Abstract
Female sex and estrogen administration are associated with increased hepatic production of triglyceride-rich lipoproteins; the basis for this has not been fully elucidated. Inasmuch as hepatic lipoprotein production is also influenced by FFA availability and triglyceride biosynthesis, we investigated sex differences in FFA utilization in rat hepatocyte suspensions and in the components of the triglyceride biosynthetic pathway.
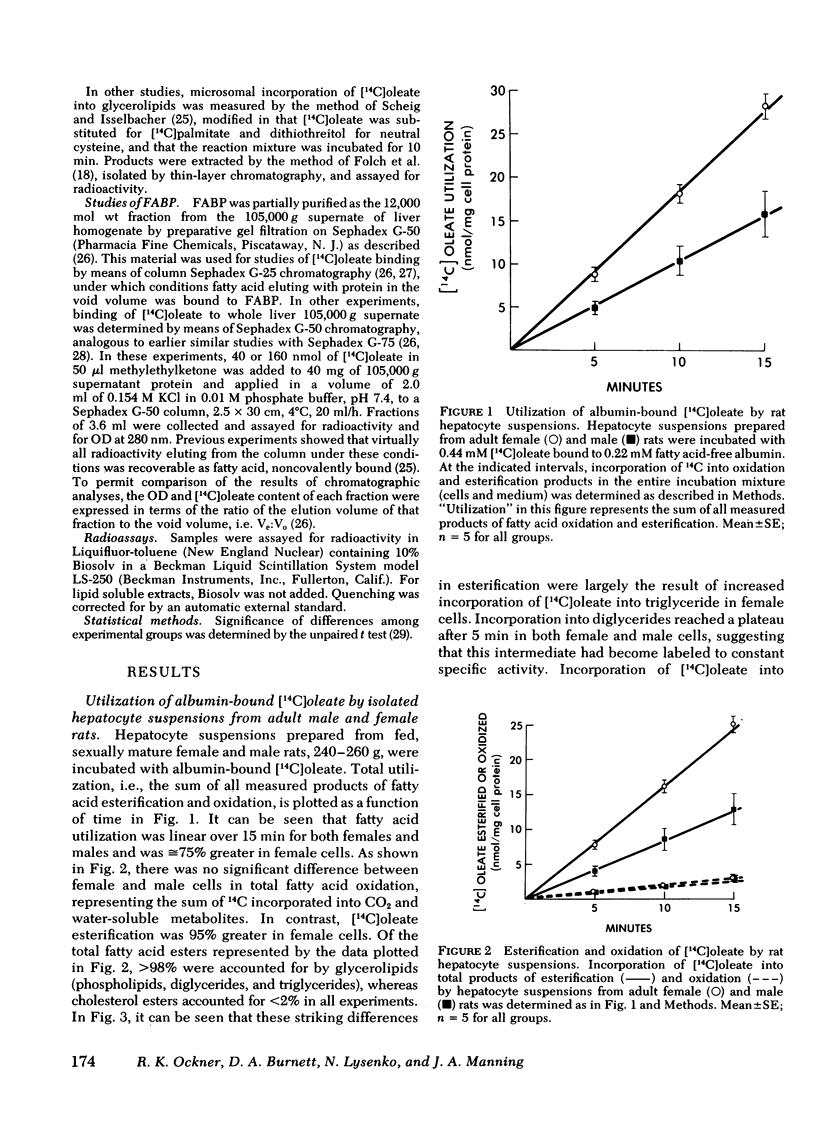
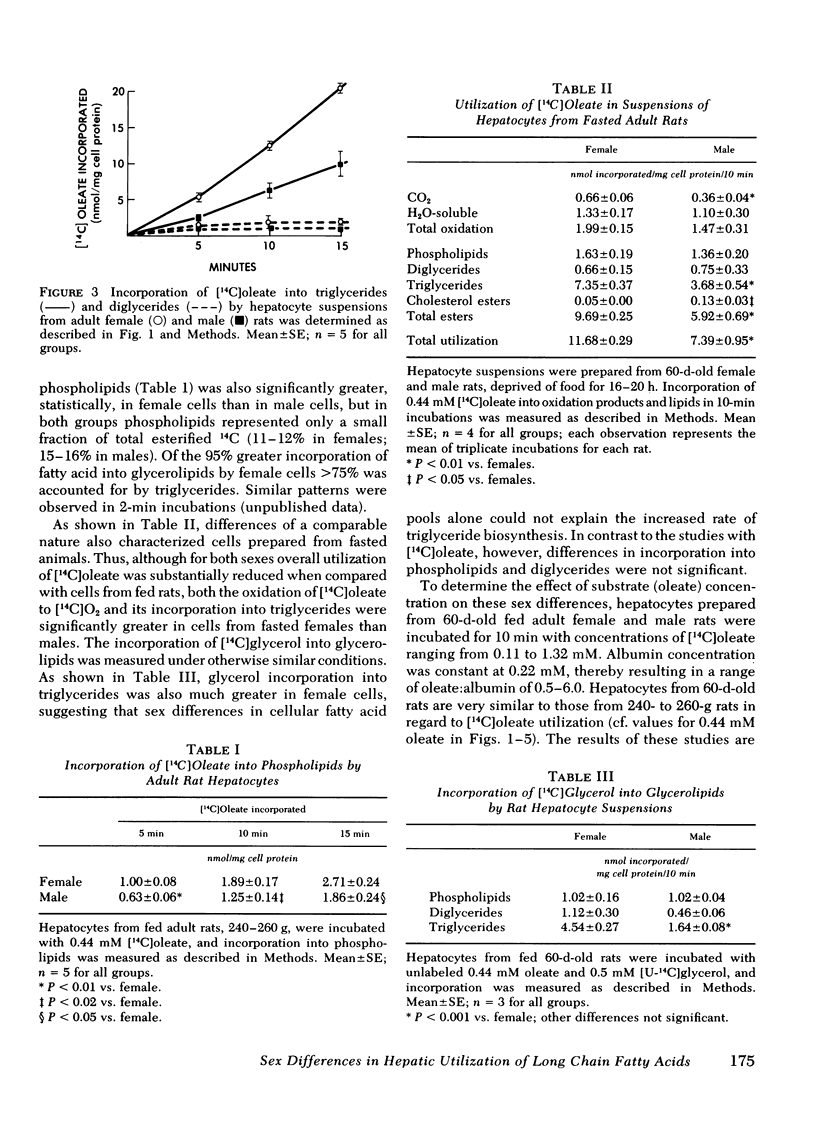
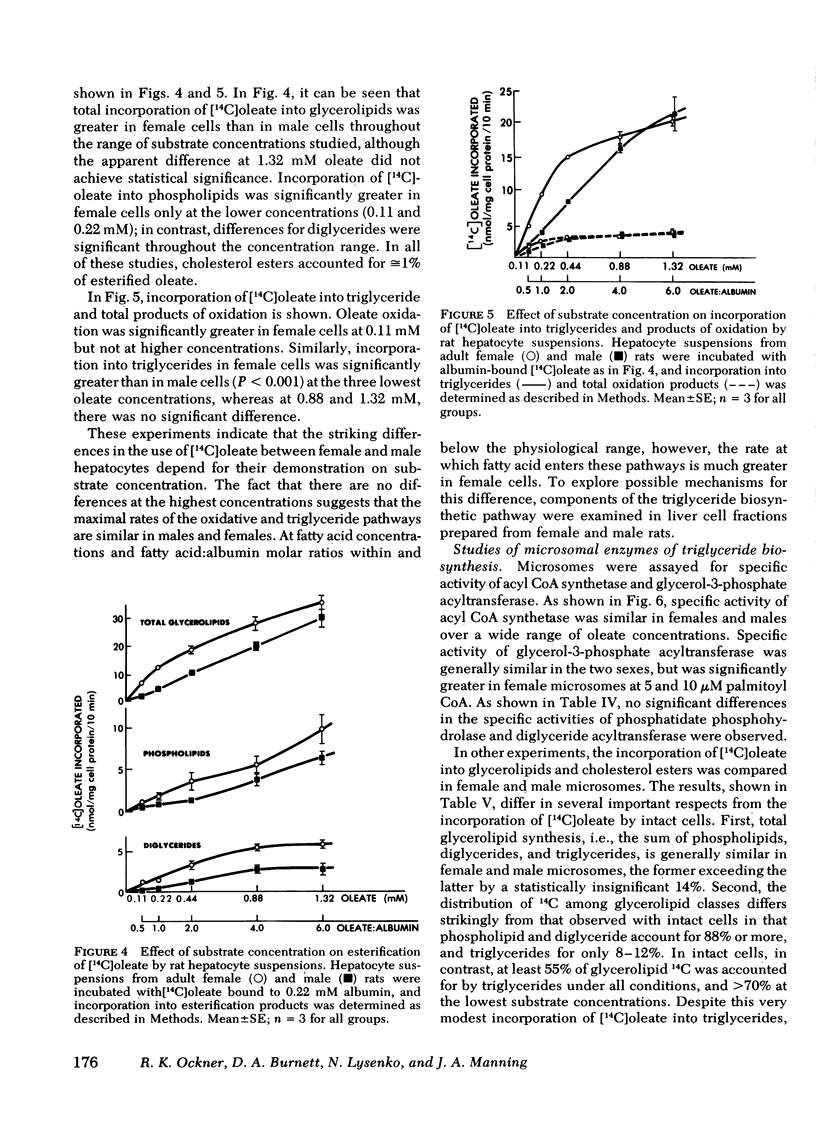
Isolated adult rat hepatocyte suspensions were incubated with albumin-bound [14C]oleate for up to 15 min. At physiological and low oleate concentrations, cells from females incorporated significantly more 14C into glycerolipids, especially triglycerides, and into oxidation products than did male cells, per milligram cell protein. At 0.44 mM oleate, incorporation into triglycerides in female cells was approximately twice that in male cells. Comparable sex differences were observed in cells from fasted animals and when [14C]-glycerol incorporation was measured. At higher oleate concentrations, i.e., fatty acid:albumin mole ratios in excess of 2:1, these sex differences were no longer demonstrable, suggesting that maximal rates of fatty acid esterification and oxidation were similar in female and male cells.
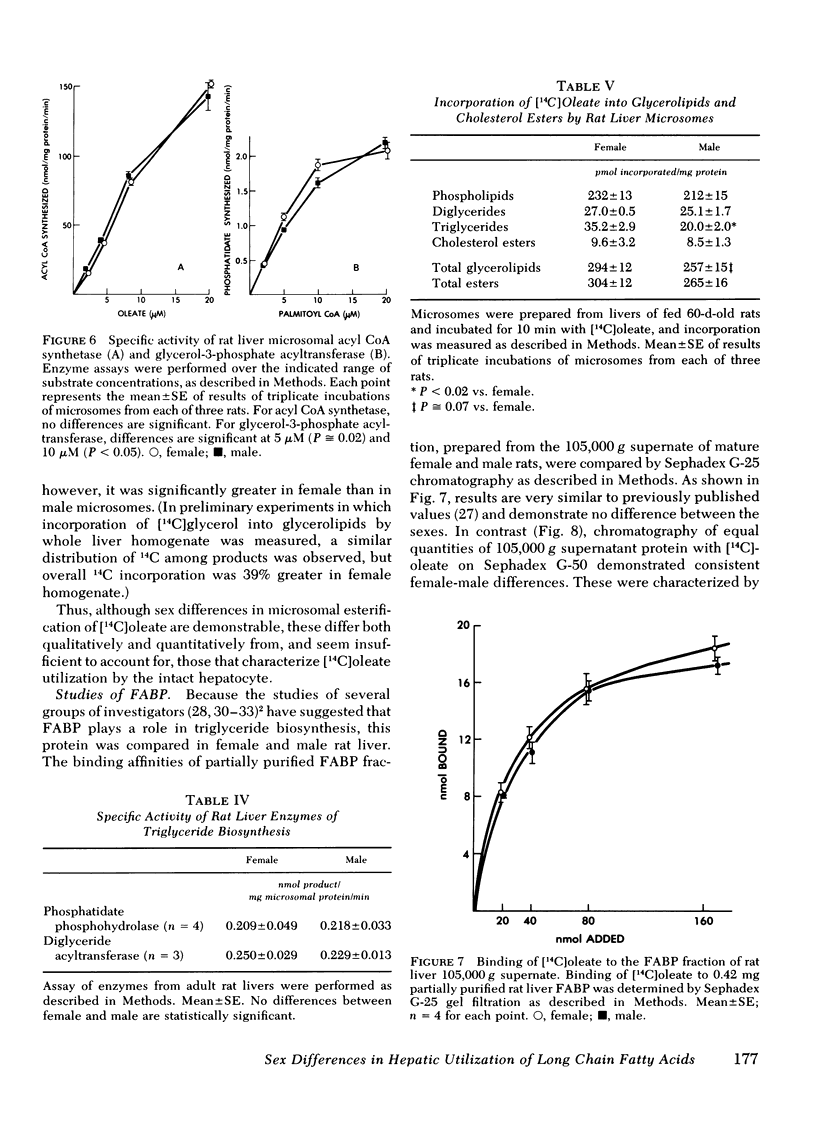
In female and male hepatic microsomes, specific activities of long chain acyl coenzyme A synthetase, phosphatidate phosphohydrolase, and diglyceride acyltransferase were similar, but glycerol-3-phosphate acyltransferase activity was slightly greater in females at certain substrate concentrations. Microsomal incorporation of [14C]oleate into total glycerolipids was not significantly greater in females. In further contrast to intact cells, microsomal incorporation of [14C]oleate into triglycerides, although significantly greater in female microsomes, accounted for only a small fraction of the fatty acid esterified.
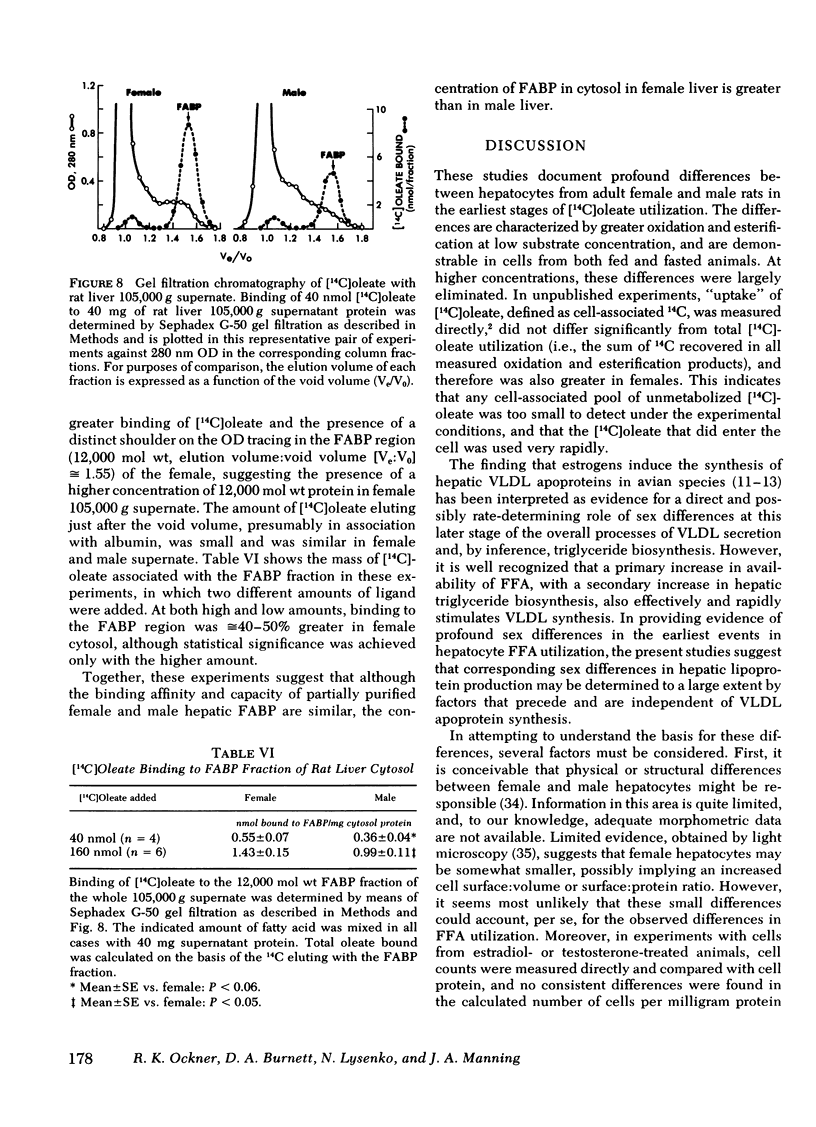
The binding affinity and stoichiometry of partially purified female hepatic fatty acid binding protein (FABP) were similar to those of male FABP. In contrast, the concentration of FABP, per milligram cytosolic protein, was 44% greater in female liver than in male, as indicated by measurement of [14C]oleate binding and of 280 nm OD in the FABP fraction of 105,000 g supernate after gel filtration chromatography.
These experiments demonstrate profound sex differences in hepatocyte utilization of long chain fatty acids at concentrations within and below the physiological range, and suggest that these are attributable at least in part to corresponding differences in cytosolic FABP concentration. At higher FFA concentrations, sex differences in hepatocyte FFA utilization are virtually eliminated, suggesting that under these conditions, differences in FABP concentration are not rate determining. Sex differences in hepatic lipoprotein production may largely reflect these important differences in the initial stages of hepatocyte FFA utilization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Goldberg A. P., Pykälistö O. J., Brunzell J. D., Hazzard W. R. Effect of estrogen on post-heparin lipolytic activity. Selective decline in hepatic triglyceride lipase. J Clin Invest. 1977 Apr;59(4):601–608. doi: 10.1172/JCI108677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J., Rose G., Shapiro B. The purification and properties of microsomal palmitoyl-coenzyme A synthetase. Biochem J. 1971 Apr;122(3):353–362. doi: 10.1042/bj1220353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL R. M., KOSTERLITZ H. W. The effects of growth and sex on the composition of the liver cells of the rat. J Endocrinol. 1950 Jan;6(3):308–318. doi: 10.1677/joe.0.0060308. [DOI] [PubMed] [Google Scholar]
- Chait A., Brunzell J. D., Albers J. J., Hazzard W. R. Type-III Hyperlipoproteinaemia ("remnant removal disease"). Insight into the pathogenetic mechanism. Lancet. 1977 Jun 4;1(8023):1176–1178. doi: 10.1016/s0140-6736(77)92717-9. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hamosh M., Hamosh P. The effect of estrogen on the lipoprotein lipase activity of rat adipose tissue. J Clin Invest. 1975 May;55(5):1132–1135. doi: 10.1172/JCI108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamdar S. C., Fallon H. J. Glycerolipid biosynthesis in rat adipose tissue. I. Properties and distribution of glycerophosphate acyltransferase and effect of divalent cations on neutral lipid formation. J Lipid Res. 1973 Sep;14(5):509–516. [PubMed] [Google Scholar]
- Kekki M., Nikkilä E. A. Plasma triglyceride turnover during use of oral contraceptives. Metabolism. 1971 Sep;20(9):878–889. doi: 10.1016/0026-0495(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kalkhoff R. K. Sex steroid influence on triglyceride metabolism. J Clin Invest. 1975 Oct;56(4):888–896. doi: 10.1172/JCI108168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973 May;5(3):184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- Kudzma D. J., Hegstad P. M., Stoll R. E. The chick as a laboratory model for the study of estrogen-induced hyperlipidemia. Metabolism. 1973 Mar;22(3):423–434. doi: 10.1016/0026-0495(73)90034-6. [DOI] [PubMed] [Google Scholar]
- Kudzma D. J., St Claire F., DeLallo L., Friedberg S. J. Mechanism of avian estrogen-induced hypertriglyceridemia: evidence for overproduction of triglyceride. J Lipid Res. 1975 Mar;16(2):123–133. [PubMed] [Google Scholar]
- Kushwaha R. S., Hazzard W. R., Gagne C., Chait A., Albers J. J. Type III hyperlipoproteinemia: paradoxical hypolipidemic response to estrogen. Ann Intern Med. 1977 Nov;87(5):517–525. doi: 10.7326/0003-4819-87-5-517. [DOI] [PubMed] [Google Scholar]
- Lamb R. G., Wyrick S. D., Piantadosi C. Hypolipidemic activity of in vitro inhibitors of hepatic and intestinal sn-glycerol-3-phosphate acyltransferase and phosphatidate phosphohydrolase. Atherosclerosis. 1977 Jun;27(2):147–154. doi: 10.1016/0021-9150(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Lunzer M. A., Manning J. A., Ockner R. K. Inhibition of rat liver acetyl coenzyme A carboxylase by long chain acyl coenzyme A and fatty acid. Modulation by fatty acid-binding protein. J Biol Chem. 1977 Aug 10;252(15):5483–5487. [PubMed] [Google Scholar]
- Luskey K. L., Brown M. S., Goldstein J. L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J Biol Chem. 1974 Sep 25;249(18):5939–5947. [PubMed] [Google Scholar]
- Mandour T., Kissebah A. H., Wynn V. Mechanism of oestrogen and progesterone effects on lipid and carbohydrate metabolism: alteration in the insulin: glucagon molar ratio and hepatic enzyme activity. Eur J Clin Invest. 1977 Jun;7(3):181–187. doi: 10.1111/j.1365-2362.1977.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Stein L., Fleischner G., Gatmaitan Z., Arias I. M. Z protein in hepatic uptake and esterification of long-chain fatty acids. Am J Physiol. 1975 Jun;228(6):1634–1640. doi: 10.1152/ajplegacy.1975.228.6.1634. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Stein L., Gatmaitan Z., Arias I. M. The binding of fatty acids to cytoplasmic proteins: binding to Z protein in liver and other tissues of the rat. Biochem Biophys Res Commun. 1972 Jun 9;47(5):997–1003. doi: 10.1016/0006-291x(72)90931-x. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Turcotte R. Stimulation of monoacylglycerophosphate formation by Z protein. Biochem Biophys Res Commun. 1974 Sep 9;60(1):376–381. doi: 10.1016/0006-291x(74)90215-0. [DOI] [PubMed] [Google Scholar]
- O'Doherty P. J., Kuksis A. Stimulation of triacylglycerol synthesis by Z protein in rat liver and intestinal mucosa. FEBS Lett. 1975 Dec 15;60(2):256–258. doi: 10.1016/0014-5793(75)80725-3. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid binding protein. Role in esterification of absorbed long chain fatty acid in rat intestine. J Clin Invest. 1976 Sep;58(3):632–641. doi: 10.1172/JCI108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Pittman J. P., Yager J. L. Differences in the intestinal absorption of saturated and unsaturated long chain fatty acids. Gastroenterology. 1972 May;62(5):981–992. [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Renaud G., Foliot A., Infante R. Increased uptake of fatty acids by the isolated rat liver after raising the fatty acid binding protein concentration with clofibrate. Biochem Biophys Res Commun. 1978 Jan 30;80(2):327–334. doi: 10.1016/0006-291x(78)90680-0. [DOI] [PubMed] [Google Scholar]
- SCHEIG R., ISSELBACHER K. J. PATHOGENESIS OF ETHANOL-INDUCED FATTY LIVER. 3. IN VIVO AND VITRO EFFECTS OF ETHANOL ON HEPATIC FATTY ACID METABOLISM IN RATS. J Lipid Res. 1965 Apr;6:269–277. [PubMed] [Google Scholar]
- Sauer F., Mahadevan S., Erfle J. D. The accumulation of citrate cycle intermediates in rat liver cells oxidizing palmitate. Biochim Biophys Acta. 1971 Jun 8;239(1):26–32. doi: 10.1016/0005-2760(71)90188-3. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Heimberg M. Comparison of metabolism of free fatty acid by isolated perfused livers from male and female rats. J Lipid Res. 1976 Nov;17(6):605–615. [PubMed] [Google Scholar]
- Wilcox H. G., Woodside W. F., Breen K. J., Knapp H. R., Jr, Heimberg M. The effect of sex on certain properties of the very low density lipoprotein secreted by the liver. Biochem Biophys Res Commun. 1974 Jun 18;58(4):919–926. doi: 10.1016/s0006-291x(74)80231-7. [DOI] [PubMed] [Google Scholar]


