Abstract
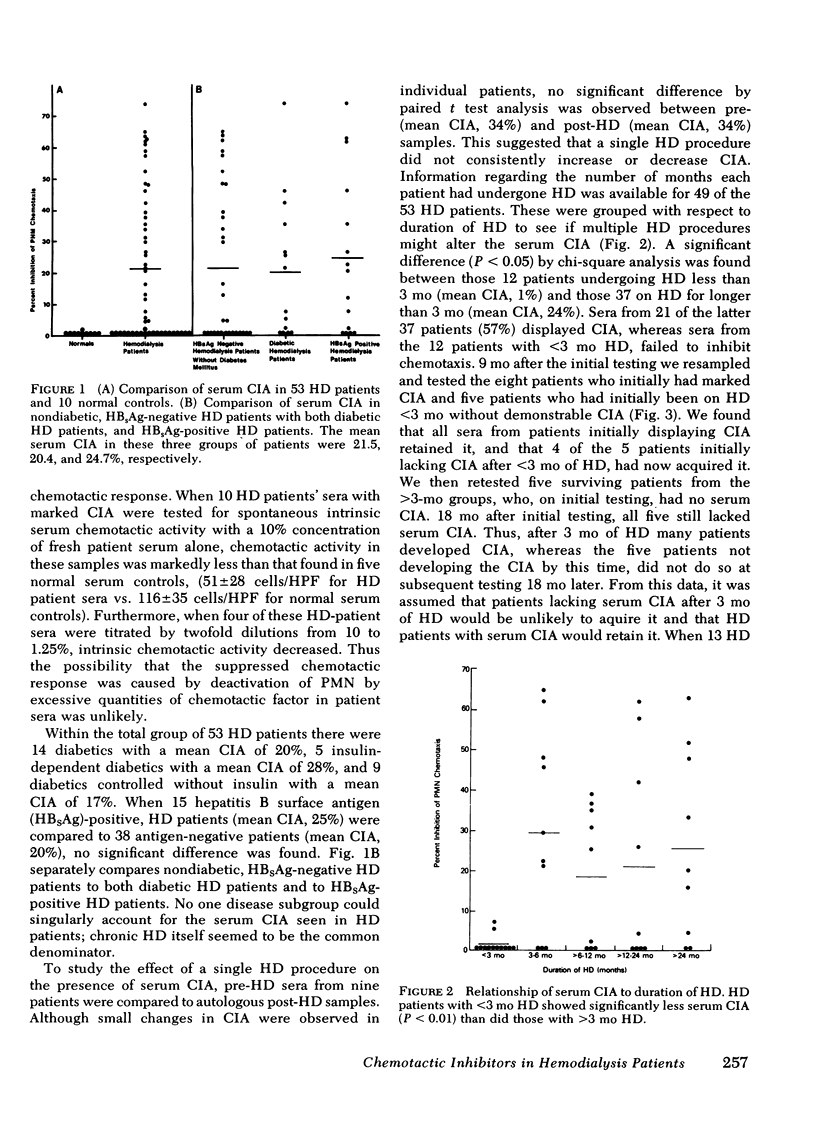
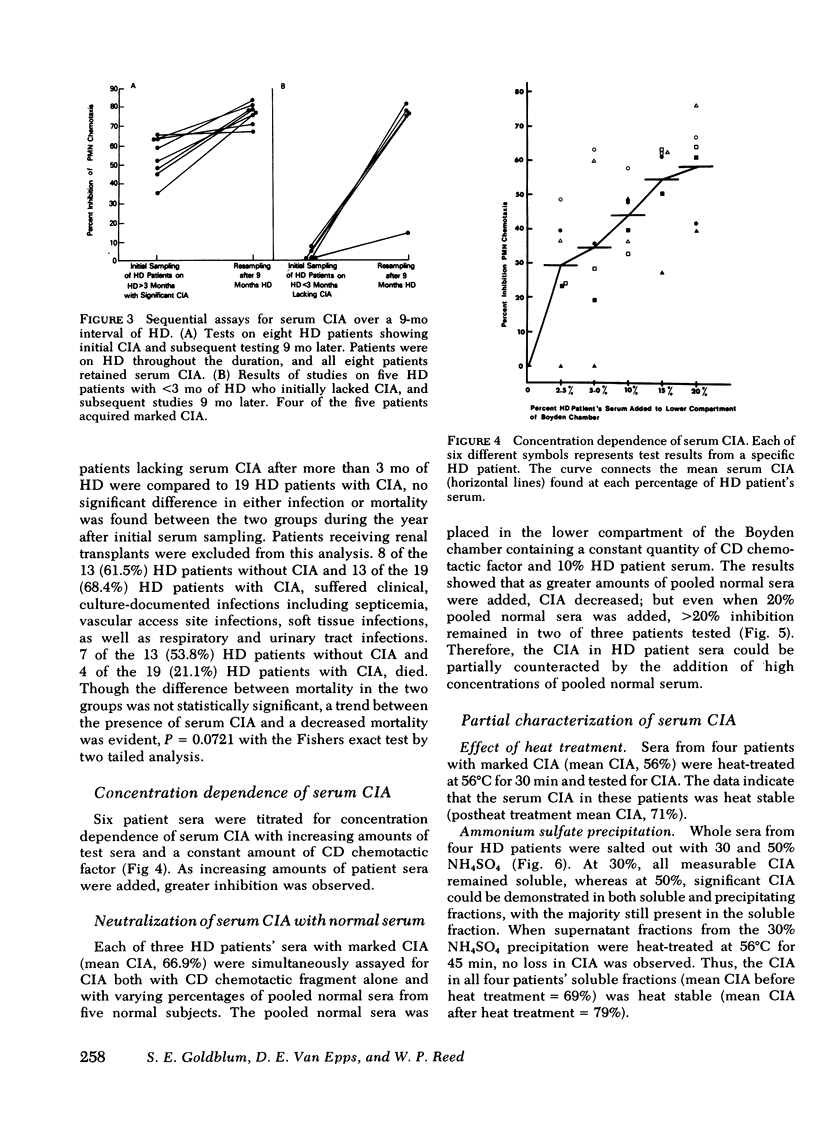
Abnormal granulocyte chemotaxis has been described in chronic hemodialysis patients. In this study, sera from 53 hemodialysis patients were tested for chemotactic inhibitory activity by a modified Boyden technique. Chemotactic inhibitory activity, defined as >20% inhibition of normal granulocyte chemotaxis, was found in 45% of patients. Only sera from patients having undergone >3 mo hemodialysis displayed chemotactic inhibitory activity and retained this inhibitory activity when retested 9 mo later. Four of five patients who had initially undergone <3 mo hemodialysis and lacked serum chemotactic inhibitory activity developed inhibitory activity when tested 9 mo later. Clinical evaluation of patients with serum chemotactic inhibitory activity showed that these patients did not have a significantly increased incidence of infection, although a trend toward decreased mortality during the time of study was observed (P = 0.0721).
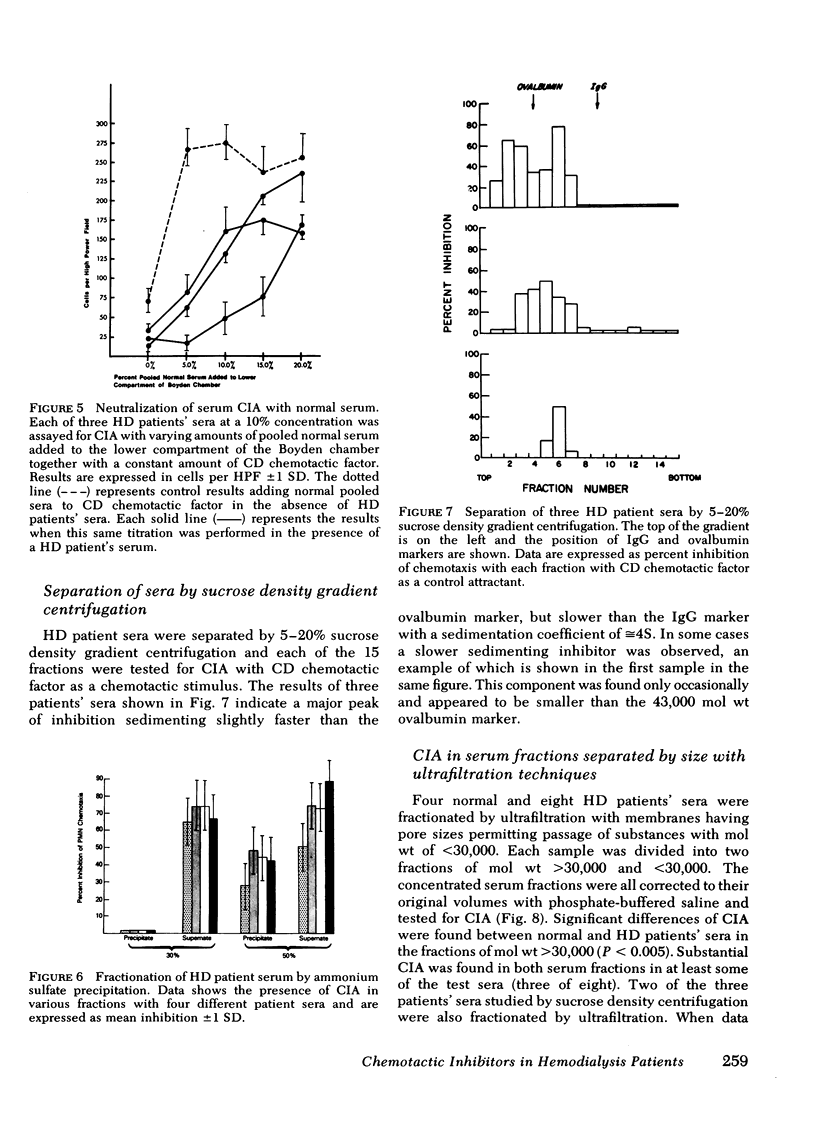
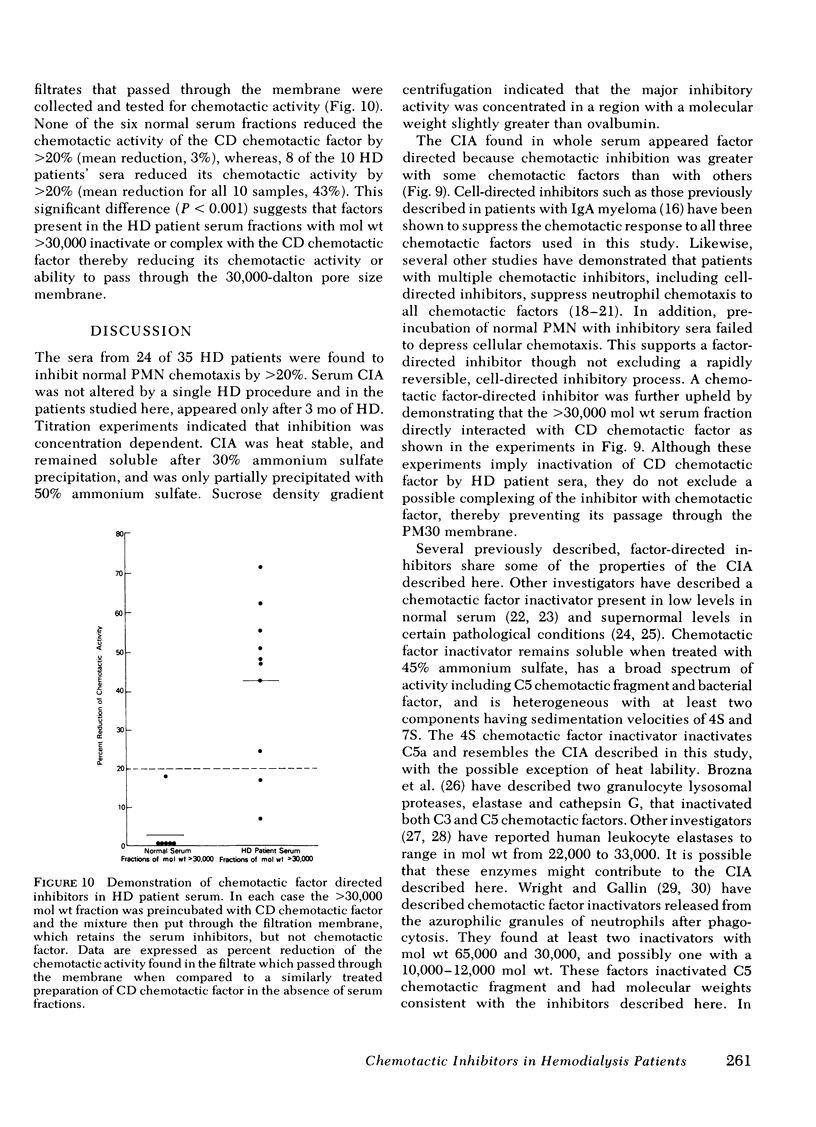
Serum chemotactic inhibitory activity was heat stable at 56°C for 30 min and concentration dependent. The major inhibitory component was found to have a sedimentation coefficient of 4S by sucrose density gradient centrifugation. The chemotactic inhibitory activity was not precipitated by 30% ammonium sulfate, but was partially precipitated by 50% ammonium sulfate.
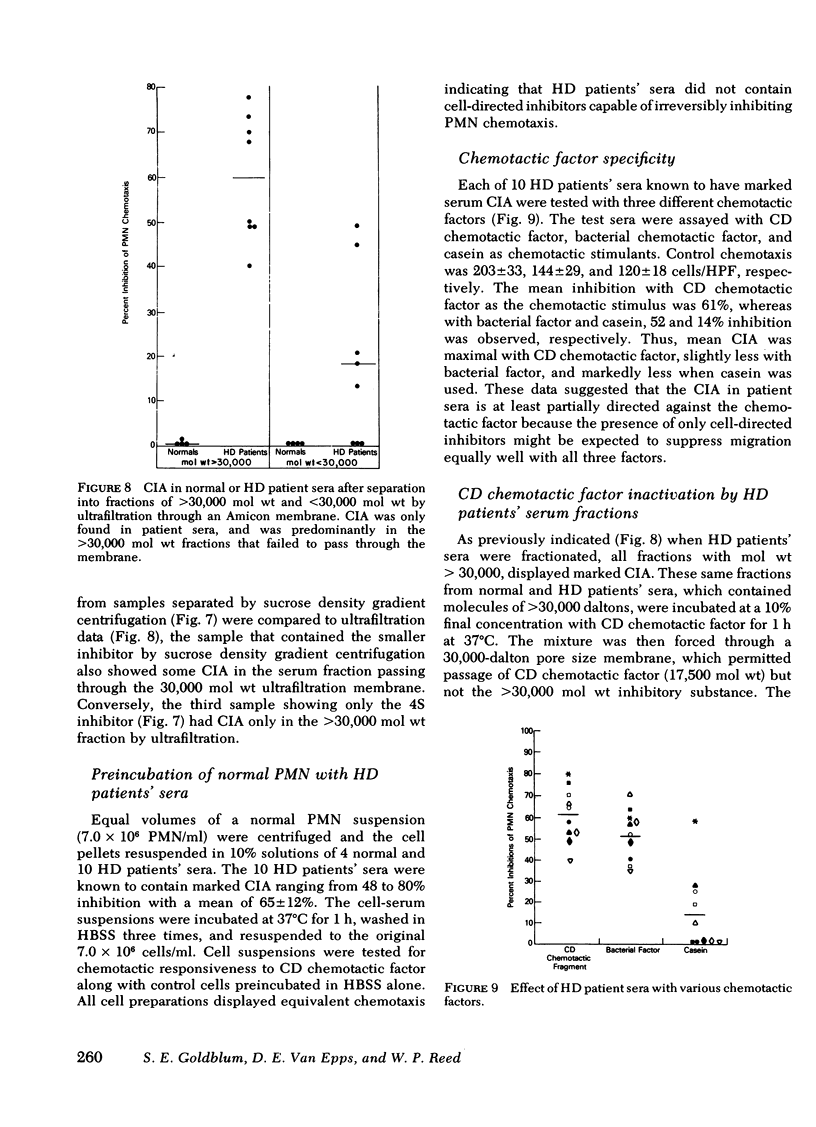
Inhibitory sera effectively suppressed neutrophil migration in response to chemotactic C5 fragment and Escherichia coli derived chemotactic factor but was least effective in a system mediated by casein. Furthermore, normal neutrophils preincubated in hemodialysis patient sera displayed normal chemotactic responsiveness indicating a lack of cell-directed inhibition. Serum fractions that contained the inhibitor were found to directly act on the chemotactic C5 fragment, reducing its chemotactic activity. This study indicates that a circulating 4S, heat-stable, factor-directed inhibitor of granulocyte chemotaxis is present in the sera of many hemodialysis patients and probably results from the hemodialysis procedure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antibodies in schizophrenics. Br Med J. 1967 Sep 2;3(5565):569–570. [PMC free article] [PubMed] [Google Scholar]
- BALCH H. H. The effect of severe battle injury and of post-traumatic renal failure on resistance to infection. Ann Surg. 1955 Aug;142(2):145–163. doi: 10.1097/00000658-195508000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Baum J., Cestero R. V., Freeman R. B. Chemotaxis of the polymorphonuclear leukocyte and delayed hypersensitivity in uremia. Kidney Int Suppl. 1975 Jan;(2):147–153. [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Mauer S. M., Mills E. L., Quie P. G. The effect of hemodialysis on neutrophil chemotactic responsiveness. Acta Med Scand. 1978;203(1-2):67–70. doi: 10.1111/j.0954-6820.1978.tb14833.x. [DOI] [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson R. L. Reversible inhibition of phagocytosis in anephric uremic patients. Surg Forum. 1973;24:75–77. [PubMed] [Google Scholar]
- Burton B. T., Krueger K. K., Bryan F. A., Jr National registry of long-term dialysis patients. JAMA. 1971 Nov 1;218(5):718–722. [PubMed] [Google Scholar]
- Buscarini L., Bassi F. Leucocyte loss in haemodialysis. Acta Haematol. 1972;48(5):278–282. doi: 10.1159/000208470. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Hamory B. H., Ford G. H., Kimball H. R. Chemotaxis in acute renal failure. J Infect Dis. 1972 Oct;126(4):460–463. doi: 10.1093/infdis/126.4.460. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps D. E., Williams R. C., Jr Serum chemotactic inhibitory activity: heat activation of chemotactic inhibition. Infect Immun. 1976 Mar;13(3):741–749. doi: 10.1128/iai.13.3.741-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Page A. R., Pickering R. J., Good R. A. Complement activity and inflammatory neutrophil exudation in man. Studies in patients with glomerulonephritis, essential hypocomplementemia and agammaglobulinemia. Int Arch Allergy Appl Immunol. 1967;32(1):64–90. doi: 10.1159/000229917. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. H., Ray C., Mauer S. M., Quie P. G. The effect of hemodialysis on neutrophil chemotactic responsiveness. J Lab Clin Med. 1976 Dec;88(6):971–974. [PubMed] [Google Scholar]
- Guckian J. C., Karrh L. R., Copeland J. L., McCoy J. Phagocytosis by polymorphonuclear leukocytes in patients with renal failure on chronic hemodialysis. Tex Rep Biol Med. 1971;29(2):193–198. [PubMed] [Google Scholar]
- Henderson L. W., Miller M. E., Hamilton R. W., Norman M. E. Hemodialysis leukopenia and polymorph random mobility-a possible correlation. J Lab Clin Med. 1975 Feb;85(2):191–197. [PubMed] [Google Scholar]
- Howell E. D., Perkins H. A. Anti-N-like antibodies in the sera of patients undergoing chronic hemodialysis. Vox Sang. 1972;23(4):291–299. doi: 10.1111/j.1423-0410.1972.tb03463.x. [DOI] [PubMed] [Google Scholar]
- Hoy W. E., Cestero R. V., Freeman R. B. Deficiency of T and B lymphocytes in uremic subjects and partial improvement with maintenance hemodialysis. Nephron. 1978;20(4):182–188. doi: 10.1159/000181220. [DOI] [PubMed] [Google Scholar]
- Kaehny W. D., Miller G. E., White W. L. Relationship between dialyzer reuse and the presence of anti-N-like antibodies in chronic hemodialysis patients. Kidney Int. 1977 Jul;12(1):59–65. doi: 10.1038/ki.1977.79. [DOI] [PubMed] [Google Scholar]
- Lowrie E. G., Lazarus J. M., Mocelin A. J., Bailey G. L., Hampers C. L., Wilson R. E., Merrill J. P. Survival of patients undergoing chronic hemodialysis and renal transplantation. N Engl J Med. 1973 Apr 26;288(17):863–867. doi: 10.1056/NEJM197304262881701. [DOI] [PubMed] [Google Scholar]
- Marshall J. W., Ahearn D. J., Nothum R. J., Esterly J., Nolph K. D., Maher J. F. Adherence of blood components to dialyzer membranes: morphological studies. Nephron. 1974;12(3):157–170. doi: 10.1159/000180372. [DOI] [PubMed] [Google Scholar]
- McLeish W. A., Brathwaite A. F., Peterson P. M. Anti-N antibodies in hemodialysis patients. Transfusion. 1975 Jan-Feb;15(1):43–45. doi: 10.1046/j.1537-2995.1975.15175103509.x. [DOI] [PubMed] [Google Scholar]
- Montgomerie J. Z., Kalmanson G. M., Guze L. B. Leukocyte phagocytosis and serum bactericidal activity in chronic renal failure. Am J Med Sci. 1972 Nov;264(5):385–393. doi: 10.1097/00000441-197211000-00006. [DOI] [PubMed] [Google Scholar]
- Montgomerie J. Z., Kalmanson G. M., Guze L. B. Renal failure and infection. Medicine (Baltimore) 1968 Jan;47(1):1–32. doi: 10.1097/00005792-196801000-00001. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Sanford J. P. Defective cellular immunity in renal failure: depression of reactivity of lymphocytes to phytohemagglutinin by renal failure serum. J Clin Invest. 1971 Jun;50(6):1262–1271. doi: 10.1172/JCI106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolph K. D., Husted F. C., Sharp G. C., Siemsen A. W. Antibodies to nuclear antigens in patients undergoing long-term hemodialysis. Am J Med. 1976 May 10;60(5):673–676. doi: 10.1016/0002-9343(76)90502-7. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Lipton M., Goldstein I. M. A specific inhibitor of complement (C5)-derived chemotactic activity in serum from patients with systemic lupus erythematosus. J Clin Invest. 1978 Jul;62(1):29–38. doi: 10.1172/JCI109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley E. J., Huang S. W., Plaut J., Morris N. Defective phagocyte adherence in acute poststreptococcal glomerulonephritis: clinical and laboratory observations. J Pediatr. 1976 Nov;89(5):748–754. doi: 10.1016/s0022-3476(76)80796-2. [DOI] [PubMed] [Google Scholar]
- Salant D. J., Glover A. M., Anderson R., Meyers A. M., Rabkin R., Myburgh J. A., Rabson A. R. Depressed neutrophil chemotaxis in patients with chronic renal failure and after renal transplantation. J Lab Clin Med. 1976 Oct;88(4):536–545. [PubMed] [Google Scholar]
- Steinman C. R., Ackad A. Appearance of circulating DNA during hemodialysis. Am J Med. 1977 May;62(5):693–697. doi: 10.1016/0002-9343(77)90872-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. C., Crawford I. P. Purification and preliminary characterization of human leukocyte elastasel. Arch Biochem Biophys. 1975 Jul;169(1):91–101. doi: 10.1016/0003-9861(75)90320-3. [DOI] [PubMed] [Google Scholar]
- Till G., Ward P. A. Two distinct chemotactic factor inactivators in human serum. J Immunol. 1975 Feb;114(2 Pt 2):843–847. [PubMed] [Google Scholar]
- Vallota E. H., Müller-Eberhard H. J. Formation of C3a and C5a anaphylatoxins in whole human serum after inhibition of the anaphylatoxin inactivator. J Exp Med. 1973 May 1;137(5):1109–1123. doi: 10.1084/jem.137.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Palmer D. L., Williams R. C., Jr Characterization of serum inhibitors of neutrophil chemotaxis associated with anergy. J Immunol. 1974 Jul;113(1):189–200. [PubMed] [Google Scholar]
- Van Epps D. E., Strickland R. G., Williams R. C., Jr Inhibitors of leukocyte chemotaxis in alcoholic liver disease. Am J Med. 1975 Aug;59(2):200–207. doi: 10.1016/0002-9343(75)90354-x. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON W. E., KIRKPATRICK C. H., TALMAGE D. W. IMMUNOLOGIC STUDIES IN HUMAN ORGAN TRANSPLANTATION. 3. THE RELATIONSHIP OF DELAYED CUTANEOUS HYPERSENSITIVITY TO THE ONSET OF ATTEMPTED KIDNEY ALLOGRAFT REJECTION. J Clin Invest. 1964 Oct;43:1881–1891. doi: 10.1172/JCI105062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Berenberg J. L. Defective regulation of inflammatory mediators in Hodgkin's disease. Supernormal levels of chemotactic-factor inactivator. N Engl J Med. 1974 Jan 10;290(2):76–80. doi: 10.1056/NEJM197401102900203. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. J Lab Clin Med. 1976 Jun;87(6):1025–1032. [PubMed] [Google Scholar]
- Webel M. L., Ritts R. E., Jr, Briggs W. A., Light J. A. Lymphocyte blastogenesis in patients receiving hemodialysis. Arch Intern Med. 1976 Jun;136(6):682–687. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. A functional differentiation of human neutrophil granules: generation of C5a by a specific (secondary) granule product and inactivation of C5a by azurophil (primary) granule products. J Immunol. 1977 Sep;119(3):1068–1076. [PubMed] [Google Scholar]


