Abstract
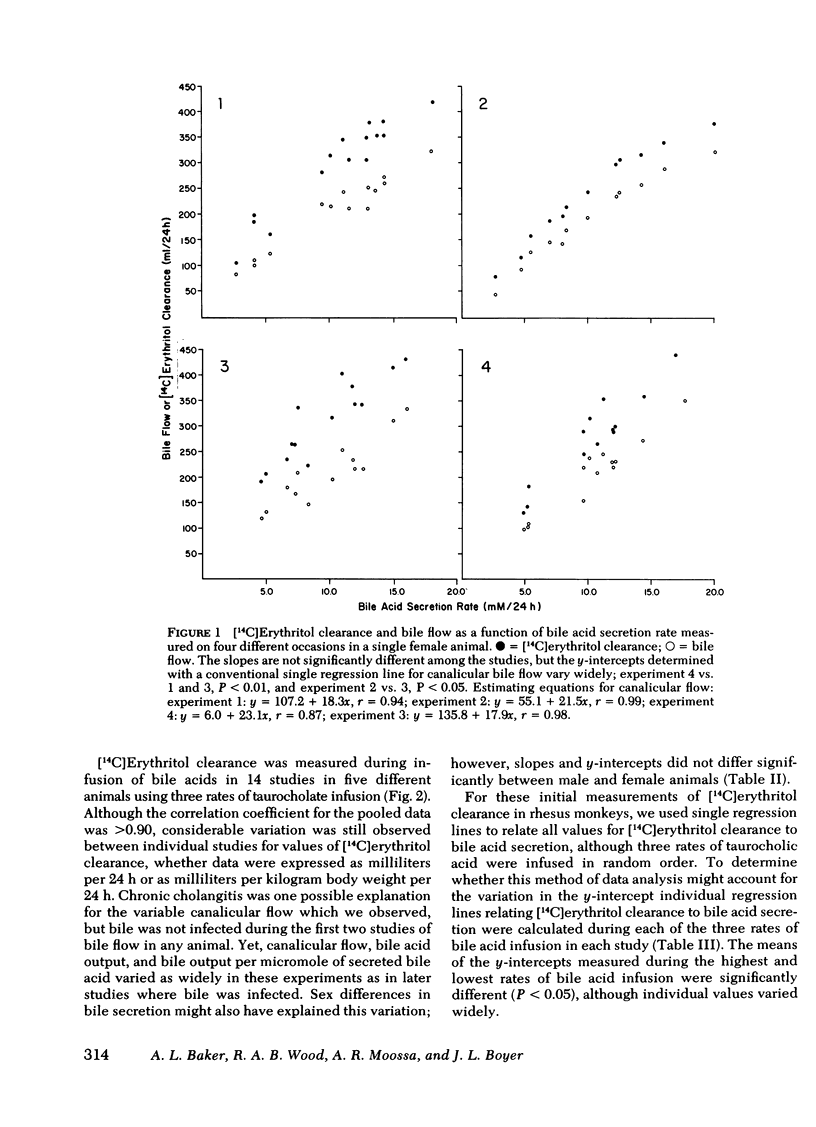
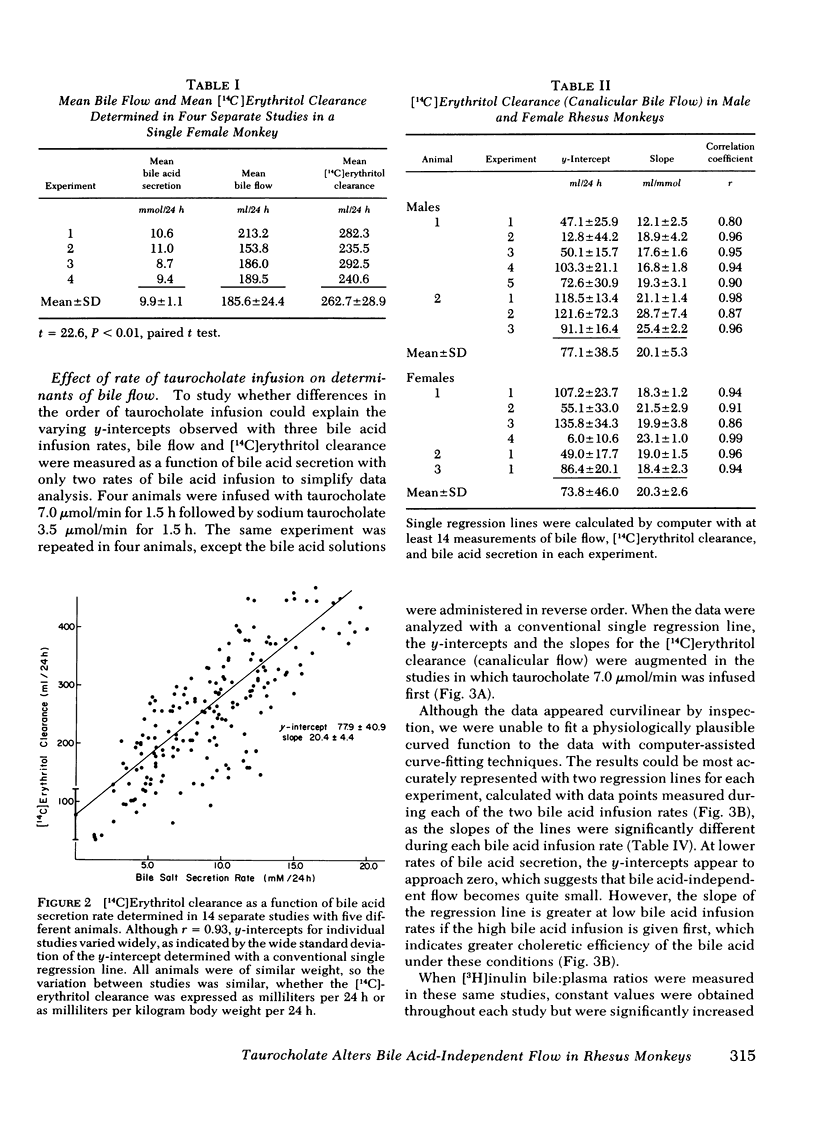
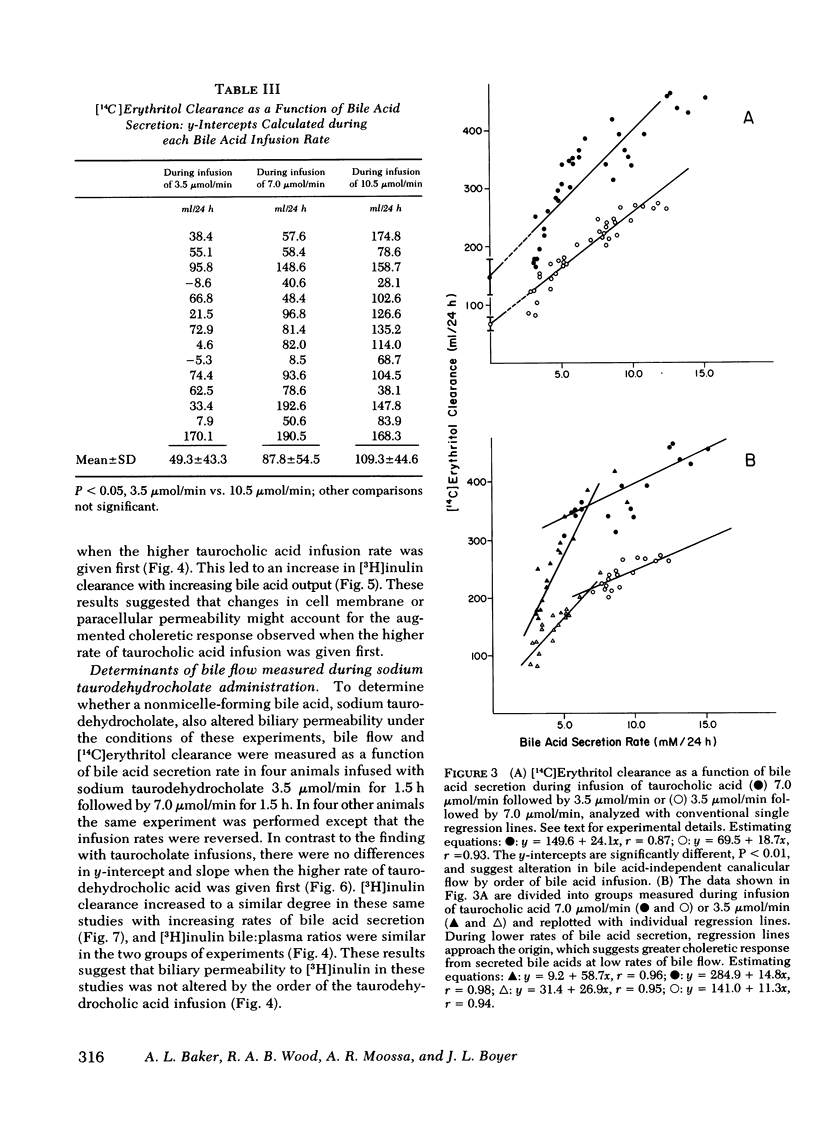
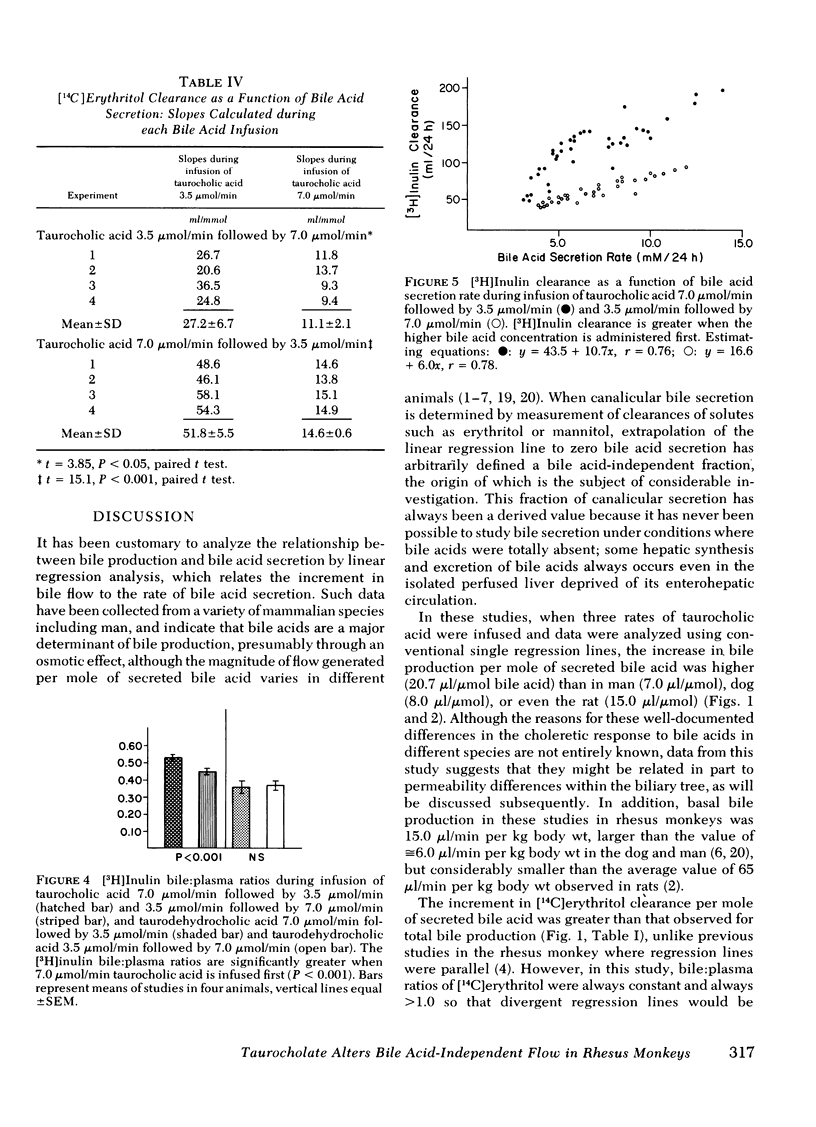
Bile acid-independent secretion and the choleretic response to taurocholate were determined in rhesus monkeys fitted with indwelling silastic cannulas in the common bile ducts. Bile acids were infused intravenously in random order at 3.5, 7.0, or 10.5 μmol/min for 1.5 h each. When data were analyzed with a single regression line, bile flow increased in proportion to the level of bile acid secretion, although the y-intercepts (the conventional measurement of bile acid-independent secretion) varied widely (77.9±40.9 ml/24 h). The variation in y-intercepts was observed between animals and with repeated studies in the same animal and could not be explained by sex differences or the effects of the indwelling silastic cannulas, but seemed to be related to the order of bile acid infusion. With only two taurocholic acid infusion rates (7.0 and 3.5 μmol/min), [14C]erythritol clearance was greater per mole of secreted bile acid when the initial bile acid infusion was at the high level, but approached zero at low bile acid secretion rates, which suggests that so-called bile acid-independent canalicular flow is closely related to bile acid secretion or is small in size. The augmentation in [14C]erythritol clearance when the high infusion rate was given first was also associated with an increase in biliary clearance of [3H]inulin, which indicates that the premeability to inulin was also enhanced. Identical experiments which substituted equimolar infusions of a nonmicelle-forming bile acid (taurodehydrocholate) for taurocholate failed to demonstrate any difference in choleretic response or biliary clearance of [3H]inulin with the order of bile acid infusion. These experiments demonstrate that a micelleforming bile acid, taurocholate, can increase the permeability of the biliary system to large molecular weight solutes and simultaneously modify the y-intercept and the volume of bile secreted in response to the transported bile acid. Taurocholate may, therefore, modify its own choleretic response, perhaps by altering the structure or function of bile secretory membranes, and appears to be a major determinant of so-called bile acid-independent flow in rhesus monkeys.
Full text
PDF

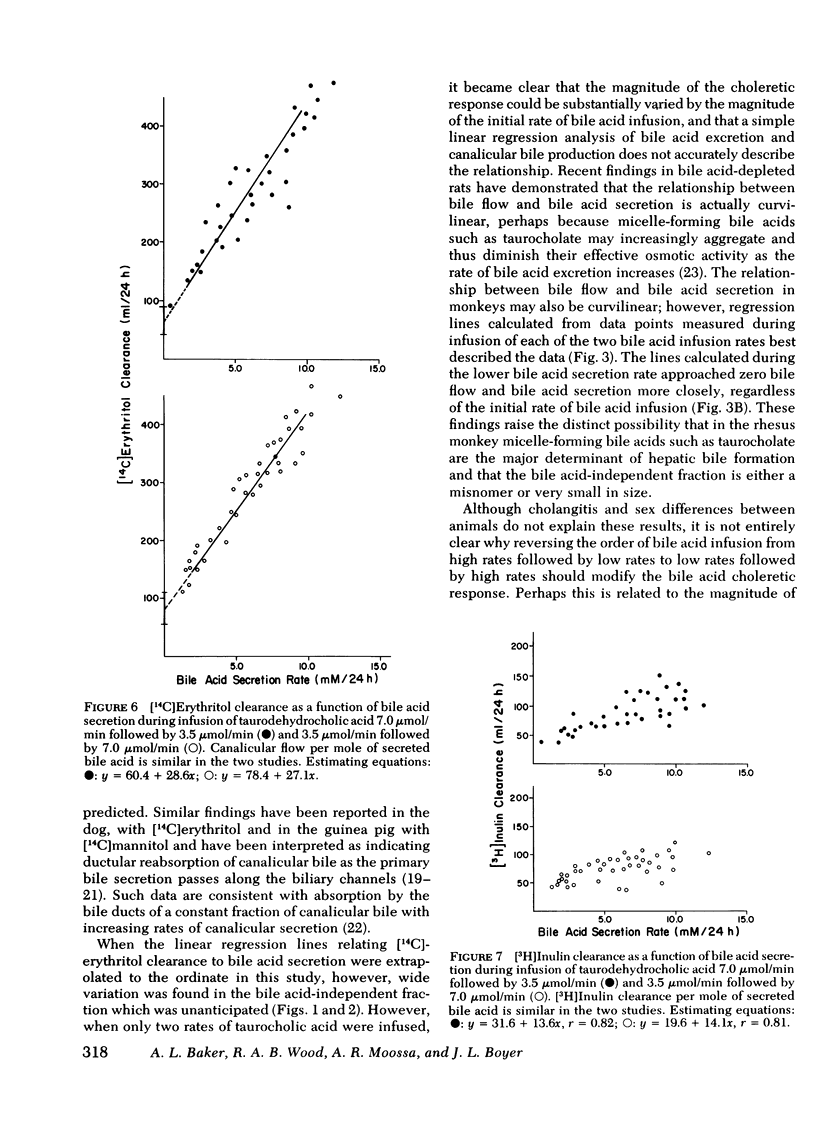
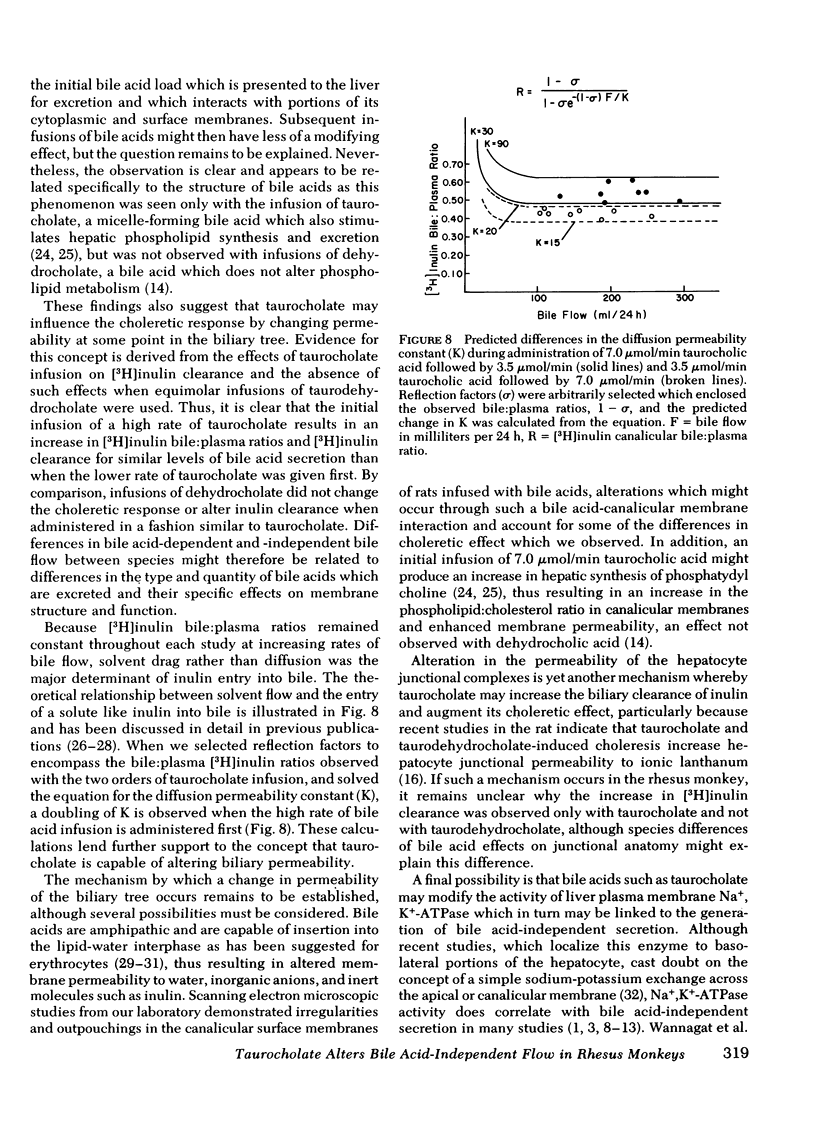

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balabaud C., Kron K. A., Gumucio J. J. The assessment of the bile salt-nondependent fraction of canalicular bile water in the rat. J Lab Clin Med. 1977 Feb;89(2):393–399. [PubMed] [Google Scholar]
- Balint J. A., Beeler D. A., Kyriakides E. C., Treble D. H. The effect of bile salts upon lecithin synthesis. J Lab Clin Med. 1971 Jan;77(1):122–133. [PubMed] [Google Scholar]
- Berthelot P., Erlinger S., Dhumeaux D., Preaux A. M. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970 Sep;219(3):809–813. doi: 10.1152/ajplegacy.1970.219.3.809. [DOI] [PubMed] [Google Scholar]
- Blitzer B. L., Boyer J. L. Cytochemical localization of Na+, K+-ATPase in the rat hepatocyte. J Clin Invest. 1978 Nov;62(5):1104–1108. doi: 10.1172/JCI109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Bloomer J. R. Canalicular bile secretion in man. Studies utilizing the biliary clearance of (14C)mannitol. J Clin Invest. 1974 Oct;54(4):773–781. doi: 10.1172/JCI107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971 Oct;221(4):1156–1163. doi: 10.1152/ajplegacy.1971.221.4.1156. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Reno D. Properties of (Na+ plus K+)-activated ATPase in rat liver plasma membranes enriched with bile canaliculi. Biochim Biophys Acta. 1975 Aug 5;401(1):59–72. doi: 10.1016/0005-2736(75)90341-7. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Diloy Puray M., Lando P., Greenverg M. S. An analysis of lipoproteins, bile acids, and red cell membranes associated with target cells and spur cells in patients with liver disease. J Clin Invest. 1972 Dec;51(12):3182–3192. doi: 10.1172/JCI107145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Garcia F. A., Trey C. X. The effect of lithocholic acid on red cell membranes in vivo. J Lab Clin Med. 1972 Jan;79(1):7–18. [PubMed] [Google Scholar]
- Deuticke B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta. 1968 Dec 10;163(4):494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D., Berthelot P., Dumont M. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol. 1970 Aug;219(2):416–422. doi: 10.1152/ajplegacy.1970.219.2.416. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Bile formation in guinea pigs: analysis with inert solutes of graded molecular radius. Am J Physiol. 1968 Jul;215(1):56–62. doi: 10.1152/ajplegacy.1968.215.1.56. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Mechanisms of hepatic bile formation. Annu Rev Physiol. 1977;39:323–347. doi: 10.1146/annurev.ph.39.030177.001543. [DOI] [PubMed] [Google Scholar]
- Forker E. L. The effect of estrogen on bile formation in the rat. J Clin Invest. 1969 Apr;48(4):654–663. doi: 10.1172/JCI106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory D. H., Vlahcevic Z. R., Schatzki P., Swell L. Mechanism of secretion of biliary lipids. I. Role of bile canalicular and microsomal membranes in the synthesis and transport of biliary lecithin and cholesterol. J Clin Invest. 1975 Jan;55(1):105–114. doi: 10.1172/JCI107900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio J. J., Accatino L., Macho A. M., Contreras A. Effect of phenobarbital on the ethynyl estradiol-induced cholestasis in the rat. Gastroenterology. 1973 Oct;65(4):651–657. [PubMed] [Google Scholar]
- Javitt N. B., Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid exeretion. J Clin Invest. 1968 May;47(5):1002–1014. doi: 10.1172/JCI105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. Taurolithocholate-induced cholestasis: taurocholate but not dehydrocholate, reverses cholestasis and bile canalicular membrane injury. Gastroenterology. 1977 Jul;73(1):120–128. [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. The effect of thyroid hormone on bile salt-independent bile flow and Na+, K+ -ATPase activity in liver plasma membranes enriched in bile canaliculi. J Clin Invest. 1976 Apr;57(4):1009–1018. doi: 10.1172/JCI108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Elias E., Boyer J. L. Bile formation in the rat: the role of the paracellular shunt pathway. J Clin Invest. 1978 Dec;62(6):1375–1385. doi: 10.1172/JCI109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemchausky B. A., Layden T. J., Boyer J. L. Effects of chronic choleretic infusions of bile acids on the membrane of the bile canaliculus. A biochemical and morphologic study. Lab Invest. 1977 Mar;36(3):259–267. [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G. The secretory characteristics of dehydrocholate in the dog: comparison with the natural bile salts. J Physiol. 1976 Oct;261(2):337–357. doi: 10.1113/jphysiol.1976.sp011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandi D. Canalicular bile production in man. Eur J Clin Invest. 1975 Feb;5(1):1–6. doi: 10.1111/j.1365-2362.1975.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Simon F. R., Sutherland E., Accatino L. Stimulation of hepatic sodium and potassium-activated adenosine triphosphatase activity by phenobarbital. Its possible role in regulation of bile flow. J Clin Invest. 1977 May;59(5):849–861. doi: 10.1172/JCI108707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg S. M., Ilson R. G., Siminovitch K. A., Brenner D., Palaheimo J. E. Analysis of the components of bile flow in the rhesus monkey. Am J Physiol. 1975 Jan;228(1):115–121. doi: 10.1152/ajplegacy.1975.228.1.115. [DOI] [PubMed] [Google Scholar]
- Wannagat R. J., Adler R. D., Ockner R. K. Bile acid-induced increase in bile acid-independent flow and plasma membrane NaK-ATPase activity in rat liver. J Clin Invest. 1978 Feb;61(2):297–307. doi: 10.1172/JCI108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., Ross E. D., Bradley S. E. Canalicular bile production in dogs. Am J Physiol. 1968 Apr;214(4):866–874. doi: 10.1152/ajplegacy.1968.214.4.866. [DOI] [PubMed] [Google Scholar]
- Wood R. A., Baker A. L., Hall A. W., Boyer J. L., Moossa A. R. Evaluation of a new monkey model for the repeated study of bile secretory physiology. Ann Surg. 1977 Mar;185(3):349–355. doi: 10.1097/00000658-197703000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]


