SUMMARY
The discrete typing units (DTUs) of Trypanosoma cruzi that infect domestic dogs and cats have rarely been studied. With this purpose we conducted a cross-sectional xenodiagnostic survey of dog and cat populations residing in two infested rural villages in Pampa del Indio, in the humid Argentine Chaco. Parasites were isolated by culture from 44 dogs and 12 cats with a positive xenodiagnosis. DTUs were identified from parasite culture samples using a strategy based on multiple polymerase-chain reactions. TcVI was identified in 37 of 44 dogs and in 10 of 12 cats, whereas TcV was identified in five dogs and in two cats –a new finding for cats. No mixed infections were detected. The occurrence of two dogs infected with TcIII –classically found in armadillos– suggests a probable link with the local sylvatic transmission cycle involving Dasypus novemcinctus armadillos and a potential risk of human infection with TcIII. Our study reinforces the importance of dogs and cats as domestic reservoir hosts and sources of various DTUs infecting humans, and suggests a link between dogs and the sylvatic transmission cycle of TcIII.
Keywords: Chagas disease, Trypanosoma cruzi, Discrete Typing Units, dogs, cats, reservoir
INTRODUCTION
Trypanosoma cruzi has a large genetic diversity (Brisse et al. 2001) classified into six Discrete Typing Units (DTUs), T. cruzi I-VI (Zingales et al. 2009). DTUs are distributed differentially among vectors, mammalian hosts and geographic regions. TcIII and TcIV usually occur in sylvatic transmission cycles; TcII, TcV and TcVI occur in domestic cycles, and TcI in both cycles (Yeo et al. 2005; Miles et al. 2009).
Dogs and cats are major reservoir hosts of T. cruzi in domestic transmission cycles throughout the Americas (Mott et al. 1978; Gürtler et al. 2005, 2007; Crisante et al. 2006). Both host species usually display a large fraction of infectious individuals and persistent infectiousness to the vector (Gürtler et al. 2007). Dogs are also sensitive sentinels of domestic transmission (Castañera et al. 1998; Cardinal et al. 2006) and the household presence of dogs and cats seropositive for T. cruzi increases the risk of parasite transmission to local vectors and humans (Gürtler et al. 2005).
Despite the large contribution of both reservoir hosts to the domestic transmission of T. cruzi, very few studies have identified the DTUs infecting dogs and cats (Chapman et al. 1984; de Luca d’Oro et al. 1993; Diosque et al. 2003; Cardinal et al. 2008; Marcili et al. 2009a; Rimoldi et al. 2012). In the southern cone countries of South America, the main DTUs identified from humans, domestic dogs and triatomine bugs were TcI, TcII, TcIII, TcV and TcVI (de Luca d’Oro et al. 1993; Barnabé et al. 2001, 2011; Diosque et al. 2003; Cardinal et al. 2008; del Puerto et al. 2010; Tomasini et al. 2011; Yeo et al. 2011; Cura et al. 2012). In northern Argentina, domestic Triatoma infestans and dogs were very frequently infected with TcVI in Santiago del Estero and Chaco provinces (Diosque et al. 2003; Cardinal et al. 2008).
As part of a multi-site research program on the eco-epidemiology and control of Chagas disease in the Gran Chaco ecoregion, we sought to identify the DTUs infecting domestic dogs and cats to characterize the structure of transmission cycles of T. cruzi in a well-defined rural area of the Argentinean Chaco. Parallel efforts in our study area identified the occurrence of TcIII in Dasypus novemcinctus armadillos; TcI in Didelphis albiventris opossums and peridomestic Triatoma sordida; TcV and TcVI in domestic or peridomestic T. infestans, and TcVI in peridomestic T. sordida (Maffey et al. in press; Alvarado-Otegui et al. 2012). We found that TcVI was more prevalent in the study dogs and cats followed by TcV; and the occurrence of the typically sylvatic DTU TcIII in two domestic dogs.
MATERIALS AND METHODS
Study area
Field work was conducted in the municipality of Pampa del Indio (26° 2′ 0″ S, 59° 55′ 0″ O), Chaco Province, Argentina, located in the humid (eastern) Chaco, close to the transition to the dry (western) Chaco (Gurevitz et al. 2011). The study area included 13 contiguous villages with 323 houses inhabited by two ethnic groups, Creoles and Tobas. Houses were immersed in a landscape consisting of a mosaic of crops and small patches of native dry forest with different degrees of degradation. In most cases house compounds rarely had fences and domestic animals (dog, cat, cattle, goat and sheep) ranged freely in the nearby forest. Most houses were made of mud walls, with metal or thatched roofs, and included peridomestic structures that housed various domestic animals. A cross-sectional demographic and entomologic survey of all study villages revealed that 40% of houses were infested with T. infestans; the prevalence of house infestation was 58.8% for Toba households and 43.5% for Creoles’ (Gurevitz et al. 2011). Prior to community-wide residual spraying with pyrethroid insecticides in December 2007, 27.4% of bugs were infected with T. cruzi and bug infection was more frequent in Toba households (42.0%) than in Creoles (20.7%) (Cardinal et al. in review).
Study design
A demographic and sero-parasitological survey targeting all dogs and cats residing in seven contiguous villages in August-December 2008 revealed that the overall prevalence of T. cruzi infection was 26% in dogs and 29% in cats examined by serological methods and/or xenodiagnosis and the distribution of infected dogs or cats was aggregated at the household level (Cardinal et al. in review).
Nested within this larger survey, for the present study we selected two villages (10 de Mayo and Las Chuñas) which had high prevalence of infection with T. cruzi in T. infestans (61%) before interventions. Then we selected all houses that had at least one T. cruzi-infected T. infestans before interventions (38 of 60 inhabited houses) and conducted a xenodiagnostic survey of all dogs and cats. This procedure sought to increase the chances of isolating parasites from a large number of infected individuals. In addition, we included seven dogs and four cats from 6 neighboring communities which had a positive xenodiagnosis. Each head of household was informed on the objectives and relevance of the study; informed oral consent was requested and obtained in all cases. Processing of dogs and cats was conducted according to the protocol approved by the “Dr. Carlos Barclay” Independent Ethical Committee for Clinical Research from Buenos Aires, Argentina (IRB No. 00001678, NIH registered, Protocol N° TW-01-004).
Xenodiagnosis
Dogs were fitted with a muzzle (except newborn pups) and handled with the help of its owners when it did not entail any physical risk, whereas cats were captured by hand or with a net and anesthetized as described elsewhere (Cardinal et al. in review). All available dogs and cats were examined by xenodiagnosis using 20 uninfected fourth-instar nymphs of T. infestans or 10 nymphs for small pups and kittens (Gürtler et al. 2007). All insects were mass-reared and provided by the National Coordination of Vector Control (Cordoba, Argentina). Xenodiagnosis boxes were exposed during 25 minutes on the belly of each individual animal, and a new re-exposure period of 10 minutes followed if most bugs had not blood-fed to repletion. The boxes were held in a cooler to avoid sudden temperature changes during field work, and bugs were kept until examination with no additional blood-feeding.
Faeces from two insects fed on each animal were examined individually at optical microscope (OM) at 400× at 30 days post-exposure, and if negative, the remainder of insects fed on the same individual was analyzed in pools of 4-5 insects each. When the first two bugs or any pool were positive, faeces from of all insects in the positive pool were re-examined individually. Bugs negative at 30 days post-exposure were re-examined individually 30 days later.
Parasite isolation and DTU identification
Parasite culture in biphasic medium and isolation were performed as described elsewhere (Lauricella et al. 2005). Briefly, faeces from two OM-positive bugs from each xenodiagnosis-positive animal were separately inoculated into two culture tubes each containing biphasic medium. Parasite growth was monitored weekly during four months until reaching 3 × 105 parasites/ml.
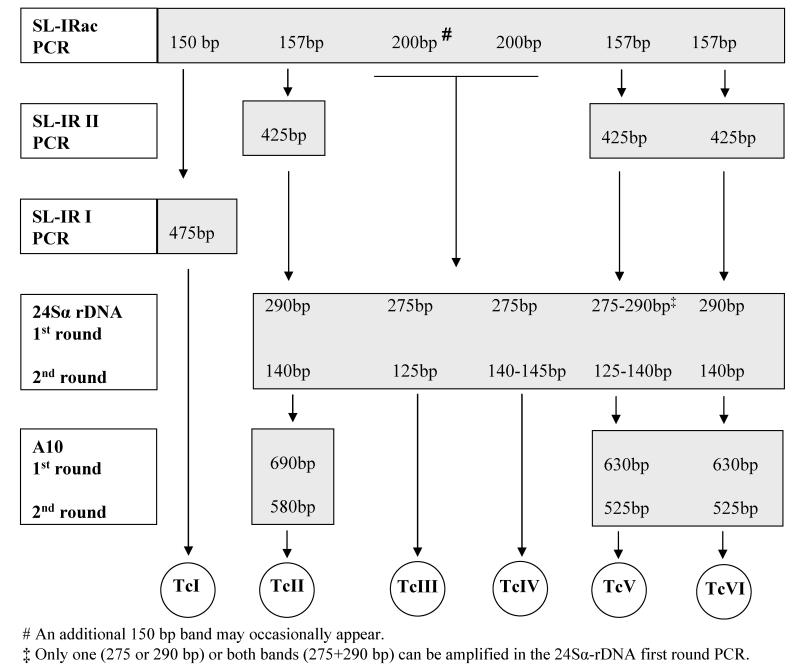
Parasites used for identification of DTUs were obtained from an aliquot of cultures at the time of cryopreservation or when a new sub-culture was initiated after cryopreservation (i.e., when parasite concentration was high). DNA was extracted by boiling parasite pellets as described by Marcet et al. (2006). T. cruzi DTUs were identified by polymerase-chain reactions (PCR) targeted to three genomic markers: spliced-leader (SL) DNA, 24Sα ribosomal RNA genes and A10 marker using Taq DNA polymerase (Invitrogen, USA) as described by Burgos et al. (2007) (Figure 1). PCR products were analyzed in 2.5% agarose gels (Invitrogen, USA) for SL-IR I and SL-IR II, in 3% agarose gels for the remainder, and all UV visualization were done after staining with Gel Red (GenBiotech).
Figure 1.
Decision key for discriminating T. cruzi DTUs following Burgos et al (2007).
Amplification of the SL-IR included the following three independent PCRs: SL IRac (using primers TCac and UTCC) to distinguish TcIII and TcIV (200 bp) from TcI (150 bp), TcII, TcV, and TcVI (157 bp); SL-IR I (using primers TC2 and UTCC) to identify TcI (475 bp); and SL-IR II (using primers TC1-UTCC) to identify TcII, TcV, and TcVI (425 bp) (Figure 1).
Regarding the 24Sα ribosomal RNA genes, a dimorphic region within the D7 domain was amplified by heminested PCR to distinguish TcV (125 or 125 + 140 bp) from TcII and TcVI (140 bp), and TcIII (125 bp) from TcIV (140 bp). The first round PCR was performed using D75 and D76 primers. The heminested round was carried out using 1 μl of the first round PCR in a 30 μl vol. reaction using primers D71 and D76 (Figure 1).
Finally, a heminested PCR targeted to the A10 genomic marker was used to discriminate TcII (580 bp) from TcV and TcVI (525 bp). The first round was carried out using Pr1 and P6 primers whereas the heminested round was carried out using 5 μl of the first round PCR in a 30 μl vol. reaction with primers Pr1 and Pr3 (Figure 1). In summary, 6 PCR assays were necessary to distinguish all UDTs.
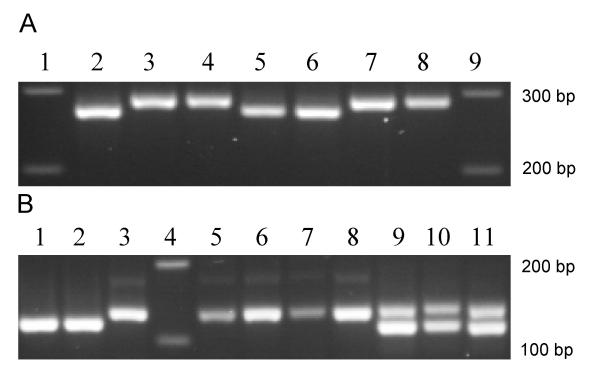
Burgos et al. (2007) reported that some TcV-infected samples amplified both 125 and 140 bp ribosomal DNA bands in the heminested 24Sα rDNA-PCR (primers D71 and D76) (Figure 2B). When this pattern appeared, it was not possible to exclude an infection with TcVI (i.e., differentiate single infections with TcV from mixed infections with both TcV and TcVI). In our samples, all parasite stocks infected with TcV amplified both bands. Consequently, we also considered the results from the first round of the 24Sα rDNA-PCR (primers D75 and D76) to distinguish infections with TcV only (showing a 275 bp band only) from those with TcV and TcVI (275 and 290 bp bands) (Figure 2A). T. cruzi reference stocks used as controls were TcI (X-10 and CA-1 K98); TcII (Tu 18); TcIII (M5631); TcIV (Can III); TcV (PAH 265); and TVI (Cl-Brener).
Figure 2.
Example of 24Sα-rDNA PCR product size polymorphism A) First round PCR, primers D75 and D76 Lane (1) 100 bp ladder; lane (2) reference stock PAH 265 (TcV); lane (3) reference stock CL-Brener (TcVI); lane (4) reference stock Tu 18 (TcII); lane (5) sample of dog with identified DTU (TcV); lane (6) sample of cat with identified DTU (TcV); lane (7) sample of dog with identified DTU (TcVI); lane (8) sample of cat with identified DTU (TcVI); lane (9) 100 bp ladder. B) Heminested PCR, primers D71 and D76 Lane (1) sample of dog with identified DTU (TcIII); lane (2) reference stock M5631 (TcIII); lane (3) reference stock Can III (TcIV); lane (4) 100 bp ladder; lane (5) sample of dog with TcVI identified; lane (6) reference stock Tu 18 (TcII); lane (7) sample of cat with identified TcVI; lane (8) reference stock CL-Brener (TcVI); lane (9) reference stock PAH 265 (TcV); lane (10) sample of dog with TcV identified; lane (11) sample of cat with TcV identified.
RESULTS
A total of 39 of 100 dogs and 8 of 29 cats examined with xenodiagnosis were positive at the two study villages. Parasites were successfully isolated from 37 of 39 dogs and from all 8 cats positive by xenodiagnosis, as well as from seven dogs and four cats residing in neighbouring communities. DTUs were identified in 56 culture-derived DNA samples (Table 1). TcVI was found in 37 of 44 dogs and in 10 of 12 cats. TcV was identified in five dogs and two cats, whereas TcIII was found only in two dogs. No mixed infections were detected.
Table 1.
Distribution of Discrete Typing Units (DTUs) of T. cruzi in isolates from dogs and cats, Pampa del Indio, Chaco, 2008
| No. of individuals with DTU identified | ||||
|---|---|---|---|---|
|
|
||||
| Host | TcIII | TcV | TcVI | Total |
| Dog | 2 | 5 | 37 | 44 |
| Cat | 0 | 2 | 10 | 12 |
| Total | 2 | 7 | 47 | 56 |
At the household level, TcVI predominated in 26 of 30 houses with DTUs identified in dogs or cats (Table 2). TcVI was found alone in 22 houses and co-circulated with TcV in four houses. TcIII and TcV occurred in two houses each, in none of which TcVI was identified.
Table 2.
Distribution of Discrete Typing Units (DTUs) in house compounds where T. cruzi was isolated from dogs or cats
| Number of houses | ||||
|---|---|---|---|---|
|
|
||||
| DTU | Only in dogs | Only in cats | Both hosts | Total |
| Only TcVI | 14 | 5 | 3 | 22 |
| Only TcIII | 2 | 0 | 0 | 2 |
| Only TcV | 1 | 0 | 1 | 2 |
| TcV and TcVI† | 2 | 0 | 2‡ | 4 |
| Total | 19 | 5 | 6 | 30 |
Single infections with TcV and TcVI were identified in different hosts residing at the same house compound.
Includes one house where TcV was identified in one cat and TcVI in both a cat and a dog, and another house with TcV in a dog and TcVI in a cat.
DISCUSSION
Our study documents the predominance of TcVI and the occurrence of TcV in xenodiagnosis-positive dogs and cats in a rural endemic area in the humid Argentinean Chaco, in the largest survey of DTUs infecting dogs and cats conducted so far. We also provide the first report of two cats infected with TcV.
Although previously reported in the Gran Chaco, the occurrence of TcV and TcVI has rarely been documented in non-humans hosts (Miles et al. 2009; Zingales et al. 2012). The predominance of TcVI in parasite stocks from dogs and cats is consistent with the predominance of TcVI in local T. infestans, especially in bugs collected from peridomestic kitchens and storerooms where they usually rested (Maffey et al. in press). The role of dogs and cats as sources of T. cruzi was also evidenced by the finding of a strong association between dog and bug infection at the household level (Gürtler et al. 2005). Given the relevance of peridomestic foci in the process of house reinfestation with T. infestans after residual spraying with insecticides (Cecere et al. 2004; Gurevitz et al. in press), dogs and cats may be the first sources of infection for the bugs that re-invade the house compound, and their DTUs may be involved in renewed parasite transmission after interventions.
Most houses had dogs and cats infected with TcVI only, despite the study identified DTUs from up to 2-5 animals at the same house. The predominance of TcVI in domestic dogs also occurred in other areas in the dry Argentinean and Paraguayan Chaco (Chapman et al. 1984; Diosque et al. 2003; Cardinal et al. 2008), suggesting an association between TcVI and peripheral blood infection of dogs and cats. We cannot exclude the occurrence of undetected mixed infections produced by histotropism within the mammalian host (Vago et al. 2000; Burgos et al. 2010) or by selection of genotypes during parasite isolation and culture, as was revealed by parasite cloning in agar plates (Yeo et al. 2007).
DTUs typically associated with the sylvatic transmission cycle have been identified in dogs with very low frequencies. In the Argentinean dry Chaco, TcIII was identified in 3 of 31 infected dogs from Santiago del Estero Province (Cardinal et al. 2008) whereas no TcIII infections were recorded in 16 infected dogs from Chaco and in two dogs from Santiago del Estero (de Luca d’Oro et al. 1993; Diosque et al. 2003). In the Paraguayan Chaco, TcIII was identified in 3 of 7 infected dogs (Barnabé et al. 2001, based on isoenzyme patterns in Chapman et al. 1984). Cats have been found to be infected only with TcVI prior to our current study (Cardinal et al. 2008). In Pampa del Indio, parallel surveys identified TcIII in armadillos Dasypus novemcinctus and TcI in Didelphis albiventris and in adult Triatoma sordida collected in peridomestic structures (Alvarado-Otegui et al. 2012; Maffey et al. in press). In spite of the local occurrence of these DTUs, we only detected TcIII in two of 44 infected dogs. Regardless of the exact mode of transmission underlying such infections (i.e., oral transmission from hunting and eating armadillos, vector-mediated transmission or vertical transmission), these findings demonstrate the occurrence of a typically sylvatic DTU in domestic dogs and a potential risk of human infection with TcIII because T. infestans and other domestic bug species frequently blood-feed on dogs (Gürtler et al. 2007).
This study documents the finding of both domestic and sylvatic DTUs infecting domestic dogs. Previous studies in South America have rarely found TcIII in the domestic environment, where it is more prevalent in dogs than in humans, whereas TcIII occurs more frequently in domestic hosts than TcV or TcVI in sylvatic mammals (Miles et al. 2009; Zingales et al. 2012). These results imply a great versatility of the dog reservoir in harbouring a broad genetic diversity of T. cruzi, and the need for more research on the role of dogs in T. cruzi transmission across the Americas up to the US.
With the aim of fully describing the genetic diversity of T. cruzi, high-resolution methods have recently been applied to investigate parasite populations at the infra-DTU level, such as microsatellites (Llewellyn et al. 2009) or multilocus sequence typing (Yeo et al. 2011). Our study may be considered a first step toward the understanding of the structure of T. cruzi transmission cycles through the distribution of DTUs in dogs and cats in a well-defined area. Those novel tools are needed to probe the local genetic structure of T. cruzi with greater detail, and examine the putative sylvatic origin of dog infections with TcIII and TcI.
Previous studies conducted in Argentina recorded TcI, TcV and TcVI in human patients affected by Chagas disease (Schijman et al. 2004; Burgos et al. 2007, 2010; Cura et al. 2012). All of these human-infecting DTUs were found in domestic dogs and cats from Chaco and Santiago del Estero Provinces further evidencing their relevance as reservoir hosts of T. cruzi in the domestic transmission.
ACKNOWLEDGMENTS
The authors are grateful to Marina Leporace, Francisco Petrocco, Laura Tomassone and Sol Gaspe for field and laboratory assistance. To Lucía Maffey, Julián Alvarado-Otegui, Pilar Fernández, Fernando Garelli, Juan Gurevitz, Yael Provecho, Jimena Gronzo, Carla Cecere and Romina Piccinali for valuable comments during the study, and two anonymous reviewers for helpful suggestions. Special thanks to the villagers of Pampa del Indio for kindly welcoming us into their homes and cooperating with the investigation. Reference strains of TcI-TcVI were kindly provided by Patricio Diosque, Miguel A. Basombrío and Michel Tibayrenc.
FINANCIAL SUPPORT This study received partial financial support from International Development Research Center (Ecohealth Program); the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR); the National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (to Uriel Kitron and REG), University of Buenos Aires. REG, MVC and AGS are members of CONICET Researcher’s Career.
REFERENCES
- Alvarado-Otegui JA, Ceballos LA, Orozco MM, Enriquez GF, Cardinal MV, Cura C, Schijman AG, Kitron U, Gürtler RE. The sylvatic transmission cycle of Trypanosoma cruzi in a rural area in the humid Chaco of Argentina. Acta Tropica. 2012;124:79–86. doi: 10.1016/j.actatropica.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabé C, Neubauer K, Solari A, Tibayrenc M. Trypanosoma cruzi: presence of the two major phylogenetic lineages and of several lesser discrete typing units (DTUs) in Chile and Paraguay. Acta Tropica. 2001;78:127–137. doi: 10.1016/s0001-706x(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Barnabé C, De Meeûs T, Noireau F, Bosseno MF, Monje EM, Renaud F, Brenière SF. Trypanosoma cruzi discrete typing units (DTUs): Microsatellite loci and population genetics of DTUs TcV and TcI in Bolivia and Peru. Infection, Genetics and Evolution. 2011;11:1752–1760. doi: 10.1016/j.meegid.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Brisse S, Verhoef J, Tibayrenc M. Characterization of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. International Journal for Parasitology. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Burgos JM, Altcheh J, Bisio M, Duffy T, Valdares HMS, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Machi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. International Journal for Parasitology. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Burgos JM, Diez M, Vigliano C, Bisio M, Risso M, Duffy T, Cura C, Brusses B, Favaloro L, Leguizamon MS, Lucero RH, Laguens R, Levin MJ, Favaloro R, Schijman AG. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clinical Infectious Disease. 2010;51:485–495. doi: 10.1086/655680. [DOI] [PubMed] [Google Scholar]
- Cardinal MV, Castañera MB, Lauricella MA, Cecere MC, Ceballos AL, Vazquez-Prokopec GM, Kitron U, Gürtler RE. A prospective study of the effects of sustained vector surveillance following community-wide insecticide application on Trypanosoma cruzi of dogs and cats in rural northwestern Argentina. American Journal of Tropical Medicine and Hygiene. 2006;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- Cardinal MV, Lauricella MA, Ceballos AL, Lanati L, Marcet PL, Levin MJ, Kitron U, Gürtler RE, Schijman AG. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. International Journal for Parasitology. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-western Argentina. Annals of Tropical Medicine and Parasitology. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- Chapman MD, Baggaley RC, Godfreyfausset PF, Malpas TJ, White G, Canese J, Miles MA. Trypanosoma cruzi from the Paraguayan Chaco isoenzyme profiles of strains isolated at Makthlawaiya. Journal of Protozoology. 1984;31:482–486. doi: 10.1111/j.1550-7408.1984.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Crisante G, Rojas A, Teixeira MMG, Añez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Tropica. 2006;98:247–254. doi: 10.1016/j.actatropica.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Cura CI, Lucero RH, Bisio M, Oshiro E, Formichelli LB, Burgos JM, Lejona S, Brusés BL, Hernández DO, Severini GV, Velazquez E, Duffy T, Anchart E, Lattes R, Altcheh J, Freilij H, Diez M, Nagel C, Vigliano C, Favaloro L, Favaloro RR, Merino DE, Sosa-Estani S, Schijman AG. Trypanosoma cruzi Discrete Typing Units in Chagas disease patients from endemic and non-endemic regions of Argentina. Parasitology. 2012;139:516–521. doi: 10.1017/S0031182011002186. [DOI] [PubMed] [Google Scholar]
- de Luca d’Oro GM, Cardenal CN, Pret B, Crisci JV, Montamat EE. Genetic structure of Trypanosoma cruzi populations from Argentina estimated from enzyme polymorphism. Parasitology. 1993;107:405–410. doi: 10.1017/s0031182000067755. [DOI] [PubMed] [Google Scholar]
- del Puerto R, Nishizawa JE, Kikuchi M, Iihoshi N, Roca R, Avilas Alberto, Gianella C, Lora J, Gutierrez Velarde FU, Renjel LA, Miura S, Higo H, Komiya N, Maemura K, Hirayama K. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Neglected Tropical Diseases. 2010;4:e687. doi: 10.1371/journal.pntd.0000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diosque P, Barnabé C, Padilla A, Marco J, Cardozo R, Cimino R, Nasserd J, Tibayrenc M, Basombrío M. Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas’ disease in Argentina. International Journal for Parasitology. 2003;33:997–1003. doi: 10.1016/s0020-7519(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enríquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Neglected Tropical Diseases. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitz JM, Gaspe MS, Enriquez GF, Vassena C, Alvarado-Otegui JA, Provecho YM, Mougabure-Cueto G, Picollo MI, Kitron U, Gürtler RE. Unexpected pyrethroid resistance and control failures of Chagas disease vector in Argentina. Journal of Medical Entomology. doi: 10.1603/me11157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Canale D, Castanñera MB, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern. Argentina. American Journal of Tropical Medicine and Hygiene. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauricella MA, Stariolo RL, Riarte AR, Segura EL, Gürtler RE. Distribution and pathogenicity of Trypanosoma cruzi isolated from peridomestic populations of Triatoma infestans and Triatoma guasayana from rural western Argentina. Memórias do Instituto Oswaldo Cruz. 2005;100:123–129. doi: 10.1590/s0074-02762005000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathogens. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffey L, Cardinal MV, Ordóñez-Krasnowski PC, Lanati LA, Lauricella MA, Schijman AG, Gürtler RE. Direct molecular identification of Trypanosoma cruzi Discrete Typing Units in domestic and peridomestic Triatoma infestans and Triatoma sordida from the Argentine Chaco. Parasitology. doi: 10.1017/S0031182012000856. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology. 2006;132:57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira ACV, Souza AI, da Rosa JA, Campaner M, Lewis MD, Llewellyn MS, Miles MA, Teixeira MMG. Comparative phylogeography of Trypanosoma cruzi TcIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infection, Genetics and Evolution. 2009;9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R, Fitzpatrick S, Gaunt MW, Mauricio IL. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology. 2009;136:1509–1528. doi: 10.1017/S0031182009990977. [DOI] [PubMed] [Google Scholar]
- Mott KE, Mota EA, Sherlock I, Hoff R, Muniz TM, Oliveira TS, Draper CC. Trypanosoma cruzi infection in dogs and cats and household seroreactivity to T. cruzi in a rural community in northeast Brazil. American Journal of Tropical Medicine and Hygiene. 1978;27:1123–1127. doi: 10.4269/ajtmh.1978.27.1123. [DOI] [PubMed] [Google Scholar]
- Rimoldi A, Tomé Alves R, Ambrósio DL, Fernandes MZ, Martinez I, De Araújo RF, Cicarelli RM, Da Rosa JA. Morphological, biological and molecular characterization of three strains of Trypanosoma cruzi Chagas, 1909 (Kinetoplastida, Trypanosomatidae) isolated from Triatoma sordida (Stål) 1859 (Hemiptera, Reduviidae) and a domestic cat. Parasitology. 2012;139:37–44. doi: 10.1017/S0031182011001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijman AG, Vigliano CA, Viotti RJ, Burgos JM, Brandariz S, Lococo BE, Leze MI, Armenti HA, Levin MJ. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic chagas heart disease. American Journal of Tropical Medicine and Hygiene. 2004;70:210–220. [PubMed] [Google Scholar]
- Tomasini N, Lauthier JJ, Monje Rumi MM, Ragone PG, Alberti D’Amato AA, Pérez Brandan C, Cura CI, Schijman AG, Barnabé C, Tibayrenc M, Basombrío MA, Falla A, Herrera C, Guhl F, Diosque P. Interest and limitations of Spliced Leader Intergenic Region sequences for analyzing Trypanosoma cruzi I phylogenetic diversity in the Argentinean Chaco. Infection, Genetics and Evolution. 2011;11:300–307. doi: 10.1016/j.meegid.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Vago AR, Andrade LO, Leite AA, d’Avila Reis D, Macedo AM, Adad SJ, Tostes S, Jr., Moreira MC, Filho GB, Pena SD. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. American Journal of Pathology. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M, Llewellyn M, Sánchez H, Adamson S, Miles G, López E, González N, Patterson J, Gaunt M, Rojas de Arias A, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. International Journal for Parasitology. 2005;35:225–223. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, Valente SAD, Valente VD, Rojas de Arias A, Miles MA. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. International Journal for Parasitology. 2007;37:111–120. doi: 10.1016/j.ijpara.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Yeo M, Mauricio IL, Messenger LA, Lewis MD, Llewellyn MS, Acosta N, Bhattacharyya T, Diosque P, Carrasco HJ, Miles MA. Multilocus sequence typing (MLST) for lineage assignment and high resolution diversity studies in Trypanosoma cruzi. PLoS Neglected Tropical Diseases. 2011;5:e1049. doi: 10.1371/journal.pntd.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memórias do Instituto Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infection, Genetics and Evolution. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]