Abstract
Loss of p53 tumor suppressor function is the most common abnormality in human cancer, which can result in enhanced presentation to immune cells of wild type sequence (wt) peptides from tumor p53 molecules, provides the rationale for wt p53 peptide-based cancer vaccines. We review evidence from preclinical murine tumor model and preclinical studies that led to the clinical introduction of wt p53 peptide-based vaccines for cancer immunotherapy. Overall, this review illustrates the complex process of wt p53 epitope selection and the issues and concerns involved in the application of p53-based vaccines for patients with cancer.
Keywords: p53, wild type sequence peptides, multiepitope vaccines, T cells, dendritic cells, immunomonitoring
Introduction
The development of therapeutic anti-cancer vaccines is based on the premise that tumors express MHC allele-restricted epitopes which are antigenic, i.e., can be recognized by host’s immune T cells and can induce their expansion as well as differentiation into cytolytic tumor-specific effector and memory T cells [1, 2]. In recent years, it has become clear that tumor-associated epitopes are largely self, and while they can prime T cells, especially when cross-presented on dendritic cells (DC), these interactions result in the induction of epitope-specific T cells with low to intermediate affinity. Such T cells will not induce autoimmunity but neither are they likely to be effective in tumor cell elimination. Another important principle that has emerged relatively recently concerns the ability of the tumor to avoid recognition by immune cells, i.e., to escape from the host’s immune system. Tumor escape can be mediated by a variety of mechanisms, including the loss of epitopes recognized by T cells [3]. In many instances, such an epitope loss makes tumor cells resistant to immune lysis by effector cells. However, when immune cells are present and are able to eliminate tumor cells expressing the relevant antigenic epitope, they leave behind tumor cells that do not. As a result of this process called “immunoediting,” tumor cells that are not recognized by T cells expand and thrive. Thus, the host immune system participates in the selection of immuno-resistant tumor variants. Still another insight into the immune system-tumor interaction suggests that tumor epitopes that are essential for tumor cell survival are the optimal targets for immune intervention, since tumor cells would not survive if they downregulated or lost expression of these epitopes as part of a tumor immune escape. The emerging recommendations for the development of antitumor vaccines emphasize the need for a judicious selection of epitopes endowed with properties of strong immunogenicity, low likelihood of inducing the epitope loss and uniformly strong expression in the majority, if not all, tumor cells. Only a few tumor-derived epitopes fit the above described profile. Among them is p53, a tumor suppressor, which appears to be an interesting exception, and it represents an attractive target for antitumor vaccines [4, 5].
Mutations in the p53 gene resulting in a loss of function are the most common abnormality in epithelial tumors, which occurs in >80% of all cases [6–8]. Missense mutations in p53 usually increase its stability and can lead to the protein accumulating in the cytosol of tumor cells, and counterintuitively for reasons not yet defined, to its enhanced processing by antigen processing machinery (APM) [9]. These changes in p53 favor enhanced presentation to T cells of the multiple wild-type (wt) sequence peptides, that is, non-mutated “self” p53 sequence epitopes derived from p53 molecules. A loss of p53 function in tumors can also occur due to rapid degradation of wt p53 molecules or p53 molecules genetically altered by frameshift or deletion mutations, conditions which also can promote enhance presentation of wt p53 epitopes [10–13]. In aggregate, therefore, a solid rationale can be made for the use of wt sequence p53 epitopes as components of antitumor vaccines.
In this review, we first present evidence derived from various animal tumor models for successful tumor rejection in animals immunized with wt p53 peptide-based vaccines in the prophylactic and therapeutic settings. Translation of these in vivo findings in mice to human tumors and immune cells in a series of ex vivo experiments is then summarized. This part of the review provides further support for the concept of vaccination with wt p53 epitopes in humans with cancer. Finally, we describe the phase I clinical trials recently performed or currently in progress, in which wt p53 peptide vaccines have been used for immunotherapy of patients with cancer. Overall, this review is designed to illustrate the complex process of wt sequence p53 epitope selection and the application of selected p53 epitopes to antitumor vaccine development for patients with cancer.
Processing and presentation of wt sequence p53 eptiopes
All vaccination strategies depend on successful processing of antigenic epitopes by APC and presentation of the epitope-MHC complexes to T cells. Processing and presentation to T cells of antigenic epitopes is a complex process involving antigen processing machinery (APM) components located in the endoplasmic reticulum of the APC, such as dendritic cells or B cells [14]. Tumor cells can also function as APC. Endogenously-derived epitopes are usually but not exclusively processed by the class I MHC pathway, while exogenous antigens are directed into class II MHC pathway [15]. As a result of processing, an epitope emerges on the surface of an APC, whether tumor or DC, and is displayed in association with MHC molecules to T cells expressing a cognate T cell receptor [16]. Processing of wt p53 epitopes follows the same rules, and because p53 is a source of numerous peptides that can presumably be processed in the tumor cell or DC via the MHC class I and/or MHC class II pathway, an opportunity exists for generating wt sequence p53 epitope-specific T cells that have helper (“Th1”) or cytotoxic (“Tc1”) activities [17]. These peptide-specific T cells collaborate in mounting an anti-p53 immune response, and the ability to reliably induce both effector and helper T cells is a crucial factor in any successful vaccination strategy [17]. Wild type (wt) sequence p53 epitopes which induce CD8+ cytotoxic T cells and those which induce CD4+ helper T cells have been identified (see Table 1) and can be combined for delivery as a multi-epitope vaccine.
Table 1.
Well-characterized T cell-defined wt p53 peptides available for vaccine use
| HLA class I-restricted CD8+ T cell-defined peptides | ||
|---|---|---|
| wt p53 peptide | sequences | reference |
| HLA-A2 restricted | ||
| 65-73 | RMPEAAPPV | [58] |
| 149-157 | STPPPGTRV | [10, 40] |
| 187-197 | GLAPPQHLIRV | [55] |
| 217-225 | VVPYEPPEV | [59] |
| 264-272 | LLGRNSFEV | [10, 12, 40] |
| HLA-A24-restricted | ||
| 125–134 | TYPALNKMF | [22] |
| 161–169 | AIYKQSQHM | [60] |
| 204–212 | EYLDDRNTF | [22] |
|
| ||
| HLA class II-restricted CD4+ T cell-defined peptides | ||
| HLA-DR1/4 | ||
| 108-122 | GFRLGFLHSGTASV | [60] |
| 110-124 | RLGFLHSGTASVTC | [20] |
| HLA-DR7/11 | ||
| 25-35 | LWKLLPENNVLSP | [21] |
| HLA-DP5 | ||
| 108-122 | GFRLGFLHSGTAKSV | [44] |
| 153-166 | PGTRVRAMAIYKQS | [44] |
Selection of wild-type sequence p53 epitopes as a vaccine target
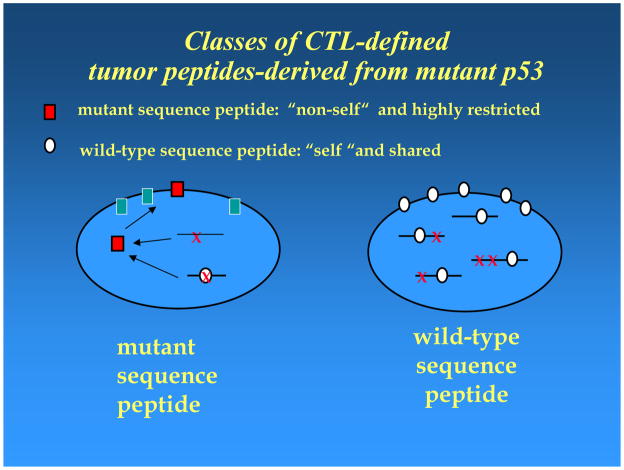
Although p53 was initially identified as a tumor antigen using antibodies present in the sera of mice immune to chemically-induced tumors, the potential use of wt sequence p53 in peptide-based vaccines for cancer was not considered until much later when the significance of accumulated p53 became understood. In normal cells, wt p53 molecules are sequestered in the nucleus and have a relatively short half-life. A missense mutation in p53 is frequently associated with the stabilization (i.e., increased half-life) of mutated p53 molecules in the tumor cytosol referred to as p53 “accumulation or overexpression.” This accumulation resembles overexpression or de-repression of several tumor-associated antigens, such as HER-2 or MAGE, selected for use in cancer vaccines. In contrast to these shared tumor antigens, however, two types of p53 epitopes exist: (i) mutated, which are “foreign or non-self,” and (ii) non-mutated or “self” wt p53 peptides, which are derived from the unaltered portions of p53 protein in the tumor. Although mutated “foreign” epitopes are likely to be strongly immunogenic, they are not attractive as vaccine components, since a vaccine targeting the mutated epitope is applicable only to one or very few individuals harboring an identical mutation. Furthermore, in order to be processed and presented, a p53 mutation must occur within or create an epitope that can fit into and be presented by MHC alleles expressed by the tumor. Consequently, targeting mutant p53 epitopes would require the preparation of “custom made” vaccines [18]. In contrast, wt p53 epitopes, although “self,” have properties similar to those of shared tumor antigens and would be processed and presented by most tumors with a loss of p53 function due to direct or indirect genetic events affecting this tumor suppressor gene product [13, 19]. Such vaccines would be broadly applicable. However, the development and clinical application of wt p53-based vaccines need to confront numerous difficulties associated with tolerance to self, which is difficult to overcome.
An important advantage of wt sequence p53 peptide-based vaccination strategy is that helper and cytotoxic peptides have been identified and can be selected for immunization based on in vitro pre-clinical studies with human T cells, as previously described [20]. The wt p53 Th1 epitopes induce CD4+ T cells that enhance activity and generation of wt p53 epitope-specific CD8+ effector T cells [20, 21]. Their presence may also be necessary for the maintenance of immunologic memory. Therefore, multi-epitope p53-based vaccines should be designed to include epitopes best able to induce cytotoxic as well as helper T cells. Multi-epitope vaccines are considered to be superior to single-epitope vaccines, largely because they induce stronger and broader immune responses. This widely-accepted principle is especially relevant to antitumor vaccines, where a generated response has to be robust and able to progress despite tolerance to “self” which is associated with most tumor-derived epitopes. The wt p53 epitopes selected for use in a vaccine are, of course, MHC allele-restricted. For this reason, wt p53 epitope characterization has been mostly focused on HLA-A2-restricted peptides. Among Caucasians, this MHC class I allele is expressed in about 50% of individuals, who, therefore, would be candidates for the vaccine. Similarly, in Asian populations, frequent expression of the HLA-A24 allele predicates the use of HLA-A24-restricted wt p53 peptides in antitumor vaccines [22, 23].
One of the more intriguing aspects of wt sequence p53 epitope processing is the possibility that it might avoid the generation of peptide-specific regulatory T cells (Treg) or type 1 regulatory T cells (Tr1). While normal p53 is processed by APC with the goal of maintaining tolerance, differential processing of wt sequence p53 epitopes derived from genetically altered protein could lead to immunity by down-regulation of Treg. While speculative, this possibility fits well with the concept of Treg being in control of responses to “self” vs. “altered self,” as represented by wt sequence p53 epitopes.
Tumors share antigens and these “shared antigens” are products of genes which become overexpressed or de-repressed in tumor cells. In contrast to a majority of tumor-associated antigens, p53 is genetically altered. It is now known that genetic abnormalities in p53 can involve a mutation, deletion or frameshift, each leading to a loss of p53 function [6]. Processing of p53 and presentation of p53 epitopes to T cells can differ depending on the type and site of the genetic lesion in the tumor cell. Obviously, a deletion or frameshift mutation can eliminate epitopes, whereas a peptide encoded adjacent to a mutation, for example, may be processed by the APM differently than the same peptide encoded in a p53 molecule mutated at a different site. The precedent for blocking epitope processing by a tumor with a missense mutation at the hotspot codon 273, which flanks wt sequence p53264-272 epitope, was described by Theobald et al [24]. It is thus possible that additional instances of blocked, altered or incomplete processing of this as well as other wt p53 epitopes exist and influence the generation and frequency of epitope-specific T cells. As a result, wt sequence p53 epitopes can be handled by the APM components in a way that decreases or enhances their immunogenicity. However, enhanced processing and presentation of wt sequence p53 epitopes need not be directly associated with mutations, as other genetic events or viral integration can also influence the peptide route via the APM. The available data suggest that selection of the optimally immunogenic wt sequence p53 epitope for use in antitumor vaccines may not be simple and may require extensive pre-clinical evaluations.
Pre-clinical studies in mice
The demonstration that a wt p53 peptide-based dendritic cell vaccine induced rejection in mice of a transplanted chemically-induced sarcoma in the protection and therapy settings established the efficacy of p53-based vaccines and their potential for use in immunotherapy of cancer [25]. Since for any vaccine development, selection of the immunogen and vaccine vehicle are critical, a wide range of p53-based vaccines and immunization protocols have been subsequently evaluated for efficacy. These studies showed that despite evidence of tolerance in mice to “self” murine wt p53 epitopes, presumably due to anergy or deletion of high affinity anti-wt p53 peptide-specific CD8+ T cells [26–28], effective T cell-mediated antitumor immunity can be induced by wt p53-based vaccines. Vaccines comprised of (i) wt p53 peptides or recombinant p53 protein admixed with chemical adjuvants or pulsed onto DC; (ii) DC transfected with non-viral plasmids or viruses encoding intact p53 or fragments; (iii) DNA vaccines delivered via a gene gun as well as recombinant viral vectors expressing p53- were all effective in inducing tumor rejection [5, 25, 29]. Critical to their translational relevance, these pre-clinical vaccines, delivered by themselves or in combination with immunomodulators to mice [30], consistently have been shown to induce low to intermediate affinity anti-wt p53 CTL, which had antitumor effects in the absence of inducing deleterious autoimmune side effects.
Most of vaccination studies in mice with transplantable tumors utilized either tumor-immunized mice in the prevention setting or mice bearing small tumors to demonstrate the efficacy of wt p53 peptide-based vaccines. These studies, however, cannot evaluate the vaccine’s efficacy in a clinically-relevant situation, namely, in the primary tumor-bearing host displaying various defects in immune defense. Polycyclic aromatic hydrocarbons, such as methylcholanthrene (MCA) and benzopyrene, are potent carcinogens and have been traditionally used to induce tumors in mice. Nearly all of these tumors express genetic alterations in p53. These carcinogens also have potent immunosuppressive activity, and are known to be environmental carcinogens associated with cancer in humans. Exposure of most mice to these compounds results in tumors arising within six months at the sites of exposure. To evaluate the efficacy of wt p53-based vaccines in mice bearing primary tumors, we recently analyzed the effects on MCA-treated mice of wt p53-based immunization given in the protection/therapy or therapy-only setting [31]. The results indicated that the efficacy of the vaccines relative to tumor incidence was severely compromised by the outgrowth of “epitope-loss” tumors. Regardless of when the vaccine was administered, “epitope-loss” occurred either by downregulation or loss of expression of the MHC allele required for presentation of the targeted wt p53 peptide or by a mutation within the epitope [31]. Despite the fact that MCA is immunosuppressive, the vaccines did induce anti-wt p53 immune responses consisting of low- to intermediate-affinity CTL. While these findings might argue against the use of p53-based vaccines for protection due to their ability to promote “epitope-loss” tumors, the experimental conditions (i.e., subcutaneous injection of MCA in oil emulsion) were ideal for immunoselection of “epitope loss” tumors. A continuous exposure of newly transformed cells with a p53 mutation to freshly generated anti-wt p53 peptide-specific CTL is likely to especially favor immunoselection of “epitope loss” tumor cells. It seems unlikely that this type of wt p53 vaccination translated to therapy of human tumors would similarly favor appearance of “epitope loss” tumor cells. Unlike the intense, rapid cancer development in MCA-conditioned mice, the initiation and progression of human tumors occurs over many years, decreasing the potential for immunoselection and tumor escape.
Pre-clinical studies in humans
Evidence for the existence of immune responses to p53 in patients with cancer includes the presence of T cells specific for p53 in the circulation of these patients [32] or at the tumor site and/or of antibodies recognizing p53 in the serum [33]. Once the concept of wt sequence p53 epitopes as potential immunogens in antitumor vaccines was introduced and its validity confirmed in mice, studies in man were initiated to determine whether antitumor immune responses could be generated in vivo, searching for the presence of anti-p53 antibodies or assessing frequencies of p53 epitope-specific T cells in the circulation of patients with cancer. In addition, ex vivo experiments using these epitopes and human peripheral blood mononuclear cells (PBMC) were conducted to test for the presence of immune precursor cells able to expand upon stimulation with wt sequence p53 epitopes presented on autologous DC and to eliminate tumor targets accumulating p53 [34].
A search for antibodies to p53 in the sera of patients with cancer by Bourhis et al identified nearly 20% as seropositive [33]. Surprisingly however, these patients had poorer rather than better prognosis, despite the evidence for antitumor immunity presumably mediated by antibodies [35]. This finding suggested that p53-specific antibodies alone might not be sufficient for effective immune response against the tumor. Therefore, the attention turned to the presence of p53-specific CTL in patients with cancer whose tumors accumulated p53.
The expectation was that p53 accumulation in the tumor cells provided an opportunity for presentation to T cells of immunogenic wt sequence p53 epitopes and generation of p53 epitope-specific T cells in the circulation. Therefore, patients with tumors accumulating p53 were expected to have a relatively high frequency of such T cells. Contrary to expectations, however, we initially found that CTL specific for the p53264-272 epitope could be generated only from PBMC of the patients whose tumors either did not accumulate p53 or accumulated but could not present this epitope [34]. In a subsequent study, we evaluated the frequency of wt sequence p53264-272 peptide-specific CD8+ T cells in the circulation of patients with cancer and correlated it with the level p53 expression in each tumor, its HPV status and the presence of p53 antibodies in the serum [32]. Using HLA-A2.1/wt p53264-272 peptide tetramers, we found the highest frequency of wt sequence p53264-272-specific CD8+ T cells in a subset of patients whose tumors did not accumulate p53 and had a wt p53 genotype [32]. Furthermore, in a subset of patients with tumors accumulating mutant p53, the mean frequency of p53264-272-specific T cells was not significantly different from that in healthy controls [32]. The interpretations for these intriguing observations are many. First, it is possible that processing of mutated p53 and/or its presentation (applicable in this case to the wt sequence p53264-272 peptide) is blocked or otherwise deranged in tumor cells as discussed above and also suggested by Vierboom et al [13]. Second, it is equally possible that the peptide-specific T cells are generated but do not survive, being sensitive to activation induced cell death (AICD) and/or apoptosis induced by tumor-associated mechanisms [36]. As discussed above, murine studies suggest that deletion of high-avidity T cells and retention of low- to intermediate-affinity effector cells may be due to tolerance to “self” [26, 27]. It is also possible, but not proven, that the paucity of wt sequence p53264-272-specific T cells in the circulation of cancer patients is under control of Treg, which are known to be increased in frequency and suppressor function in these patients [37, 38]. Finally, the presence of HPV E6 in tumor cells (for tumors such as cervical carcinoma or SCCHN) could influence p53 processing and CTL generation [13].
Given the complexity of tumor-host interactions, the development and clinical introduction of p53-based vaccines represent a challenge that requires attention to several specific issues as follows.
(a) Composition of p53-based vaccines
In selecting p53-based vaccine components, a source of immunogenic epitopes as well as the vehicle for their delivery need to be considered. It is possible to select recombinant p53 as an immunogen. Advantages would be the availability, relatively low cost and utilization without any HLA restrictions. However, the lack of defined epitopes could impede adequate monitoring of immune responses to the vaccine. Also, p53 would have to be processed by endogenous APC, which may be dysfunctional in patients with advanced malignancy [39], or delivered following ex vivo loading of autologous DC, which is feasible but not convenient. For these reasons, a more advantageous approach is to use wt sequence p53 peptides. However, selection of the peptides and their delivery as a vaccine require a careful strategy.
Most wt p53 peptides that might be useful for vaccination have been identified by “reverse immunology,” as sequence motifs that best fit and bind HLA-A2 molecules. Hence, the immunogenic properties of these peptides have to be determined in in vitro studies designed to confirm that the naturally processed peptides induce HLA-A2-restricted CTLs capable of targeting tumors with accumulated p53 and wt p53 epitope-specific Th1 T cells able to provide immune help. As discussed above, HLA-A2neg and HLA-A24-restricted wt p53 epitopes are being preferentially selected as vaccine components because of the frequent expression of these MHC alleles in Caucasian and Asian populations, respectively. To date, a number of HLA-A2-restricted wt p53 peptides have been tested in IVS cultures with human PBMC, and their potential for inducing anti-wt p53 CTLs confirmed in vitro (Table 1). Likewise, a number of HLA class II-restricted wt p53 peptides have been identified (Table 1). Pre-clinical testing of other potentially useful peptides is in progress.
(b) Conditions for the presentation of wt p53 epitopes
Our observations using various human squamous cell carcinomas of the head and neck (SCCHN) indicated that human tumor accumulating p53 were not necessarily sensitive to anti-p53 CTLs [40]. Also, the nature as well as the site of p53 missense mutation can influence antigen processing of p53, as discussed above. In addition, the presence of HPV or mdm2 amplification can influence processing of wt p53 epitopes for T-cell recognition.
(c) Breaking immunologic tolerance to self
As previously mentioned, because p53 is expressed in all nucleated cells, including those in the thymus, peripheral and central tolerance have to be broken to allow for the development of immune responses to wt p53. Thus, especially powerful adjuvant effects are necessary to overcome tolerance to wt p53 and induce antitumor immunity. We have determined that PBMC in only 1/3 of normal donors or patients with SCCHN are responsive to autologous DC pulsed with wt p53 peptides in IVS cultures, despite the fact that DC are capable of priming naïve T cells in culture [34, 40]. Further, T cells induced under these conditions were of low-to-intermediate affinity, a finding similar to that reported earlier in mice [26].
(d) Possibilities for by-passing unresponsiveness to wt p53 peptides
One effective strategy for enhancing peptide immunogenicity is to identify and implement an amino acid exchange in the peptide sequence that will enhance its binding to HLA molecules and/or to the cognate T cell receptor on T cells recognizing the parental peptide. We and others have used this strategy to augment immunogenicity of wt p53264-272 and p53149-157 peptides and demonstrated substantially higher responder T cell reactivity in ELISPOT and cytotoxicity assays with optimized rather than parental wt p53 peptides [41, 42]. The reliable identification of the potential of optimized p53264-272 peptides able to induce responsiveness of patients’ PBMC required IVS-based analysis performed under defined conditions as described by us earlier [42] and depended on the availability of PBMC obtained from a cohort of normal subjects as well as a cohort of patients with cancer. Using this approach, it was possible to induce CTL from PBMC obtained from nearly half of donors whose PBMC were non-responsive to the parental wt p53264-272 and augment the total number of donors that could be potential candidates for vaccination from 3/10 to 7/10 [34,42]. Interestingly, anti-wt p53264-272 peptide CD8+ T cells induced from a donor unresponsive to the parental but responsive to the optimized peptide utilized the same TCR Vβ family and identical variable TCR Vβ sequences as did CD8+ T cells induced from PBMC of a different donor which were responsive to the parental peptide. This finding points out the limited TCR repertoire available for anti-wt p53 peptide-specific CD8+ T cells in humans [42].
(e) Generation of helper T-cell responses
The identification of helper wt p53 epitopes and their use to enhance Th1-type responses is desirable in cancer. Fortunately, it appears that tolerance of CD4+ T cells to wt p53 peptides is not as strict as it is for CD8+ T cells, which enhances the potential efficacy of vaccines incorporating wt p53 helper peptides [43]. To this end, we identified wt p53110-124 peptide as naturally-presented HLA-DRB1*040-binding 15-mer peptide and showed not only that this peptide presented on DC induced CD4+ T cells, but that the addition of the generated CD4+. T cells to tumor-specific CTL significantly enhanced the number and activity of CD8+ T effector cells in vitro [20]. We and others have identified additional immunogenic HLA class II-restricted wt p53 peptides (see Table 1), including multiple HLA-DR allele-restricted wt p53 peptides, such as the HLA-DR7/11-restricted, wt p5325-35 peptide [21, 44]. Thus, an opportunity exists to combine CTL-defined cytotoxic and Th1-defined helper wt p53 peptides in multiepitope vaccines which are expected to be more broadly immunogenic than a single-epitope wt p53 vaccine.
(f) Elimination of wt p53-specific Treg
We and others have described accumulation of regulatory T cells (Treg) at the tumor site and in the peripheral circulation of patients with cancer [36, 37, 45]. Some of these Treg are likely tumor antigen-specific Tr1 cells [46], and although wt p53-specific Tr1 have not been reported as yet, natural Treg (nTreg), which originate in the thymus [47] could be responsible for maintaining tolerance to p53. Thus, the strategy to eliminate Treg prior to, or concomitantly with, vaccination against wt p53+ tumors might be a crucial factor in achieving success. This could be accomplished by delivery of antibodies to CD25 [48], ONTAK™ (denileukin diftitox) which is currently in clinical trials [49, 50]. Elimination of Treg by use of chemotherapy has also been previously utilized in the clinic [51].
(g) Protection of activated wt p53-specific T cells from apoptosis
Provided that immunization targeting wt p53 epitopes is successful and leads to an increased frequency of wt p53-specific CTL, it is essential to assure these effector cells do not succumb to AICD or tumor-induced apoptosis. Their survival and functions are essential for elimination of occult tumor or micrometastases. In this respect, combination of cytokine therapies with vaccine delivery might be effective. For example, we have recently showed that activated T cells can be protected from apoptosis induced by tumor-derived exosomes or microvesicles isolated form sera of patients with cancer by pretreatment with a cocktail of natural cytokines [52].
(h) Maintenance of memory responses
The major objective of antitumor vaccines is to induce effective immunologic memory in order to prevent tumor recurrence. Therefore, the effective wt p53 vaccine has to induce wt p53-specific memory T cells and assure their survival. To do so, all of the above listed conditions may have to be satisfied, including the administration of cytokines known to promote survival and maintenance of memory T cells such as IL-7, IL-12, and IL-15 [53], none of which are currently approved for immune support of therapeutic antitumor vaccines.
Clinical trials of p53-based immunotherapy in patients with cancer
Evidence from human pre-clinical studies that T cells specific for wt p53 epitopes are present in the circulation of some, but not all, cancer patients and antibodies to p53 are detectable in a subset of these patients, suggests that these epitopes can be immunogenic. Clearly, these epitopes can activate and expand subsets of wt p53 epitope-specific effector T cells and contribute to shaping of immunologic memory, provided they are delivered to patients with cancer in appropriately designed vaccines. Pre-clinical results with wt p53 peptide-based immunization in murine tumors as well as in vitro experiments utilizing human PBMC indicate that p53 protein-, peptide- as well as recombinant viral vector-based p53 vaccines are effective in inducing anti-tumor immunity. However, as most anti-tumor vaccines, those targeting wt p53 epitopes are still under development and have not been optimized.
A wide range of developmental approaches to p53 vaccines have been implemented in murine models of tumor growth. For example, it has been shown that recombinant p53 protein admixed with chemical adjuvants or p53 peptides pulsed onto bone-marrow derived DC are effective in inducing anti-wt p53 T-cell mediated anti-tumor responses [25, 31]. DC transfected with non-viral plasmids or viruses encoding p53 or p53 fragments have been used for vaccinations [29]. In vitro co-culture studies using PBMC of normal donors or patients with cancer and DC pulsed with p53, p53 peptides or transfected with recombinant constructs expressing p53 convincingly show induction and expansion of anti-wt p53 CTL and Th cells [20, 21, 54].
Based on these pre-clinical studies and a wealth of information gathered from vaccines in murine models of tumor growth, four therapeutic vaccination trials in patients with cancer were initiated and are either completed or almost completed (Table 2). We wish to feature these vaccination trials, as they incorporate most of the strategies discussed above and represent the current state-of-the-art in wt p53 peptide-based vaccines.
Table 2.
Clinical trials of wt p53 peptide-based immunotherapy
| Vaccine | Phase | Patient Numbera | Responsesb | ref |
|---|---|---|---|---|
| Breast | ||||
| wt p5365-73, 1877-197, and 264-272 peptides, optimized p53149-157, 103-111, and 139-147 peptides and PADRE pan-helper peptide/DC | I | 6 (3) | 3/6 immune responses 2/3 stable disease |
(55) |
| same as above/IL-2 | II | 26 (19) | 6/19 immune responses 8/19 stable disease |
(56) |
| Ovarian | ||||
| p53264-272 peptide/DC/IL-2 | II | 7 | 3/7 immune responses | (57) |
| p53264-272 peptide/ISA-51/GM-CSF/IL-2 | 13 | 5/13 immune responses | ||
| SCCHN | ||||
| optimized p53149-157 and 264-272 peptides plus wt p53110-124 or tetanus toxoid helper peptide or no helper peptide/DC | I | 24 (15) | in progress [UPCI protocol #03-156] | |
number of patients enrolled in trial; number of patients completing therapy and evaluatable shown in parenthesis.
Immune responses indicate vaccine-induced responses detected in ELISPOT IFN-γ assays
Phase I and Phase II trials of p53 peptide pulsed DC-based vaccine for patients with advanced breast cancer-M. H. Claesson, Principal Investigator [55, 56]
The both of these multiepitope DC-based vaccine trials of heavily pretreated HLA-A2+ subjects with progressive advanced breast cancer have been completed. The vaccine used consisted of autologous DC pulsed with the CTL-defined wt p53 65-73, 187-197 and 264-272 peptides, as well as optimized peptides for the wt p53 149-157, 103-111 and 139-147 epitopes together with the pan-HLA-DR Th peptide, PADRE. A total of 6 subjects were recruited for the first trial which consisted of 10 planned immunizations. 3/6 completed the trial; the remaining subjects were withdrawn from the trial after 7 or 8 immunizations due to progressive disease. ELISPOT analyses indicated that the vaccine-induced CD8+ T cells responsive to wt and modified p53 peptides in 3/6 subjects, 2/3 showing disease stabilization. The second trial, a Phase II trial, used an immunization protocol comparable to that employed in the Phase I trial with the exception that IL-2 was administered s.c. 19/26 subjects recruited for the trial were evaluatable after the first 6 immunizations with 8/19 showing stable disease, while the remainder had progressive disease. 4/7 subjects with stable disease showed vaccine-induced wt p53 peptide-specific CD8+ T cell responses, whereas only 2/9 subjects with progressive disease did. The results of these trials clearly indicate that p53 peptide-specific immune responses can be achieved in subjects with cancer and appear to be associated with stabilization of their disease.
A randomized phase II p53 vaccine trial comparing subcutaneous direct administration with intravenous peptide-pulsed dendritic cells in high risk ovarian cancer patients – S.N. Khleif, Principal Investigator [57]
This clinical protocol for patients with ovarian carcinoma who are at very high risk of relapse but who had no visible disease on CT scan (i.e., had microscopic disease present) targets a wt p53264-272 epitope. Eligible patients had stage III, IV or recurrent disease and were clinically NED at study entry. They all had prior chemotherapy, and some were very heavily pre-treated. Elevated CA125 was allowed. Patients had to be HLA-A2.1+ and have tumors overexpressing p53. The vaccine utilized a wt epitope (264-272) with high HLA-A2.1 affinity. Two methods of vaccination were randomly compared: subcutaneous (SQ) peptide admixed with ISA-51 and GM-CSF adjuvants or peptide-pulsed autologous dendritic cells given intravenously (IV). The vaccine was administered every three weeks on day 1 of each cycle. Patients in both arms also received six million IU/m2 IL-2 SQ for 10 days beginning with cycle 3 for two consecutive cycles in a recurring pattern (i.e., IL-2 was given with cycles 3 and 4; 7 and 8; 11 and 12, etc.).
The trial, now completed, enrolled 21 patients, of whom 20 were evaluable (13 on SQ arm and 7 on IV arm). After reaching statistical significance, accrual to the more technically difficult IV dendritic cell arm was halted. The primary endpoint was the immune response to wt p53264-272 epitope, and ELISPOT and tetramer analyses were used for immune monitoring. The frequency of Treg (CD4+CD25highFoxp3+) cells in the circulation was also determined. Results are encouraging. In spite of previous chemotherapy, an immune response to wt p53264-272 epitope was generated. On the SQ arm, 11/14 patients had positive ELISPOT and 14/14 were tetramer positive following vaccination vs. 6/14 and 5/14 pre-vaccine, respectively. On the IV arm, 6/7 had detectable immune responses pre-vaccine vs. 3/7 pre-vaccine. Grade III/IV toxicities were observed in 42% of patients on each arm and included elevated liver enzymes in the SQ arm and fatigue and lymphopenia in the IV arm. Only one patient disenrolled due to toxicity (a cardiac arrhythmia exacerbated by IL-2). The currently available data analyses are preliminary. In 2007, the mean overall survival (OS) on the SQ arm was >42 months, on the IV arm, it was >38 months. The OS data are not yet mature for final analysis and long-term survivors are being followed.
In summary, this trial demonstrated that cellular immune response to a vaccine targeting a wt p53 epitope can be generated in nearly all enrolled patients with ovarian carcinoma (80–90%) who were pre-treated with chemotherapy. Definite immunologic responses were detected by ELISPOT and tetramer analyses. Furthermore, both vaccine delivery approaches appeared to be equally effective, although the SQ peptide vaccine had less toxicity and was technically much simpler. Toxicities were acceptable.
Adjuvant p53 peptide loaded DC-based therapy for subjects with SCCHN (A pilot/feasibility study) R.L. Ferris, Principal Investigator [UPCI protocol #03-156]
The objectives of this clinical protocol for patients with SCCHN are to (i) evaluate the safety and feasibility of a multi-epitope wt p53 peptide DC-based vaccine that employs CTL-defined and Th-defined peptides, and (ii) characterize the immunological responses to the vaccine. The trial is restricted to HLA-A2+ subjects, but due to the heterogeneity of SCCHN and the potential role of HPV in these tumors, enrollment was not restricted to subjects whose tumors accumulated p53 and, presumably, expressed p53 mutations. A total of 24 HLA-A2+ subjects are being recruited for the protocol, eight subjects must also be HLA-DR4+. This number permits the subjects to be divided into three cohorts designed to gain insights into whether incorporation of a Th-defined peptide in the vaccines enhances vaccine-induced p53-specific CTL responses, and whether a Th-defined wt p53 peptide is more effective than a pan-HLA-DR Th-defined tetanus peptide in this regard. All 24 subjects will receive three immunizations of autologous DC pulsed with optimized peptides for the HLA-A2 restricted, wt p53149-157 and p53264-272 epitopes, F270W and T149L. The eight HLA-DR4+ subjects receive DC pulsed with the two optimized p53 peptides and the HLA-DR4-restricted, wt p53110-124 peptide. The remaining 16 HLA-DR4- subjects are divided into two groups of eight each, one gets the vaccine incorporating the pan-HLA-DR tetanus toxoid peptide, while the other group does not. Each subject receives three immunizations at 10–14 day intervals.
PBMC obtained from the subjects prior to each immunization are evaluated for wt p53 peptide-specific CD8+ and CD4+ T cell responses in ELISPOT assays. Wt p53 peptide-specific CD8+ T cell responses are also monitored by tetramer analysis, including differential phenotype of tetramer+ cells. To date, more than 12 subjects have successfully completed the full course of the protocol, and samples from four subjects monitored. 2/4 subjects showed vaccine-induced responses to the wt p53264-272 peptide only in ELISPOT assays, whereas flow cytometry analyses indicated showed increased frequencies of CD8+ T cells to both wt p53 peptides in circulation of all four subjects.
Expert commentary and five-year view
The p53-based therapeutic vaccines for cancer have been underutilized, given the available pre-clinical and emerging clinical evidence for their in vivo effects. Human clinical trials with wt p53 peptides, while still not optimized in terms of vaccine delivery (i.e., dose, injection site(s), frequency of vaccines, duration of therapy, or choice of adjuvants) provide intriguing preliminary data. These vaccines have acceptable toxicity and are reliable in inducing anti-wt p53 peptide immune responses in patients with advanced malignancies such as ovarian carcinoma or SCCHN even in patients previously treated with chemotherapy or radiotherapy. This by itself is encouraging, because such patients are known to be refractory to immune interventions. The unique nature of p53, which normally functions as tumor suppressor, but in its mutated form is a source of multiple immunogenic cytotoxic and helper peptides, offers a special opportunity to explore. This mutated protein is essential for the tumor progression, yet its non-mutated wt sequences can be effectively targeted by immune cells in most epithelial human cancers. To accomplish this, vaccine designs will have to be selectively stringent and robust. However, advances made in the understanding of the biology of wt p53 peptide handling in APC and their presentation to T cells provide confidence that wt p53 peptide-based vaccines are here to stay, especially for tumors such as lung or ovarian carcinomas, for which a roster of immunogenic, tumor-specific peptides has remained small.
Figure 1.
Key Issues.
The vast majority of tumor antigens recognized by T cells are “self”
The most common abnormality of human cancers is loss of the tumor suppressor functions of p53.
A variety of genetic alterations occurring in tumors promote processing and presentation of wild type sequence (wt) p53 peptides (self) derived from wild type or mutant p53 molecules expressed in the tumor for T cell recognition.
wt p53 peptides are attractive targets for broadly applicable cancer vaccines.
Immunological tolerance to “self” to wt p53 peptides only permits the generation of weak to intermediate affinity anti-wt p53 T cells.
Despite tolerance, wt p53 peptide-based vaccines induce wt p53 peptide-specific CD8+ T cells which can induce tumor rejection in prevention and therapy settings using murine tumor models.
wt p53 peptides have the ability to induce in vitro antitumor CD8+ and CD4+ T cells from lymphocytes obtained from normal donors and cancer patients.
Treg play critical role in regulating immune responses, in particular, antitumor “self” responses.
Achieving beneficial responses to cancer vaccines not only involves generation, long-term survival and maintenance of tumor-specific immune responses, but minimizing the immunosuppressive effects of antigen-specific Treg.
Acknowledgments
Supported in part by NIH grants: 1PO1 CA109688 and 2PO1 DE12321 to TLW
Footnotes
Disclosure
The authors declare no potential Conflict of Interest.
References
- 1.Whiteside TL. Anti-tumor vaccines in head and neck cancer: targeting immune responses to the tumor. Curr Cancer Drug Targets. 2007;7:633–642. doi: 10.2174/156800907782418310. Vaccines need to generate long-term survival of tumor-specific immune cells to be clinical beneficial. [DOI] [PubMed] [Google Scholar]
- 2.Morris LF, Ribas A. Therapeutic cancer vaccines. Surg Oncol Clin N Am. 2007;16:819–831. doi: 10.1016/j.soc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. Immune system plays a major role in the nature of tumors that arise in humans. [DOI] [PubMed] [Google Scholar]
- 4.Nijman HW, Van der Burg SH. Vierboom MP, Houbiers JG, Kast WM, Melief CJ. p53, a potential target for tumor-directed T cells. Immunol Lett. 1994;40:171–178. doi: 10.1016/0165-2478(94)90189-9. The first report identifying CTL-defined wt p53 peptides for potential vaccine use. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann TK, Bier H, Donnenberg AD, Whiteside TL, DeLeo AB. p53 as an immuno-therapeutic target in head and neck cancer. Adv Otorhinolargyngol. 2005;62:151–160. doi: 10.1159/000082505. [DOI] [PubMed] [Google Scholar]
- 6.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. The vast extent of genetic alterations in p53 in human cancer described. [DOI] [PubMed] [Google Scholar]
- 7.Harris CC. Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst. 1996;88:1442–1455. doi: 10.1093/jnci/88.20.1442. [DOI] [PubMed] [Google Scholar]
- 8.Balz V, Scheckenbach K, Gotte K, Bockmuhl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63:1188–1191. Sequencing p53 exons (exons 2-11) as well as the intron/exon junctions required to identify p53 genetic alterations in cancer. [PubMed] [Google Scholar]
- 9.De Leo AB. p53-based immunotherapy of cancer. Adv Otorhinolaryngol. 2005;62:134–150. doi: 10.1159/000082504. Overview of the development of p53-based immunotherapy. [DOI] [PubMed] [Google Scholar]
- 10.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci USA. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. Multiple wt p53 peptides identified for vaccines using HLA-A2 transgenic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet JG, Choppin J. Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J Immunol. 1998;160:328–333. [PubMed] [Google Scholar]
- 12.Ropke M, Hald J, Guldberg P, et al. Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. Proc Natl Acad Sci USA. 1996;93:14704–14707. doi: 10.1073/pnas.93.25.14704. Wt p53264-272 peptide-specific CTL recognize and kill human tumor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vierboom MP, Zwaveling S, Bos GMJ, et al. High steady-state levels of p53 are not a prerequisite for tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:5508–5513. P53 parameters that influence processing and presentation of wt p53 peptides. [PubMed] [Google Scholar]
- 14.Allen PM. Making antigens presentable. J Immunol. 2007;179:3–4. doi: 10.4049/jimmunol.179.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Harding CV, Neefjes J. Antigen processing and recognition. Curr Opin Immunol. 2005;17:55–57. doi: 10.1016/j.coi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. Defects in antigen processing machinery components play a key role in tumor escape. [DOI] [PubMed] [Google Scholar]
- 17.Toes RE, Ossendorp F, Offringa Melief CJM. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. Critical role of CD4+ helper T cells in anti-tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099–5107. doi: 10.1200/JCO.2005.03.158. Personalized mutant p53 peptide-based vaccines for immunotherapy of patients with cancer. [DOI] [PubMed] [Google Scholar]
- 19.Nijman HW, Houbiers JG, Vierboom MP, et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 20.Chikamatsu K, Albers A, Stanson J, et al. p53110-124-specific human CD4+ T-helper cells enhance in vitro generation and antitumor function of tumor-reactive CD8+ T cells. Cancer Res. 2003;63:3675–3681. HLA-DR4 restricted wt p53110-124 peptide is a helper peptide. [PubMed] [Google Scholar]
- 21.Ito D, Albers A, Zhao YX, et al. The wild-type sequence (wt) p53(25-35) peptide induces HLA-DR7 and HLA-DR11-restricted CD4+ Th cells capable of enhancing the ex vivo expansion and function of anti-wt p53(264-272) peptide CD8+ T cells. J Immunol. 2006;177:6795–6803. doi: 10.4049/jimmunol.177.10.6795. Identification of a multi-HLA class II-wt p53 peptide for vaccine use. [DOI] [PubMed] [Google Scholar]
- 22.Eura M, Chikamatsu K, Katsura F, et al. A wild-type sequence p53 peptide presented by HLA-A24 induces cytotoxic T lymphocytes that recognize squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:979–986. [PubMed] [Google Scholar]
- 23.Sakakura K, Chikamatsu K, Furuya N, Appella E, Whiteside TL, DeLeo AB. Toward the development of multi-epitope p53 cancer vaccines: an in vitro assessment of CD8+ T cell responses to HLA class I-restricted wild-type sequence p53 peptides. Clin Immunol. 2007;125:43–51. doi: 10.1016/j.clim.2007.05.015. CD8+ T cells obtained from patients with cancer are responsive in vitro to multiple wt p53 peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theobald M, Ruppert T, Kuckelkorn U, et al. The sequence alteration associated with a mutational hotspot in p53 protects cells from lysis by cytotoxic T lymphocytes specific for a flanking peptide epitope. J Exp Med. 1998;188:1017–1028. doi: 10.1084/jem.188.6.1017. The p53 R273H mutation, the most common occurring p53 missense mutation in human cancer, blocks processing of the wt p53264-272 peptide in tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayordomo JI, Loftus DJ, Sakamoto H, et al. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med. 1996;183:1357–1365. doi: 10.1084/jem.183.4.1357. The efficacy of a wt p53 peptide-based vaccine for immunotherapy demonstrated in a preclinical murine tumor model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. Tolerance to wt p53 peptides permits only the induction of low to intermediate affinity anti-wt p53 peptide-specific CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez J, Lee PP, Davis MM, Sherman LA. The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol. 2000;164:596–602. doi: 10.4049/jimmunol.164.2.596. [DOI] [PubMed] [Google Scholar]
- 28.Vierboom MP, Nijman HW, Offringa R, et al. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuting T, Gambotto A, Robbins PD, Storkus WJ, DeLeo AB. Co-delivery of T helper 1-biasing cytokine genes enhances the efficacy of gene gun immunization of mice: studies with the model tumor antigen beta-galactosidase and the BALB/c Meth A p53 tumor-specific antigen. Gene Ther. 1999;6:629–636. doi: 10.1038/sj.gt.3300859. [DOI] [PubMed] [Google Scholar]
- 30.Hernández J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001;166:3908–3914. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 31.Cicinnati VR, Dworacki G, Albers A, et al. Impact of p53-based immunization on primary chemically-induced tumors. Int J Cancer. 2005;113:961–970. doi: 10.1002/ijc.20686. Wt p53 peptide-based immunization influences selection of sites of p53 mutation and epitope-loss tumors in chemically induced carcinogenesis in mice. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann TK, Donnenberg AD, Finkelstein SD, et al. Frequencies of tetramer+ T cells specific for the wild-type sequence p53 264-272 peptide in the circulation of patients with head and neck cancer. Cancer Res. 2002;62:3521–3529. Frequency of wt p53-peptide specific CTL present in the circulation of HLA-A2+ head and neck cancer patients and correlates to sites of p53 mutation and epitope-loss in their tumors. [PubMed] [Google Scholar]
- 33.Bourhis J, Lubin R, Roche B, et al. Analysis of p53 serum antibodies in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:1228–1233. doi: 10.1093/jnci/88.17.1228. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann TK, Nakano K, Elder EM, et al. Generation of T cells specific for the wild-type sequence p53(264-272) peptide in cancer patients: implications for immunoselection of epitope loss variants. J Immunol. 2000;165:5938–5944. doi: 10.4049/jimmunol.165.10.5938. CD8+ T cells obtained from cancer patients responsive in vitro to wt p53 peptides and relationship to p53 status of their tumors. [DOI] [PubMed] [Google Scholar]
- 35.Houbiers JG, van der Burg SH, van de Watering LM, et al. Antibodies against p53 are associated with poor prognosis of colorectal cancer. Br J Cancer. 1995;72:637–641. doi: 10.1038/bjc.1995.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteside TL. Apoptosis of immune cells in the tumor microenvironment and peripheral circulation of patients with cancer: implications for immunotherapy. Vaccine. 2002;20:A46–A51. doi: 10.1016/s0264-410x(02)00387-0. High rate of T-cell turnover might have unfavorable effects on immunotherapy. [DOI] [PubMed] [Google Scholar]
- 37.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. Oncologic therapy favors expansion of Treg. [DOI] [PubMed] [Google Scholar]
- 38.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. Critical need to address the role of Treg in immunotherapy failures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI. Mechanisms and functional significance of tumor-induced dendritic cell differentiation in cancer. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 40.Chikamatsu K, Nakano K, Storkus WJ, et al. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281–1288. [PubMed] [Google Scholar]
- 41.Petersen TR, Buus S, Brunak S, Nissen MH, Sherman LA, Claesson MH. Identification and design of p53-derived HLA-A2-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001;53:357–364. doi: 10.1046/j.1365-3083.2001.00887.x. Optimized p53149-157 peptide identified for vaccine use. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann TK, Loftus DJ, Nakano K, et al. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264-272) epitope. J Immunol. 2002;168:1338–1347. doi: 10.4049/jimmunol.168.3.1338. Optimized p53264-2727 peptide can overcome tolerance to the wt pt3 264-272 peptide in HLA-A2+ patients with cancer. [DOI] [PubMed] [Google Scholar]
- 43.Lauwen MM, Zwaveling S, de Quartel L, et al. Self-tolerance does not restrict the CD4+ T-helper response against the p53 tumor antigen. Cancer Res. 2008;68:893–900. doi: 10.1158/0008-5472.CAN-07-3166. [DOI] [PubMed] [Google Scholar]
- 44.Fujita H, Senju S, Yokomizo H, et al. Evidence that HLA class II-restricted human CD4+ T cells specific to p53 self peptides respond to p53 proteins of both wild and mutant forms. Eur J Immunol. 1998;28:305–316. doi: 10.1002/(SICI)1521-4141(199801)28:01<305::AID-IMMU305>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–1081. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vence L, Palucka AK, Fay JW, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. PNAS. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. HLA class II-restricted tumor peptides recognized by antigen-specific T reg obtained from patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. Tregs play a critical role in immunological unresponsiveness to self-antigens. [DOI] [PubMed] [Google Scholar]
- 48.Arias RS, Flanagan ML, Miller KD, et al. RA8, a human anti-CD25 antibody against human Treg cells. Hybridoma (Larchmt) 2007;26:119–130. doi: 10.1089/hyb.2006.0041. [DOI] [PubMed] [Google Scholar]
- 49.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen specific immune responses to cancer vaccines. Blood. 2008 doi: 10.1182/blood-2008-01-135319. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 51.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czystowska M, Szczepanski M, Szajnik M, Whiteside TL. AACR, Abst #2090. San Diego, CA: 2008. IRX-2, a multi-targeted biologic, protects human T-cells from tumor-induced cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikitina EY, Clark JI, Van Beynen J, et al. Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001;7:127–135. [PubMed] [Google Scholar]
- 55.Svane IM, Pedersen AE, Johnsen HE, et al. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a phase I study. Cancer Immunol Immunother. 2004;53:633–41. doi: 10.1007/s00262-003-0493-5. First report on results of a wt p53 peptide-based vaccine trial for patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svane IM, Pedersen AE, Johansen JS, et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother. 2007;56:1485–99. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrin VE, Achtar MS, Steinberg SM, et al. ASCO JCO Abst #3011. Chicago, IL: 2007. A randomized phase II p53 vaccine trial comparing subcutaneous direct administration with intravenous peptide-pulsed dendritic cells in high risk ovarian cancer patients. Two- arm wt p53 peptide-based vaccine trial for ovarian cancer patients. [Google Scholar]
- 58.Barfoed AM, Petersen TR, Kirkin AF, Thor Straten P, Claesson MH, Zeuthen J. Cytotoxic T-lymphocyte clones, established by stimulation with the HLA-A2 binding p5365-73 wild type peptide loaded on dendritic cells in vitro, specifically recognized and lyse HLA-A2 tumour cells overexpressing the p53 protein. Scand J Immunol. 2000;51:128–133. doi: 10.1046/j.1365-3083.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 59.McArdle SE, Rees RC, Mulcahy KA, Saba J, McIntyre CA, Murray AK. Induction of human cytotoxic T lymphocytes that preferentially recognise tumour cells bearing a conformational p53 mutant. Cancer Immunol Immunother. 2000;49:417–425. doi: 10.1007/s002620000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umano Y, Tsunoda T, Tanaka H, Matsuda K, Yamaue H, Tanimura H. Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br J Cancer. 2001;84:1052–1057. doi: 10.1054/bjoc.2000.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]