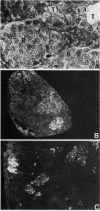
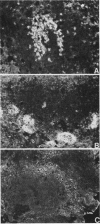
Abstract
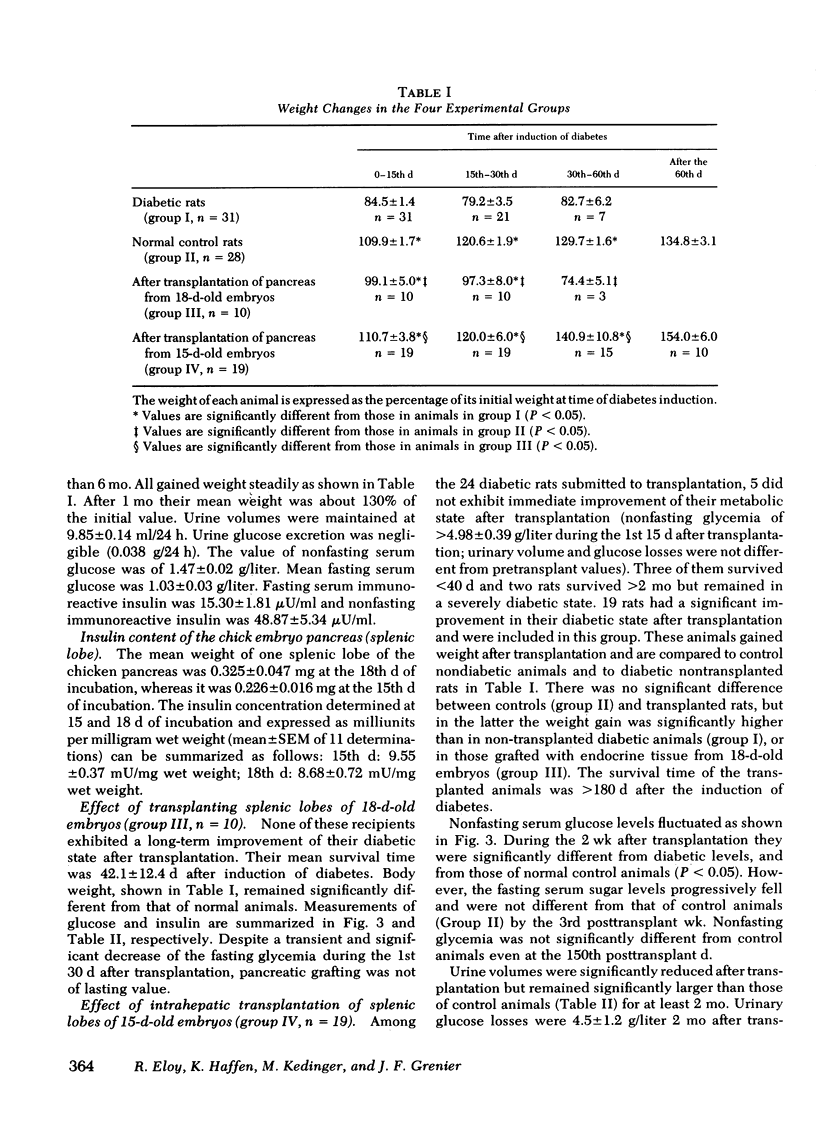
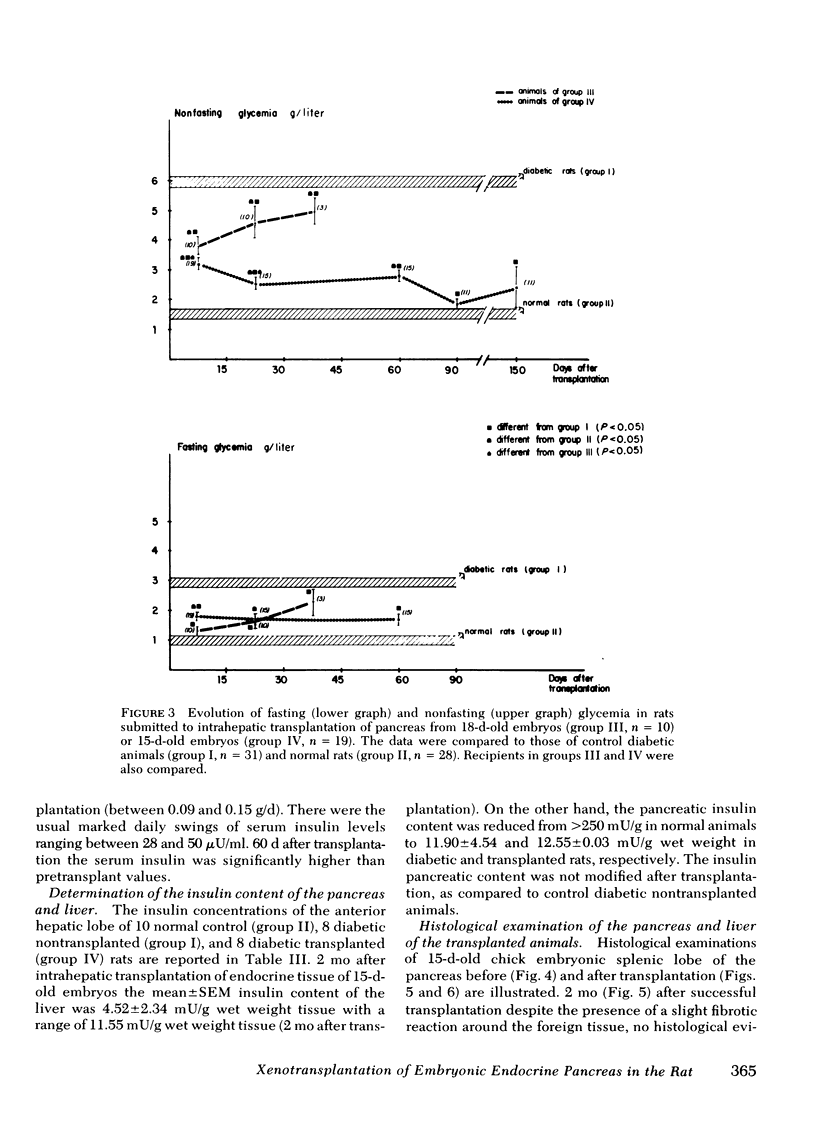
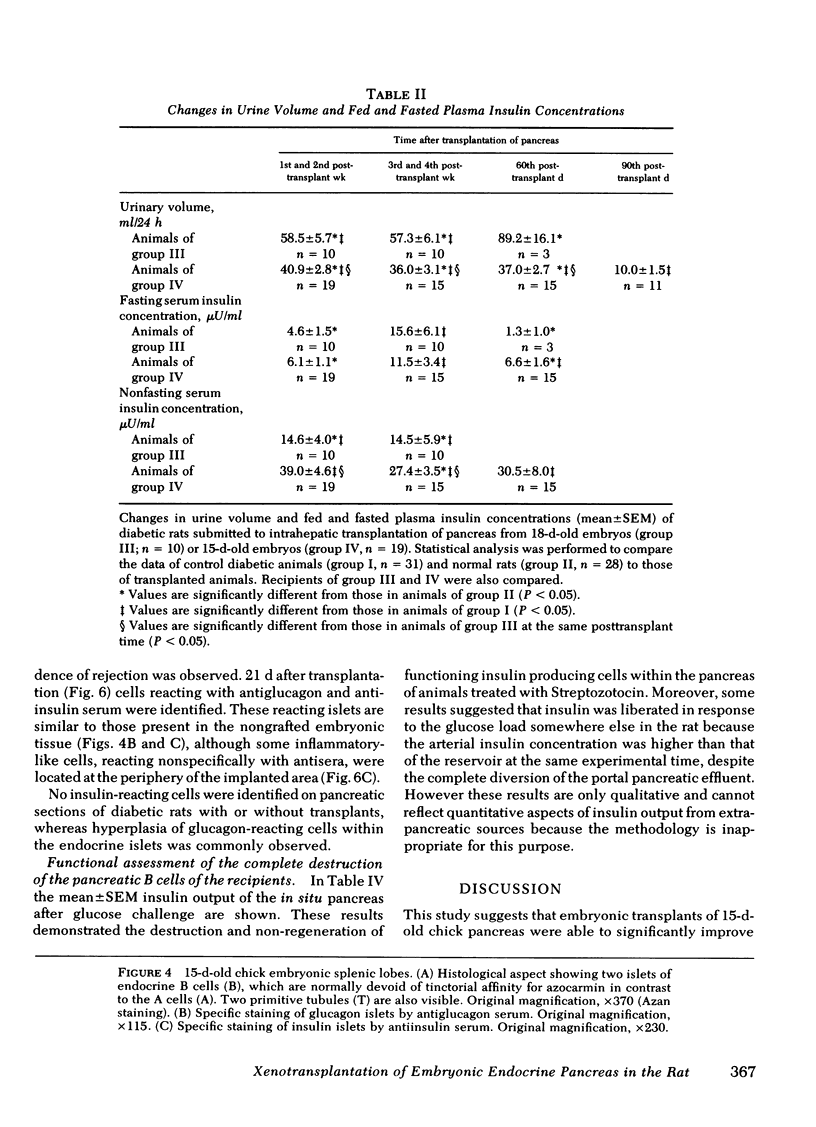
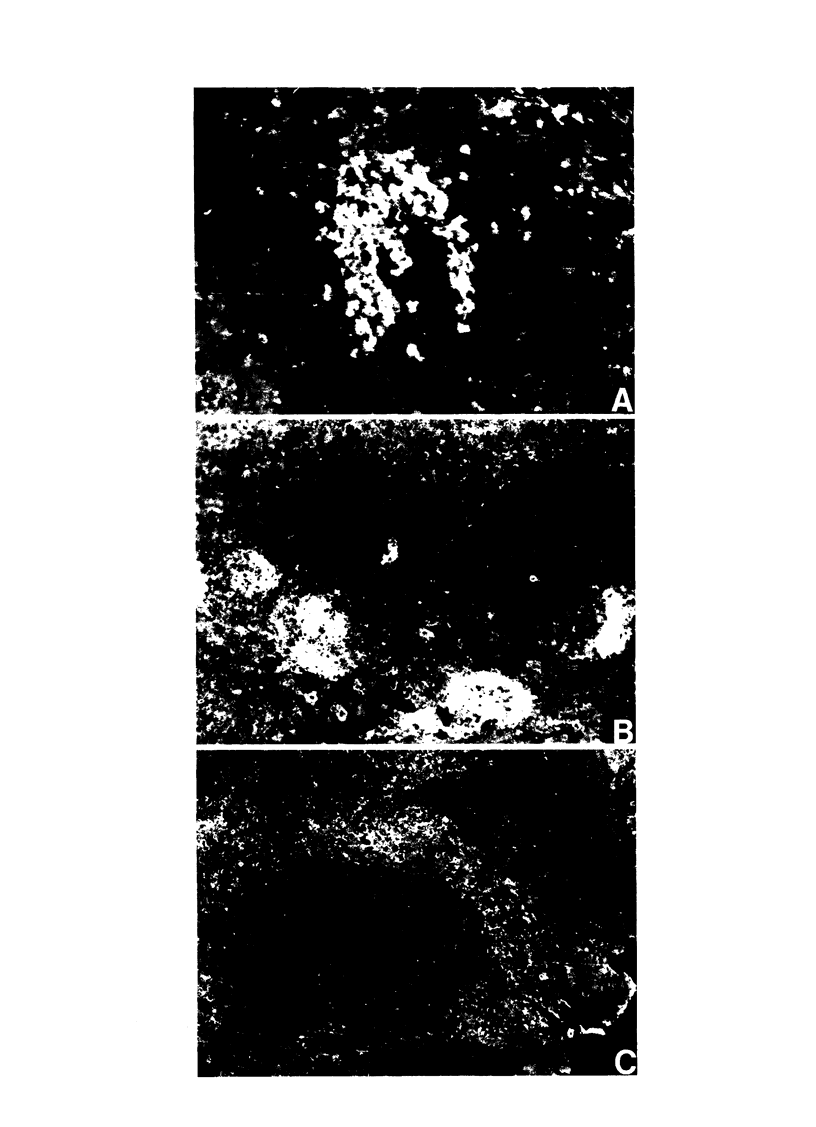
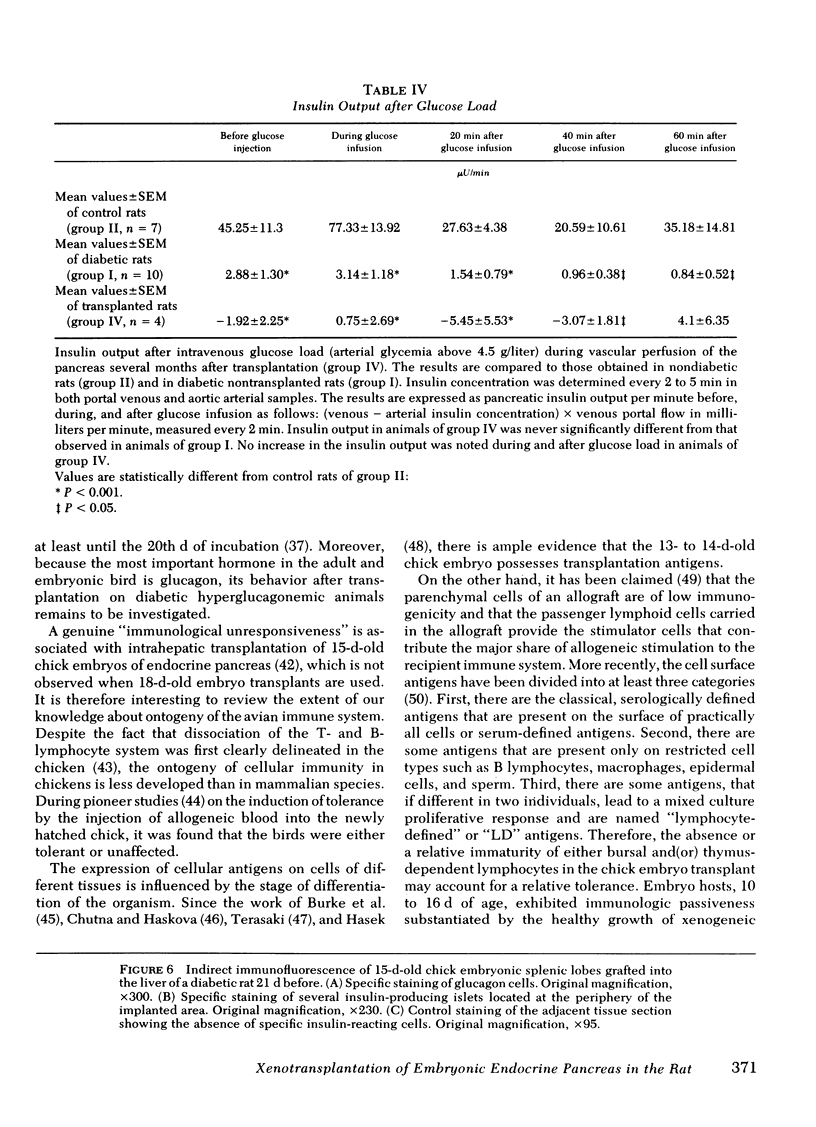
The effectiveness of xenogeneic embryonic tissue in the treatment of experimental diabetes has been investigated in rats. The splenic lobes (80) of 15- to 18-d-old chick embryos, composed almost exclusively of endocrine tissue, were implanted directly into the hepatic parenchyma of the rat recipient. The biochemical and metabolic changes in the recipients suggest that embryonic transplants of 15-d-old chick pancreases were able to significantly improve, for a prolonged period of time (18 mo), the diabetic state of nonimmunosuppressed rats. None of the recipients of 18-d-old embryos splenic lobes exhibited a long-term improvement of the diabetic state after transplantation. The complete destruction of the pancreatic B cells of the recipients was assessed by: (a) immunocytochemical investigations of the recipient's pancreas, (b) measurement of insulin in the liver and pancreas of the recipients and (c) in situ vascular perfusion of their pancreas submitted to high glucose challenge. The results suggest that pancreatic tissue of the 15-d-old embryos is immunologically immature lacking one or several lymphocyte subsets implicated in the afferent lood of "non-self" recognition.
Full text
PDF
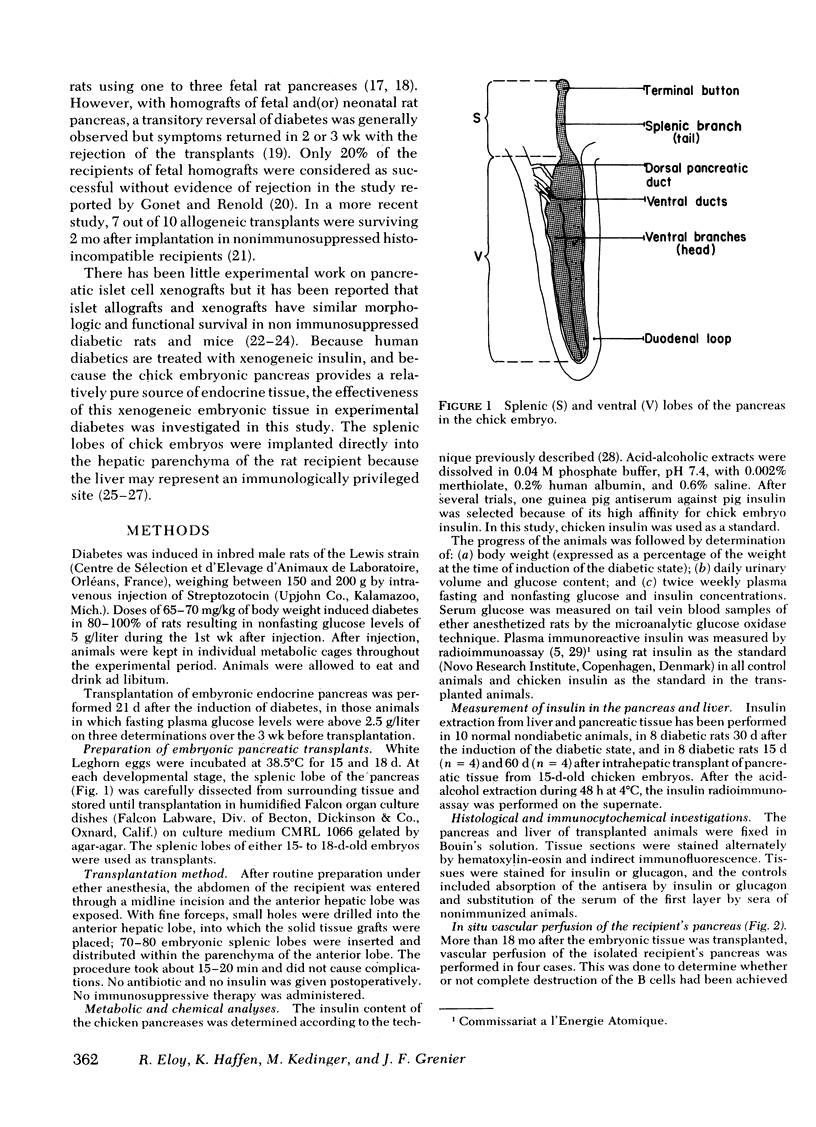
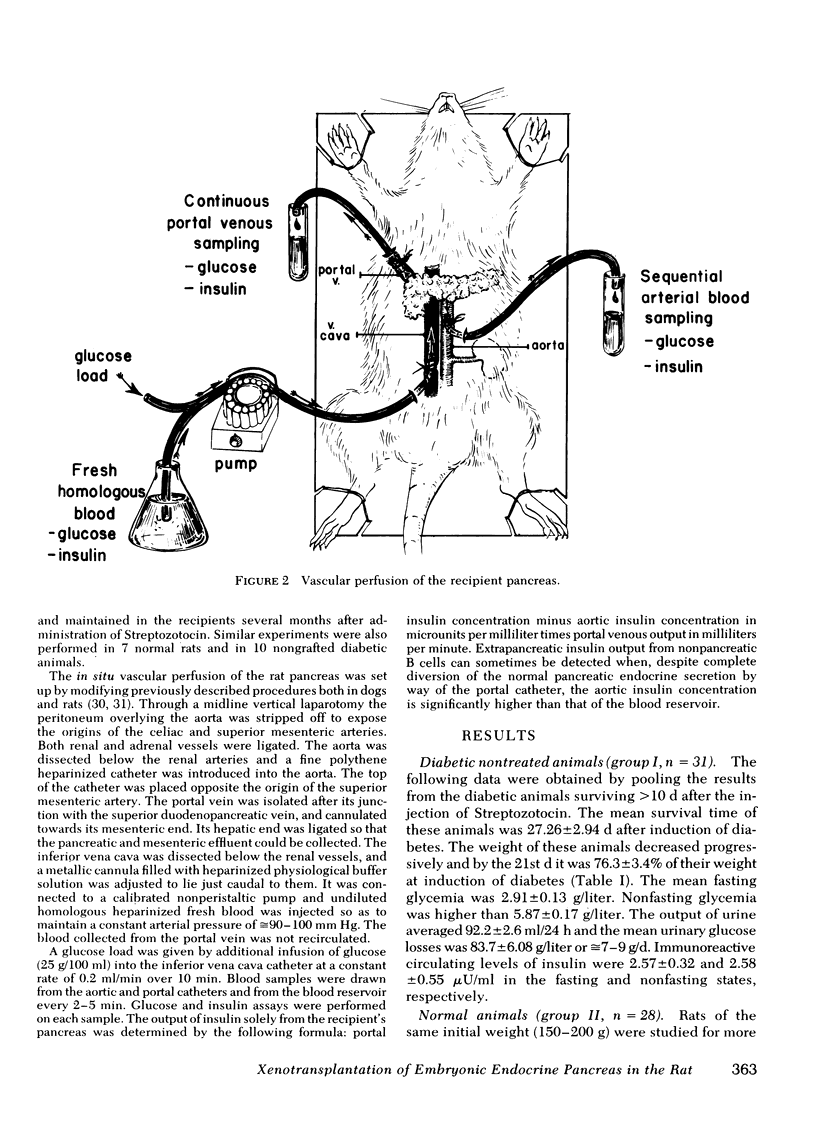




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison R. N., Ciaccio E. I., Glitzer M. S., Cassaro J. A., Pruss M. P. Light and electron microscopy of lesions in rats rendered diabetic with streptozotocin. Diabetes. 1967 Jan;16(1):51–56. doi: 10.2337/diab.16.1.51. [DOI] [PubMed] [Google Scholar]
- BROWNING H., RESNIK P. Homologous and heterologous transplantation of pancreatic tissue in normal and diabetic mice. Yale J Biol Med. 1951 Nov;24(2):140–152. [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Ballinger W. F., Lacy P. E. Transplantation of intact pancreatic islets in rats. Surgery. 1972 Aug;72(2):175–186. [PubMed] [Google Scholar]
- Barker C. F. Transplantation of the islets of Langerhans and the histocompatibility of endocrine tissue. Diabetes. 1975 Aug;24(8):766–775. doi: 10.2337/diab.24.8.766. [DOI] [PubMed] [Google Scholar]
- Benzo C. A., Green T. D. Functional differentiation of the chick endocrine pancreas: insulin storage and secretion. Anat Rec. 1974 Nov;180(3):491–496. doi: 10.1002/ar.1091800308. [DOI] [PubMed] [Google Scholar]
- Brown J., Molnar I. G., Clark W., Mullen Y. Control of experimental diabetes mellitus in rats by transplantation of fetal pancreases. Science. 1974 Jun 28;184(4144):1377–1379. doi: 10.1126/science.184.4144.1377. [DOI] [PubMed] [Google Scholar]
- Charles M. A., Imagawa W., Forsham P. H., Grodsky G. M. Islet transplantation into rat liver: in vitro secretion of insulin from the isolated perfused liver and in vivo glucagon suppression. Endocrinology. 1976 Mar;98(3):738–742. doi: 10.1210/endo-98-3-738. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966 Jan 1;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
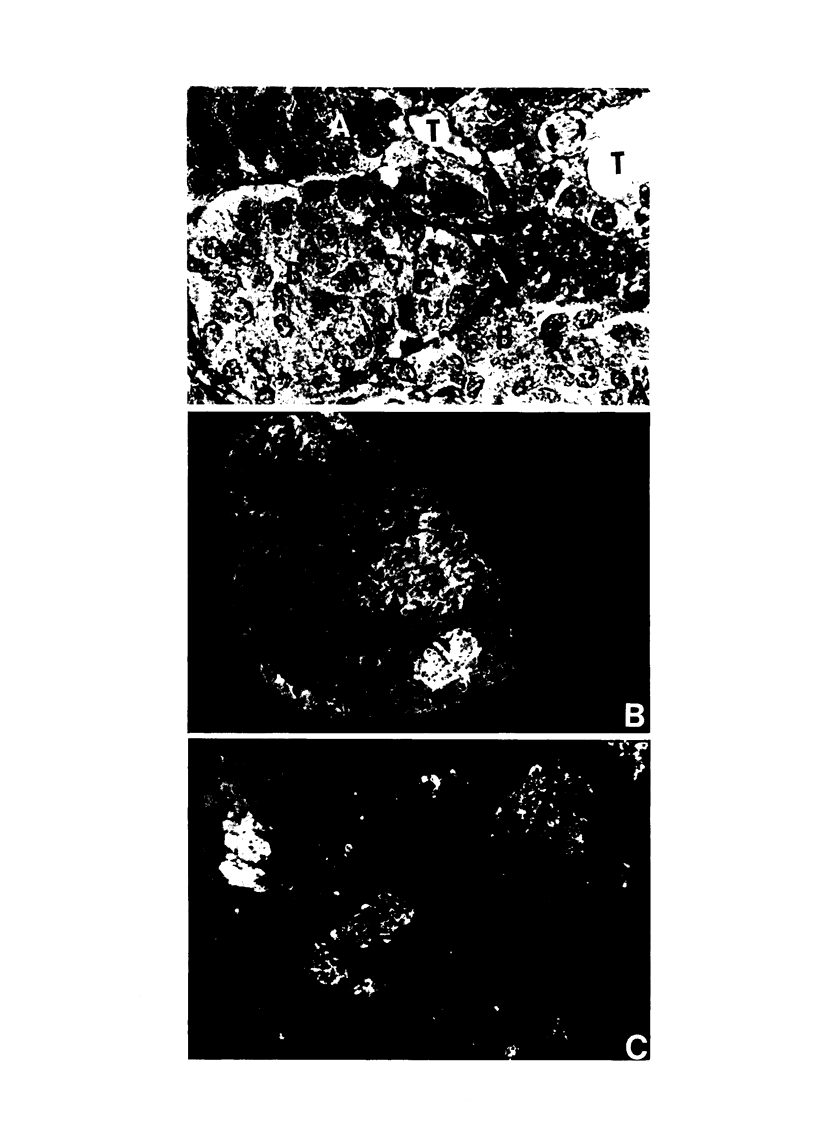
- Dieterlen-Liévre F., Beaupain D. Etude immunocytologique de la différenciation du pancréas endocrine chez l'embryon de Poulet. I. Ilots à insuline. Gen Comp Endocrinol. 1974 Jan;22(1):62–69. doi: 10.1016/0016-6480(74)90088-4. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Staskawicz M. O., Gershon R. K. Suppressor cells: dependence on assay conditions for functional activity. J Exp Med. 1976 May 1;143(5):1211–1219. doi: 10.1084/jem.143.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloy M. R., Kachelhoffer J., Pousse A., Dauchel J., Grenier J. F. Ex vivo vascular perfusion of the isolated canine pancreas. Experimental procedure, haemodynamic data and experimental applications. Eur Surg Res. 1974;6(6):341–353. doi: 10.1159/000127741. [DOI] [PubMed] [Google Scholar]
- Eloy R., Kedinger M., Garaud J. C., Haffen K., Launay J. F., Moody J., Clendinnen G., Grenier A. F. Intrahepatic transplantation of pancreatic islets in the rat. Horm Metab Res. 1977 Jan;9(1):40–46. doi: 10.1055/s-0028-1093581. [DOI] [PubMed] [Google Scholar]
- Eloy R., Raul F., Pousse A., Mirhom R., Anana A., Grenier J. F. Ex vivo vascular perfusion of the isolated rat small bowel. Importance of the intestinal brush border enzyme-release in basal conditions. Eur Surg Res. 1977;9(2):96–112. doi: 10.1159/000127930. [DOI] [PubMed] [Google Scholar]
- Eloy R., Vuitton D., Vaultier J. P., Pousse A., Grenier J. F. Decreased graft versus host reaction after intrahepatic lymphoid tissue implantation. Cell Immunol. 1976 Feb;21(2):236–242. doi: 10.1016/0008-8749(76)90052-6. [DOI] [PubMed] [Google Scholar]
- Ewald S., Freedman L., Sanders B. G. EA rosette forming lymphoid cells: distribution as to cell types and organs from chickens of different developmental stages. Cell Immunol. 1976 Apr;23(1):158–170. doi: 10.1016/0008-8749(76)90179-9. [DOI] [PubMed] [Google Scholar]
- Finch D. R., Morris P. J. Passive enhancement of isolated pancreatic islet allografts. Transplantation. 1976 Nov;22(5):508–512. doi: 10.1097/00007890-197611000-00015. [DOI] [PubMed] [Google Scholar]
- GREEN H., LORINCZ A. L. The role of a natural antibody in the rejection of mouse tumor cells by the chick embryo. J Exp Med. 1957 Jul 1;106(1):111–126. doi: 10.1084/jem.106.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood R. L., Kimmel J. R., Pollock H. G. Biological characterization of chicken insulin activity in rats and domestic fowl. Endocrinology. 1968 Dec;83(6):1331–1336. doi: 10.1210/endo-83-6-1331. [DOI] [PubMed] [Google Scholar]
- Hegre O. D., Leonard R. J., Rusin J. D., Lazarow A. Transplantation of the fetal rat pancreas: quantitative morphological analysis of islet tissue growth. Anat Rec. 1976 Jun;185(2):209–221. doi: 10.1002/ar.1091850208. [DOI] [PubMed] [Google Scholar]
- Heslop B. F. Maturation of histocompatibility antigens and host reactivity in the rat. Transplant Proc. 1969 Mar;1(1):560–563. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Orci L., Pictet R., Gonet A. E., Renold A. E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967 Oct;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Dieterlen-Lièvre F., Moody A. Données quantitatives sur la teneur en inuline du pancréas embryonnaire de poulet. C R Acad Sci Hebd Seances Acad Sci D. 1972 Oct 30;275(18):2065–2068. [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Grenier J., Eloy R. In vitro culture reduces immunogenicity of pancreatic endocrine islets. Nature. 1977 Dec 22;270(5639):736–738. doi: 10.1038/270736a0. [DOI] [PubMed] [Google Scholar]
- Kemp C. B., Knight M. J., Scharp D. W., Ballinger W. F., Lacy P. E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973 Dec;9(6):486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- Kimmel J. R., Pollock H. G., Hazelwood R. L. Isolation and characterization of chicken insulin. Endocrinology. 1968 Dec;83(6):1323–1330. doi: 10.1210/endo-83-6-1323. [DOI] [PubMed] [Google Scholar]
- King D. L., Hazelwood R. L. Regulation of avian insulin secretion by isolated perfused chicken pancreas. Am J Physiol. 1976 Dec;231(6):1830–1839. doi: 10.1152/ajplegacy.1976.231.6.1830. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Jones M. A. Reactions of the graft versus host (GVH) type. Aust J Exp Biol Med Sci. 1969 Feb;47(1):17–54. doi: 10.1038/icb.1969.3. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Misko I. S., Cooley M. A. Allogeneic stimulation modulates the in vitro response of T cells to transplantation antigen. Nature. 1974 May 17;249(454):275–276. doi: 10.1038/249275a0. [DOI] [PubMed] [Google Scholar]
- Lazarow A., Wells L. J., Carpenter A. M., Hegre O. D., Leonard R. J., McEvoy R. C. The Banting Memorial Lecture 1973: Islet differentiation, organ culture, and transplantation. Diabetes. 1973 Dec;22(12):877–912. doi: 10.2337/diab.22.12.877. [DOI] [PubMed] [Google Scholar]
- Mangnall Y., Smythe A., Slater D. N., Milner G. R., Milner R. D., Taylor C. B., Fox M. Neonatal islet cell transplantation in the diabetic rat: effect on hepatic enzyme activity and glucose homeostasis. J Endocrinol. 1977 Aug;74(2):231–241. doi: 10.1677/joe.0.0740231. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Chromosome marker studies on the development of the haemopoietic system in the chick embryo. Nature. 1965 Dec 4;208(5014):956–passim. doi: 10.1038/208956a0. [DOI] [PubMed] [Google Scholar]
- Mullen Y. S., Clark W. R., Molnar I. G., Brown J. Complete reversal of experimental diabetes mellitus in rats by a single fetal pancreas. Science. 1977 Jan 7;195(4273):68–70. doi: 10.1126/science.137524. [DOI] [PubMed] [Google Scholar]
- Naber S. P., Hazelwood R. L. In vitro insulin release from chicken pancreas. Gen Comp Endocrinol. 1977 Aug;32(4):495–504. doi: 10.1016/0016-6480(77)90233-7. [DOI] [PubMed] [Google Scholar]
- Nelken D., Friedman E. A., Morse S. I., Beyer M. M. Islet of Langerhans allotransplantation in the rat. Transplant Proc. 1977 Mar;9(1):333–336. [PubMed] [Google Scholar]
- Nelken D., Morse S. I., Beyer M. M., Friedman E. A. Prolonged survival of allotransplanted islet of langerhans cells in the rat. Transplantation. 1976 Jul;22(1):74–75. doi: 10.1097/00007890-197607000-00013. [DOI] [PubMed] [Google Scholar]
- Potworowski E. F. T and B lymphocytes. Organ and age distribution in the chicken. Immunology. 1972 Aug;23(2):199–204. [PMC free article] [PubMed] [Google Scholar]
- Reckard C. R., Ziegler M. M., Barker C. F. Physiological and immunological consequences of transplanting isolated pancreatic islets. Surgery. 1973 Jul;74(1):91–99. [PubMed] [Google Scholar]
- Reemtsma K. Heterotransplantation: theoretical considerations. Transplant Proc. 1971 Mar;3(1):49–52. [PubMed] [Google Scholar]
- Rerup C., Tarding F. Streptozotocin- and alloxan-diabetes in mice. Eur J Pharmacol. 1969 Jul;7(1):89–96. doi: 10.1016/0014-2999(69)90169-1. [DOI] [PubMed] [Google Scholar]
- Seto F. Allograft reactivity in chick embryos. J Exp Zool. 1971 Jul;177(3):343–352. doi: 10.1002/jez.1401770308. [DOI] [PubMed] [Google Scholar]
- Seto F. Enhancement of chicken hemagglutinin response by thymocytes. Exp Hematol. 1975 Sep;3(5):319–326. [PubMed] [Google Scholar]
- Steffes M. W., Sutherland D. E., Mauer S. M., Leonard R. J., Najarian J. S., Brown D. M. Plasma insulin and glucose levels in diabetic rats prior to and following islet transplantation. J Lab Clin Med. 1975 Jan;85(1):75–81. [PubMed] [Google Scholar]
- Sutherland D. E., Steffes M. W., Bauer G. E., McManus D., Noe B. D., Najarian J. S. Isolation of human and porcine islets of Langerhans and islet transplantation in pigs. J Surg Res. 1974 Feb;16(2):102–111. doi: 10.1016/0022-4804(74)90017-1. [DOI] [PubMed] [Google Scholar]
- TERASAKI P. I. Tolerance of skin grafts produced by various adutl cells, soluble extracts, and embryonic cells. J Embryol Exp Morphol. 1959 Sep;7:409–416. [PubMed] [Google Scholar]
- Tolerance and enhancement. Transplant Proc. 1975 Mar;7(1 Suppl 1):337–469. [PubMed] [Google Scholar]
- Vuitton D., Eloy R., Clendinnen G., Grenier J. F. Role of kupffer cells and suppressor T cells in the graft versus host reaction after intrahepatic lymphoid tissue implantation. Cell Immunol. 1977 Nov;34(1):138–145. doi: 10.1016/0008-8749(77)90236-2. [DOI] [PubMed] [Google Scholar]
- Vuitton D., Eloy R., Coumaros G., Grenier J. F. Effect of partial hepatectomy on the graft versus host reaction after intrahepatic lymphoid tissue implantation. Cell Immunol. 1977 Jan;28(1):51–58. doi: 10.1016/s0008-8749(77)80005-1. [DOI] [PubMed] [Google Scholar]
- Weber C., Weil R., 3rd, McIntosh R., Hogle H., Warden G., Reemtsma K. Xenotransplantation of piscine islets into hyperglycemic rats. Surgery. 1975 Feb;77(2):208–215. [PubMed] [Google Scholar]
- Weber C., Zatrigi A., Weil R., McIntosh R., Hardy M. A., Reemtsma K. Pancreatic islet isografts, allografts, and xenografts: comparison of morphology and function. Surgery. 1976 Feb;79(02):144–151. [PubMed] [Google Scholar]