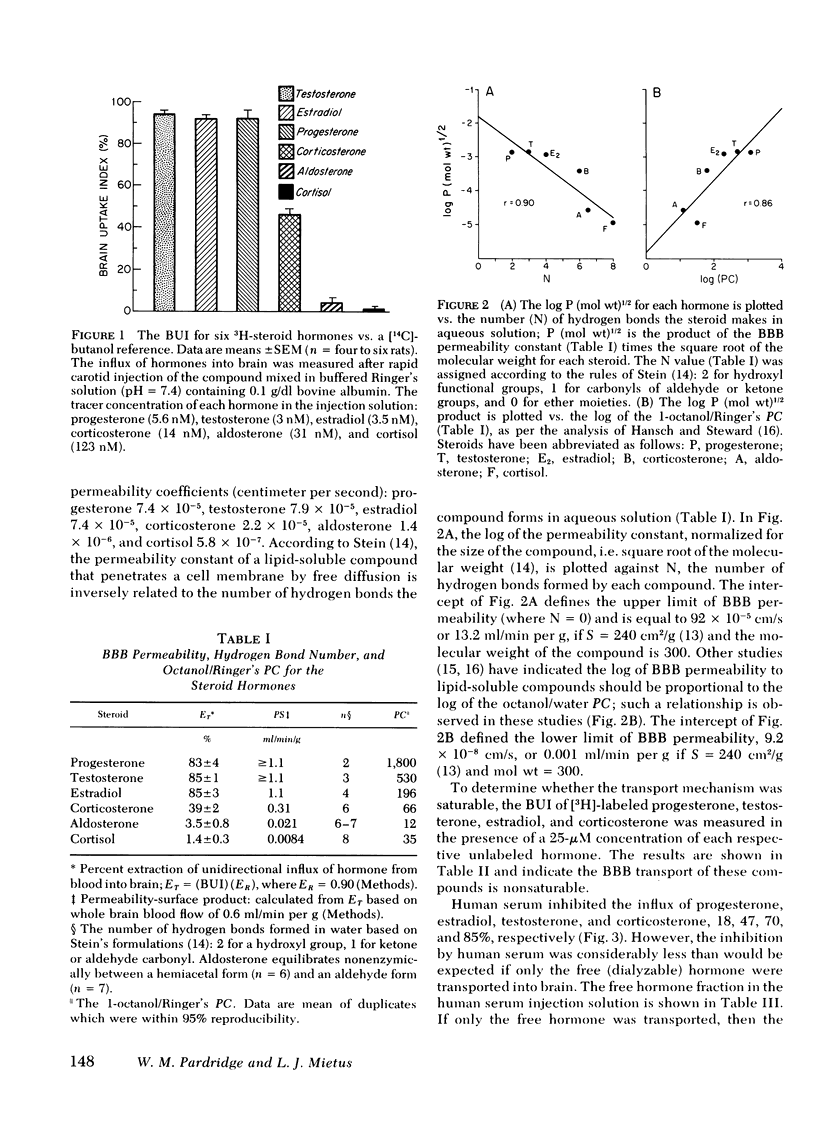
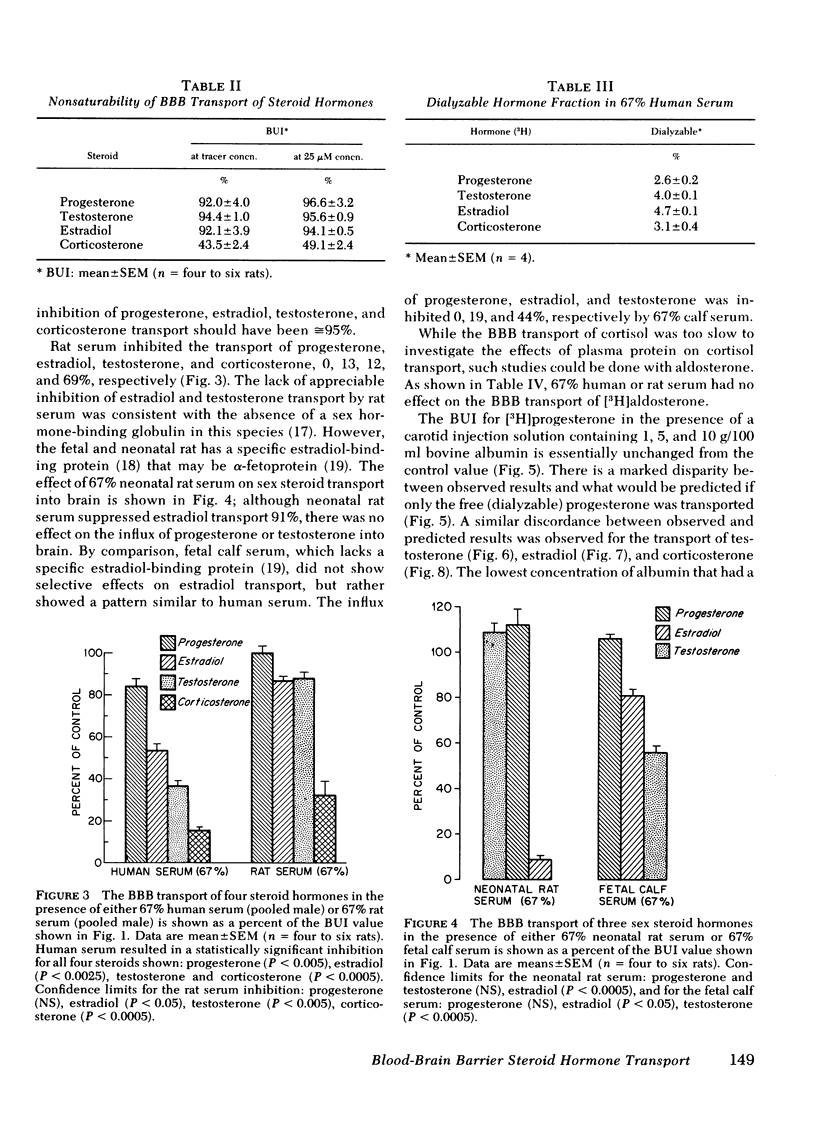
The permeability of the BBB to [3H]-labeled progesterone, testosterone, estradiol, corticosterone, aldosterone, and cortisol, was measured relative to [14C]butanol, a freely diffusable reference, in the barbiturate anesthetized rat using a tissue sampling-single injection technique. The isotopes were rapidly injected in a 200-μl bolus of Ringer's solution (0.1 g/dl albumin) via the common carotid artery and the percent extraction of unidirectional influx of hormone was determined after a single pass through brain: progesterone, 83±4%; testosterone, 85±1%; estradiol, 83±3%; corticosterone, 39±2%; aldosterone, 3.5±0.8%; and cortisol, 1.4±0.3%. The selective permeability of the BBB was inversely related to the number of hydrogen bonds each steroid formed in aqueous solution and directly related to the respective 1-octanol/Ringer's partition coefficient.
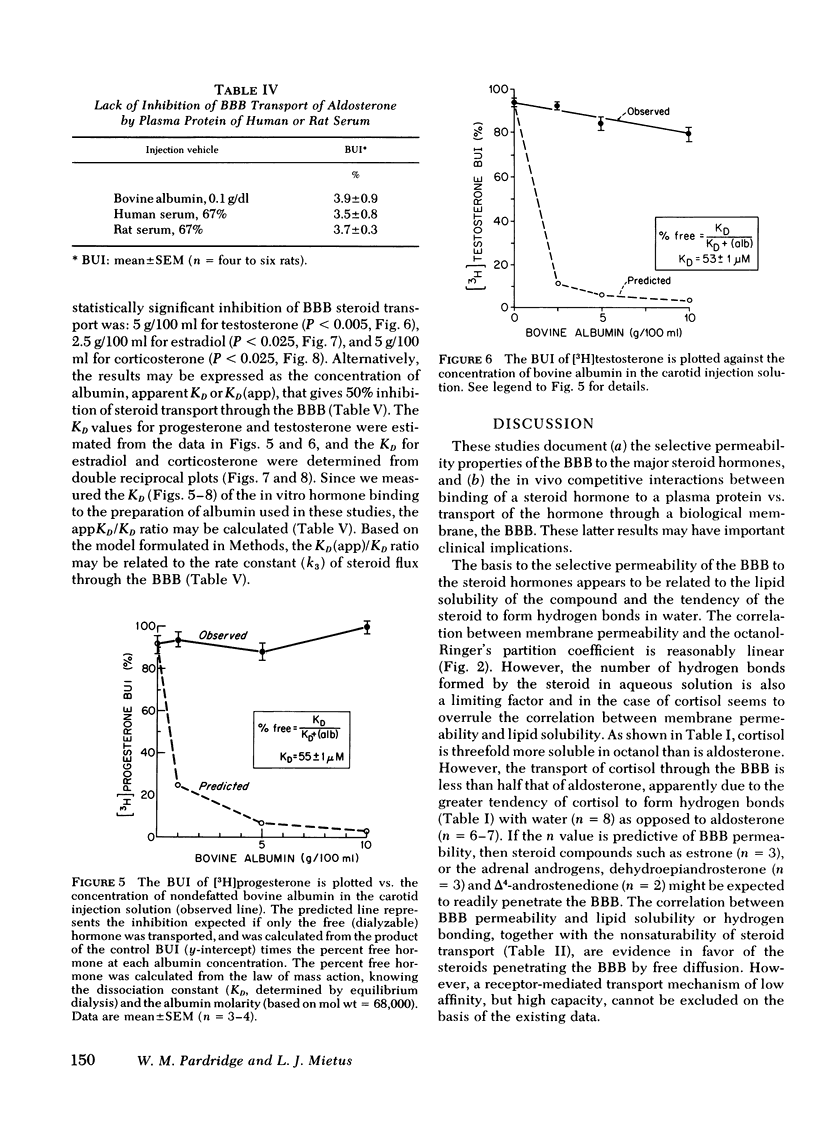
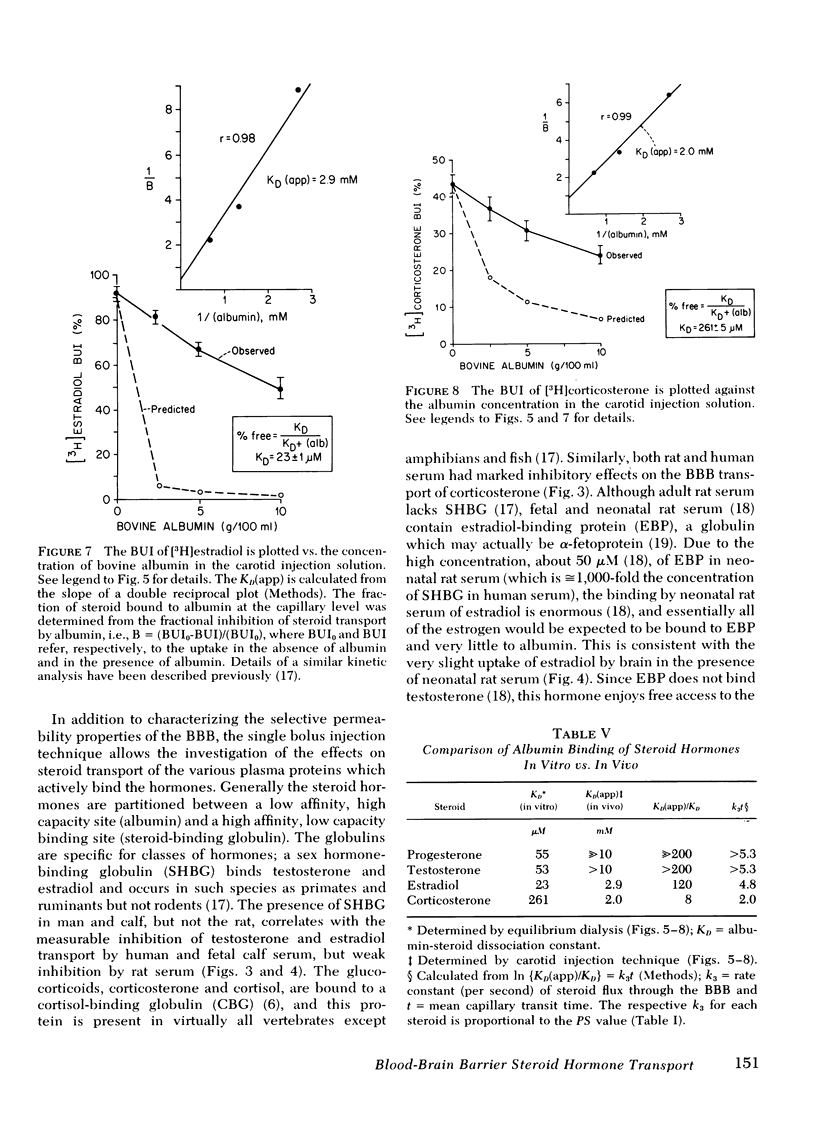
When the bolus injection was 67% human serum, >95% of the labeled steroid was bound as determined by equilibrium dialysis. However, the influx of the steroids through the BBB was inhibited by human serum to a much less extent than would be expected if only the free (dialyzable) hormone was transported; progesterone, estradiol, testosterone, and corticosterone transport was inhibited 18, 47, 70, and 85% respectively, or in proportion to the steroid binding to plasma globulins. Rat serum (67%) only inhibited the transport of these four hormones, 0, 13, 12, and 69%, respectively, reflecting the absence of a sex hormone-binding globulin in rat plasma. However, neonatal rat serum (67%) inhibited progesterone, testosterone, and estradiol transport 0, 0, and 91%, respectively, consistent with the presence of an estradiol-binding protein in neonatal rat serum.
The binding of steroid hormone to bovine albumin in vitro (as determined by equilibrium dialysis) was compared to albumin binding in vivo (as determined by the single injection technique). The ratio of apparent dissociation constant in vivo, KD(app), to the in vitro KD was: ≫200 for progesterone, >200 for testosterone, 120 for estradiol, and 7.7 for corticosterone. Assuming the steady-state condition, the KD(app)/KD was found to be proportional to the BBB permeability for each steroid.
These data demonstrate (a) the selective permeability properties of the BBB to the major steroid hormones is proportional to the tendency of the steroid to partition in a polar lipid phase and is inversely related to the number of hydrogen bond-forming functional groups on the steroid nucleus; (b) the presence of albumin in serum may bind considerable quantities of steroid hormone, but exerts little inhibitory effects on the transport of steroids into brain, whereas globulin-bound hormone does not appear to be transported into brain to a significant extent. Therefore, the hormone fraction in plasma that is available for transport into brain is not restricted to the free (dialyzable) fraction, but includes the larger albumin-bound moiety.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman M. W. Morphology of blood-brain interfaces. Exp Eye Res. 1977;25 (Suppl):1–25. doi: 10.1016/s0014-4835(77)80008-0. [DOI] [PubMed] [Google Scholar]
- Corvol P., Bardin C. W. Species distribution of testosterone-binding globulin. Biol Reprod. 1973 Apr;8(3):277–282. doi: 10.1093/biolreprod/8.3.277. [DOI] [PubMed] [Google Scholar]
- Dixon P. F. The kinetics of the exchange between transcortin-bound and unbound cortisol in plasma. J Endocrinol. 1968 Apr;40(4):457–465. doi: 10.1677/joe.0.0400457. [DOI] [PubMed] [Google Scholar]
- Ermisch A., Rühle H. J. Autoradiographic demonstration of aldosterone-concentrating neuron populations in rat brain. Brain Res. 1978 May 19;147(1):154–158. doi: 10.1016/0006-8993(78)90780-1. [DOI] [PubMed] [Google Scholar]
- Furlow T. W., Jr, Bass N. H. Cerebral hemodynamics in the rat assessed by a non-diffusible indicator-dilution technique. Brain Res. 1976 Jul 9;110(2):366–370. doi: 10.1016/0006-8993(76)90409-1. [DOI] [PubMed] [Google Scholar]
- HANSCH C., STEWARD A. R. THE USE OF SUBSTITUENT CONSTANTS IN THE ANALYSIS OF THE STRUCTURE--ACTIVITY RELATIONSHIP IN PENICILLIN DERIVATIVES. J Med Chem. 1964 Nov;7:691–694. doi: 10.1021/jm00336a001. [DOI] [PubMed] [Google Scholar]
- Keiding S., Johansen S., Winkler K., Tonnesen K., Tygstrup N. Michaelis-Menten kinetics of galactose elimination by the isolated perfused pig liver. Am J Physiol. 1976 May;230(5):1302–1313. doi: 10.1152/ajplegacy.1976.230.5.1302. [DOI] [PubMed] [Google Scholar]
- Mickelson K. E., Pétra P. H. Purification and characterization of the sex steroid-binding protein of rabbit serum. Comparison with the human protein. J Biol Chem. 1978 Aug 10;253(15):5293–5298. [PubMed] [Google Scholar]
- Ohno K., Pettigrew K. D., Rapoport S. I. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol. 1978 Sep;235(3):H299–H307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Braun L. D. [H] Tryptamine and 3H-water as diffusible internal standards for measuring brain extraction of radio-labeled substances following carotid injection. Brain Res. 1976 Aug 20;113(1):219–224. doi: 10.1016/0006-8993(76)90024-x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Lipid solubility and drug penetration of the blood brain barrier. Proc Soc Exp Biol Med. 1974 Dec;147(3):813–815. doi: 10.3181/00379727-147-38444. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970 Dec 1;24(2):372–376. doi: 10.1016/0006-8993(70)90123-x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1977 Jan;28(1):103–108. doi: 10.1111/j.1471-4159.1977.tb07714.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Kinetics of blood-brain transport of hexoses. Biochim Biophys Acta. 1975 Mar 25;382(3):377–392. doi: 10.1016/0005-2736(75)90279-5. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977 Jan;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Unidirectional influx of glutamine and other neutral amino acids into liver of fed and fasted rat in vivo. Am J Physiol. 1977 May;232(5):E492–E496. doi: 10.1152/ajpendo.1977.232.5.E492. [DOI] [PubMed] [Google Scholar]
- Raynaud J. P., Mercier-Bodard C., Baulieu E. E. Rat estradiol binding plasma protein (EBP). Steroids. 1971 Dec;18(6):767–788. doi: 10.1016/0039-128x(71)90035-3. [DOI] [PubMed] [Google Scholar]


