Summary
Sleep-disordered breathing is an increasingly recognized disorder that is particularly prevalent among stroke patients. Obstructive sleep apnea, a form of sleep-disordered breathing, is associated with multiple major stroke risk factors but is also an independent risk factor for stroke. In addition, untreated sleep apnea is associated with poor functional outcome after stroke. Sleep apnea is amenable to treatment and should be considered a modifiable stroke risk factor, though long-term compliance remains a major barrier. A better understanding of the relationship between sleep apnea and stroke may prompt providers to pursue the early diagnosis and treatment of underlying sleep-disordered breathing to both improve the chance of recovery from stroke in the short term and to reduce the risk of recurrent stroke in the long term.
Sleep apnea is a common, yet often underrecognized, risk factor for incident and recurrent stroke and may adversely affect stroke recovery. Obstructive sleep apnea (OSA) is repeated narrowing or closure of the upper airway during sleep leading to transient hypoxemia, arousals, and apneas. OSA is also highly associated with cardiovascular diseases including hypertension, atrial fibrillation, heart failure, and diabetes. Continuous positive airway pressure (CPAP) is an effective treatment for OSA. Diagnosing and treating OSA with CPAP typically occurs in only a subset of stroke survivors1 but should be more universally considered. Evidence is accumulating that CPAP is among a handful of interventions demonstrated to improve functional outcome after stroke, a leading cause of long-term disability in the United States.
The subacute inpatient management of patients with stroke involves a thorough evaluation of stroke etiology, seeking opportunities for secondary stroke prevention and an assessment of disability and therapy needs. Clinicians may be faced with an array of vascular diagnostic tests, cardiac monitoring, and imaging results to assist in determining stroke etiology. Yet the cause of stroke may remain unclear or complex, involving interplay among numerous risk factors. Depending on the postulated stroke subtype, prevention efforts typically focus on the evaluation and treatment of hypertension, hyperlipidemia, diabetes, cigarette smoking, alcohol consumption, atrial fibrillation, extracranial carotid disease, or some combination of these factors.2 Current guidelines for stroke prevention do not typically include provisions for the evaluation or treatment of OSA.
Epidemiology of OSA
Sleep apnea is a common disorder, affecting up to 20% of the general population.3 With 2 of the greatest risk factors for OSA being age and obesity, the prevalence should increase with time as the population ages and waistlines bulge. Sleep apnea severity is measured by the apnea-hypopnea index (AHI). The AHI is a measure of the number of apneas, complete cessation of airflow, and the number of hypopneas, reduction in airflow with desaturation or arousal, per hour of sleep. An AHI ≥5 events/hour with symptoms of daytime somnolence, snoring, or waking with gasping or choking or an AHI ≥15 events/hour regardless of symptoms is considered diagnostic of OSA. The prevalence of sleep apnea is high in stroke patients—estimated to be between 50% and 70%.4 The most common type of sleep-disordered breathing found after stroke or TIA is OSA. It may predate the stroke, worsen during the acute stage, and persist after the acute phase.
Case 1
A 74-year-old man was admitted to the hospital for a 3-day history of worsening dizziness, imbalance, and nausea. He had a long history of atrial fibrillation, hyperlipidemia, diabetes, and hypertension, all of which had been well controlled after a prior posterior circulation ischemic stroke. Medications on admission included both warfarin and aspirin. Brain MRI revealed several punctate infarcts within the pons and cerebellum (figure 1, A and B). Conventional catheter angiogram showed luminal irregularities involving the vertebral arteries and basilar artery, consistent with atherosclerotic narrowing (figure 1C). The patient's symptoms resolved quickly. However, over the next 6 months, he reported persistent difficulty concentrating and worsening daytime sleepiness. He underwent a polysomnogram (PSG), which revealed severe OSA with an AHI of 56 events/hour and a nadir oxygen saturation of 79%. Treatment with CPAP was initiated, his symptoms improved, and his AHI normalized. The patient had no further stroke symptoms.
Figure 1. Imaging in case 1.
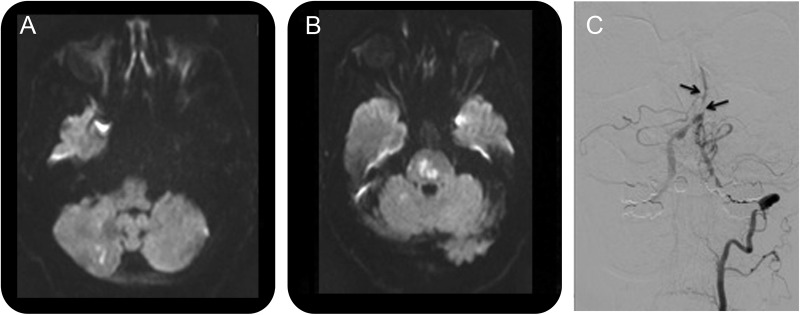
(A) Diffusion-weighted MRI demonstrates a right cerebellar infarct and (B) pontine infarcts. (C) Catheter angiogram with a left vertebral injection reveals poor flow and multifocal narrowing of the basilar artery (black arrows).
This case illustrates a patient with recurrent stroke despite optimized traditional vascular risk factors and aggressive antithrombotic therapy. The evaluation and treatment of OSA represents another area of risk factor modification that has potentially contributed to a successful secondary prevention strategy.
OSA and stroke: Shared risk factors vs independent association
The relationship be tween OSA and stroke is a complex one with shared risk factors as well as a likely independent association. Several studies have established OSA as a strong risk factor for hypertension, one of the leading risk factors for stroke.5,6 Studies have linked OSA to other major stroke risk factors, including insulin resistance,7 coronary artery disease, heart failure, and arrhythmias.8,9 Individuals with OSA have a fourfold increased risk of atrial fibrillation and a two- to threefold higher risk of other complex arrhythmias, even after adjustment for potential confounders.8 Sleep apnea has also been suggested as an independent risk factor for stroke.10–12 Postulated mechanisms of OSA as an independent risk factor for stroke include tachyarrhythmias related to sympathetic activation; impaired cerebral hemodynamics as a consequence of blood pressure fluctuations; enhanced inflammation and oxidative stress associated with hypoxemia, including evidence for OSA activating nuclear factor kappa-B-mediated inflammatory pathways leading to systemic inflammation; promotion of atherosclerosis and thrombosis; abnormal coagulation markers, including plasma fibrinogen levels and platelet reactivity; and increased right-to-left shunting through a patent foramen ovale.13–15
OSA as a predictor of poor outcome after stroke
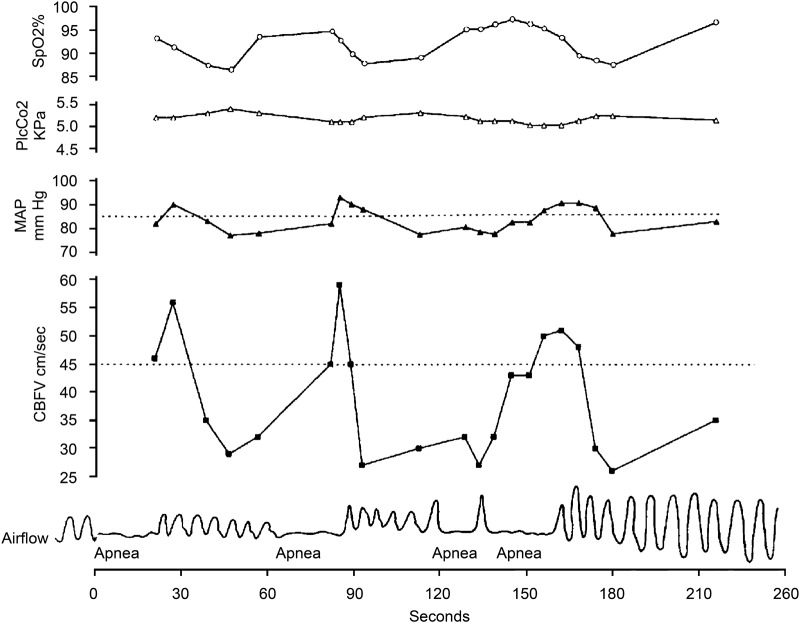
If sleep apnea increases the risk of stroke, either directly or indirectly, untreated patients with comorbid OSA may have worse functional outcomes and higher mortality after acute stroke. Several observational studies suggest that OSA is a predictor of poor functional outcome after stroke, increasing the likelihood of dependency16–18 and poststroke mortality.19,20 Potential mechanisms of OSA contributing to poor neurologic recovery include direct effects of reduced cerebral blood flow and modulation of blood pressure and oxygen saturation associated with apneic episodes, resulting in further neurologic injury due to a compromise in perfusion to the ischemic penumbra. Mean arterial pressure and cerebral blood flow velocity increase during obstructive apneas and then drop precipitously with apnea termination.e1 In patients with repetitive apneas, the subsequent hemodynamic changes can be profound (figure 2). A greater than 50% reduction in middle cerebral artery blood flow velocity has also been demonstrated by transcranial Doppler studies during obstructive apneas and hypopneas.e2 The relevant drop in systemic blood pressure and cerebral perfusion accompanied by an overall impairment of cerebral autoregulation may have deleterious consequences. Systemic blood pressure fluctuations during the acute phase of ischemic stroke, whether elevated or decreased, have been shown to portend poor outcome, with early neurologic deterioration and an increased early and late mortality.e3,e4 In the subacute setting of the recovering stroke patient, such as in our described patient, untreated OSA can also cause impaired cognitive function, decreased concentration, and excessive daytime sleepiness, prolonging hospitalizations and compromising rehabilitation participation.e5,e6 One study demonstrated that stroke patients with sleep apnea had longer hospitalizations and rehabilitation stays than those without sleep apnea.18
Figure 2. Physiologic recordings in a patient with obstructive sleep apnea.
Simultaneous recordings of arterial oxygen saturation (SaO2), transcutaneous arterial PCO2 (PtcCO2), mean arterial blood pressure (MAP), cerebral blood flow velocity (CBFV), and airflow. Note the fluctuations in MAP and CBFV away from baseline (dashed lines) during and following apnea. With repetitive apneas, as demonstrated here, CBFV remains below baseline for the majority of the recording. (Reprinted with permission of the American Thoracic Society. Copyright© 2012 American Thoracic Society. From: Balfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med 1994;150:1587–1591.)e1
Diagnosis of OSA after stroke
Given the ubiquity of OSA after stroke, clinical providers face the challenge of finding a practical and cost-effective way of evaluating stroke survivors for OSA. Clinical risk stratification for OSA using questionnaires has proven ineffective.e7,e8 In-laboratory PSG remains the accepted standard for the diagnosis and management of OSA. A full-night PSG study can simultaneously record multiple physiologic variables, including a limited EEG, electrooculogram (EOG), chin and leg EMG, airflow via thermal and pressure changes, oximetry, abdominal and thoracic movement, snoring, and ECG. Data from EEG, EOG, and EMG leads are used to determine sleep architecture and efficiency and identify arousals and limb movements. A dedicated technician is required to administer and score the in-laboratory PSG, making it more expensive and less feasible in acute stroke inpatients.
A wide variety of portable monitors are available, ranging from single-channel recorders to units that record the full montage of signals recorded during an in-laboratory PSG. Portable devices have been validated in prior clinical research studiese9 though data collected from inpatient unattended PSG compared to an attended in-laboratory PSG in stroke patients has not been extensively evaluated. The possible cost-effectiveness of portable device testing and its potential to enable patient access to early diagnosis and treatment have made this approach appealing for ambulatory and in-hospital diagnosis. Out-of-center sleep testing using portable devices allows patients to remain on the acute care floor while undergoing a study with more rapid results at less cost. However, portable devices are less sensitive and specific for the diagnosis of OSA than a formal PSG and more likely to yield inadequate data. The feasibility of the routine early use of portable devices among stroke patients has not been studied, although it may be a cost-effective alternative.
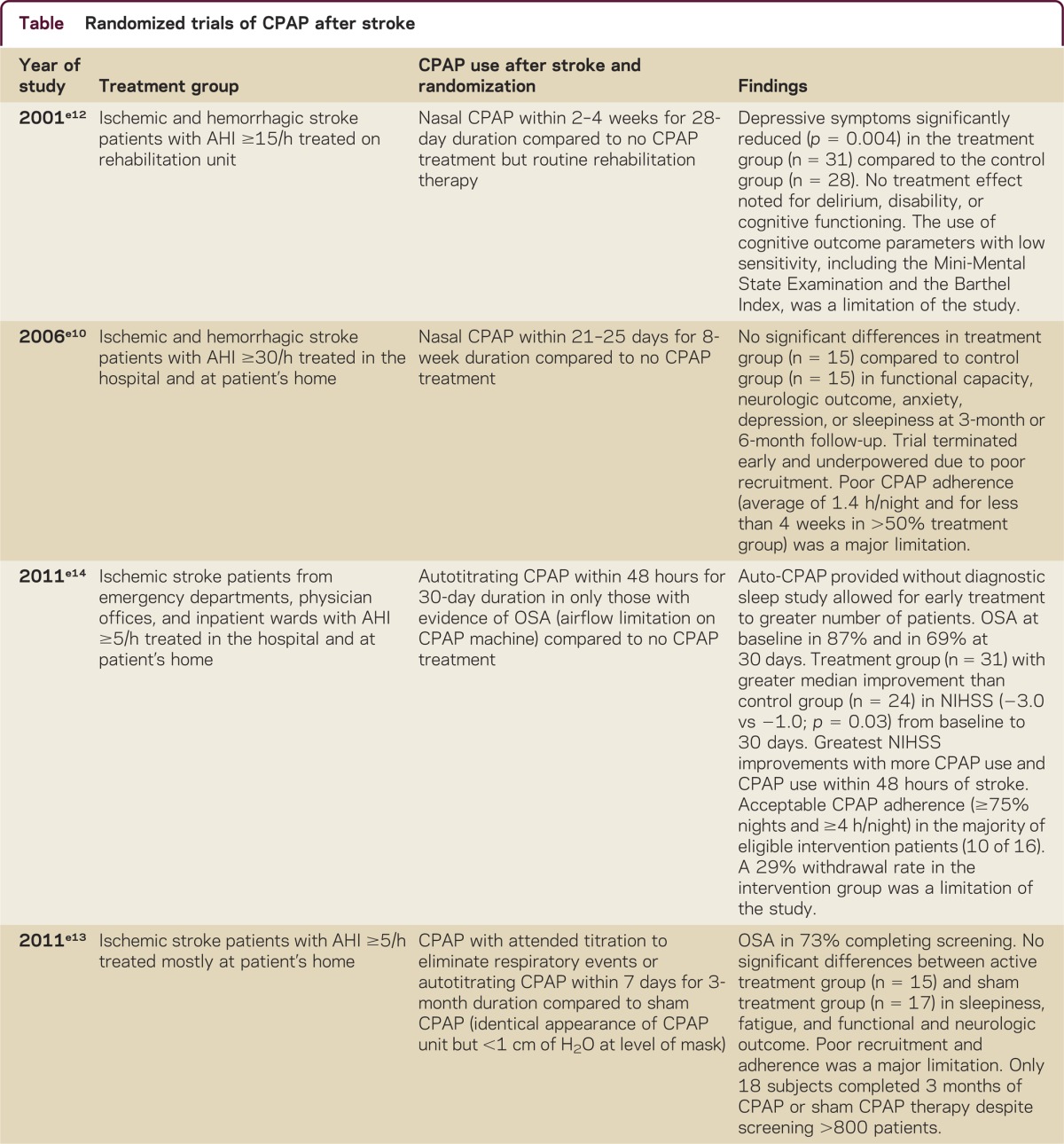
Randomized trials of CPAP after stroke (table) suggest identifying OSA may be best accomplished with portable devices.e10–e15 The SATS triale13 proposed screening with full polysomnography, but switched to portable monitoring early in the study due to intolerance and logistical challenges of coordinating an in-laboratory PSG during the acute stroke period. Clinicians can reasonably pursue portable device testing with the goal of increasing patient tolerance and accomplishing timelier testing.
Table.
Randomized trials of CPAP after stroke

Treatment of OSA after stroke
Treatment with CPAP has the unique potential to improve the recovery from stroke and to reduce the risk of recurrent stroke both indirectly through better control of multiple modifiable risk factors and directly through a variety of proposed mechanisms. It is an effective treatment for OSA, reducing OSA-associated hypertension, atrial fibrillation, cardiovascular morbidity and mortality, exclusive of stroke, and reducing daytime somnolence. CPAP provides a constant, positive pressure to the airway throughout the respiratory cycle, serving to splint open the upper airways to prevent narrowing or collapse. The impact of early treatment of OSA with CPAP on neurologic recovery after stroke is just beginning to be studied. Research studies on whether CPAP improves outcome from stroke are more amenable to randomized trials than studies of whether CPAP reduces the risk of recurrent stroke. Preliminary randomized trials of CPAP therapy after ischemic stroke have shown an improvement in stroke impairment scales, depressive symptoms, motor recovery, sleepiness, and the time until the appearance of cardiovascular events (table).e11,e12,e14–e16 Other randomized trials of CPAP have shown no difference in functional and neurologic outcome, anxiety, depression, sleepiness, and fatigue.e10,e13 Limitations of some of these studies (table) may be related to the lack of sensitivity of chosen patient outcome parameters as well as poor recruitment and retention.
Although results across the studies appear inconsistent, a few conclusions may guide neurologists considering whether to initiate CPAP in a stroke patient with OSA. First, the studies that have shown increased CPAP adherence have demonstrated greater improvements in stroke symptom recovery,e15,e16 while those limited by poor recruitment or adherence have been underpowered to show any significant CPAP benefit.e10,e13 Further, the reasons for poor treatment adherence often included patient selection factors, including advanced agee11,e15 and stroke severity,e16 as well as stroke or hospitalization-related deficits, including delirium, cognitive impairment, neglect, and depression.e10,e12,e15 Finally, high rates of early CPAP use have been demonstrated when study personnel regularly encouraged treatment adherencee14 or when nurses were trained to administer CPAP treatment.e11,e16 Thus, a close examination by clinicians of patient motivation, stroke-related impairments, and caregiver or nursing assistance with CPAP may lead to a better selection of patients likely to benefit from treatment. Given the high prevalence of OSA after stroke, another practical approach for future researchers that was utilized in recent studiese14,e15 is to treat all stroke survivors with CPAP using autotitration during the acute hospitalization to allow early treatment for a greater number of patients.
Treatment with CPAP may also reduce the risk of recurrent stroke in patients with OSA directly. An observational study suggested that treating stroke patients with CPAP prevented recurrent stroke.e17 Stroke patients with moderate to severe sleep apnea (AHI ≥20 events/hour) who did not tolerate CPAP showed an increased adjusted incidence of nonfatal cardiovascular events, especially for new ischemic stroke, during a 7-year follow-up period (hazard ratio 2.87; 95% confidence interval 1.11–7.71) compared to those who tolerated CPAP and compared to patients with mild disease (AHI 10–19 events/hour) or without OSA (AHI <10 events/hour). However, as CPAP therapy was not randomized, this observational study is limited by possible confounding. Another trial that randomized patients with an AHI ≥20 events/hour to conventional stroke treatment plus CPAP or conventional treatment without CPAP revealed a delayed time until appearance of cardiovascular events in the CPAP group, though no difference at 2 years in mortality or new cardiovascular events, including stroke recurrence and cardiovascular death, was noted.e11
CPAP and the challenges of adherence
If CPAP proves effective in improving recovery from stroke or preventing recurrent stroke, its efficacy would be limited by poor acceptance and adherence, as has been demonstrated in multiple randomized trials.e12,e13 Poor treatment adherence, 12% in one studye18 and 15% in another,19 is a major limitation among stroke patients due to overall poor tolerance of CPAP. Compared to the general OSA population, patients with acute stroke are typically older with more functional disability. Stroke patients may also have more difficulty tolerating the CPAP mask, due to underlying extremity or facial paresis, dysphagia, aphasia, or neglect. Other well-established stroke preventative measures also suffer from poor adherence though not to the degree that is seen with CPAP. In a study of patients with ischemic stroke or TIA, approximately one-third of patients discontinued one or more secondary prevention medications (antithrombotics, antihypertensive, lipid-lowering, and diabetes medications) within 1 year of hospital discharge.e19 Initial acceptance and adherence may be greater when CPAP is initiated in the hospital under the guidance of respiratory therapists and nurses. Early CPAP treatment has been shown to be feasible as early as the first night after stroke without neurologic deterioration or an increase in nursing workload, and several recent trials demonstrated improved CPAP adherence with early initiation with acceptance rates ranging from 40% to 88%.e15,e16 Efforts to improve CPAP adherence during hospitalization may include patient education, mask and humidity adjustments, and encouragement. Close monitoring of CPAP tolerance while patients are hospitalized, possibly during their inpatient stroke rehabilitation, may also influence long-term adherence.
OSA: An underrecognized and modifiable stroke risk factor
Evidence that sleep apnea is a risk factor for stroke is strong and its prevalence among stroke survivors is high. After stroke, OSA may be an underappreciated risk factor for both poor functional outcome and stroke recurrence. Until better evidence accumulates, providers are left having to decide how aggressively to pursue a diagnosis of OSA and treatment with CPAP in patients who have had a stroke. One conclusion could be that all stroke survivors should be screened for sleep apnea and should have it treated if it is found. Compliance is an issue for all interventions aimed at secondary stroke prevention. Does that knowledge mean that we should not try to treat hypertension when we find it in a patient because patients are often not compliant with their antihypertensive medications? Current studies suggest that treatment with CPAP may be beneficial in patients after stroke. When to treat sleep apnea in patients after stroke is in many ways the main issue. Treatment would ideally start during hospitalization or as soon as the patient can tolerate CPAP to maximize effects on recovery and to improve long-term CPAP adherence and tolerability. Given the potential short- and long-term benefits and the low risk of the intervention, early evaluation and treatment for OSA in this population seems easily justified.
Supplementary Material
Footnotes
Supplemental data: neurology.org/cp
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/cp for full disclosures.

REFERENCES
- 1.Skolarus LE, Lisabeth LD, Morgenstern LB, Burgin W, Brown DL. Sleep apnea risk among Mexican American and non-Hispanic white stroke survivors. Stroke 2012;43:1143–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276 [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009;108:246–249 [PMC free article] [PubMed] [Google Scholar]
- 4.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology 2009;73:1313–1322 [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384 [DOI] [PubMed] [Google Scholar]
- 6.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA 2000;283:1829–1836 [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530 [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25 [DOI] [PubMed] [Google Scholar]
- 9.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006;173:910–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053 [DOI] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 13.Brown DL. Sleep disorders and stroke. Semin Neurol 2006;26:117–122 [DOI] [PubMed] [Google Scholar]
- 14.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol 2004;3:333–342 [DOI] [PubMed] [Google Scholar]
- 15.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath 2006;10:43–50 [DOI] [PubMed] [Google Scholar]
- 16.Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med 2009;10:717–719 [DOI] [PubMed] [Google Scholar]
- 17.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke 1996;27:252–259 [DOI] [PubMed] [Google Scholar]
- 18.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep 2003;26:293–297 [DOI] [PubMed] [Google Scholar]
- 19.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006;37:967–972 [DOI] [PubMed] [Google Scholar]
- 20.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med 2008;168:297–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.