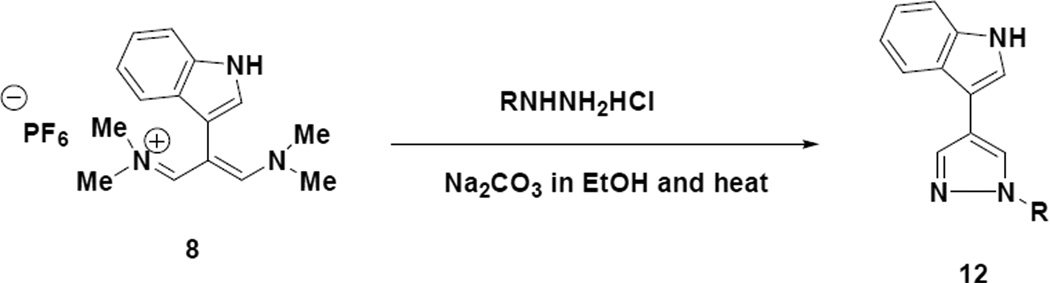Abstract
The preparation of an indole appended vinamidinium salt, an indole appended vinylogous amide and an indole appended chloroenal are described. The subsequent regiospecific conversion of these indole containing building blocks to functionalized pyrazoles and pyrroles is detailed.
Keywords: Vinamidinium salt, Vinylogous amide, Chloroenal, Pyrrole, Pyrazole, Indole
1. Introduction
The indole group has long been considered a privileged structure1 as it relates to the discovery of new medicinally active substances. The term privileged structure2 refers to the ability of “a single molecular framework able to provide ligands for diverse receptors”. It has been suggested2 that “indoles represent the most important of all structural classes in drug discovery” due to their extremely wide range of pharmacological activity. Some relevant examples of bioactive indole containing substances are presented in Figure 1.
Figure 1.
Biologically Active Indole Derivatives
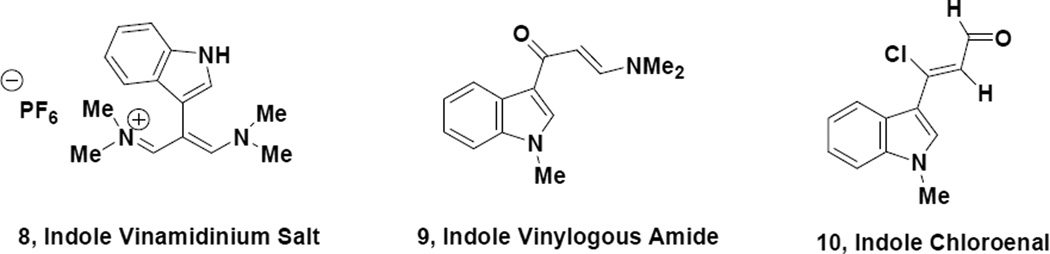
The range of compounds represented in Fig. 1 include Lycogallic acid3 natural products (1 and 2), which have been reported to be biosynthetic precursors to the antitumor agent Staurosporine4 (3), Chalcone-like antitumor agents5 (4), Meridianin type kinase inhibitors6 (5), microtubule inhibitors7 (6) and COX-2 inhibitors8 (7). Humphrey and Kuethe1 have previously reviewed practical methods for the synthesis of indole containing substances and a variety of useful methodologies are available. Our research group has been interested for some time in the use of vinylogous iminium compounds9 and their derivatives for the construction of important bioactive heterocycles and we envisioned using such systems as building blocks for the construction of indole appended heterocyclic motifs. Padwa10 and coworkers have recently pointed out the advantage of having access to versatile indole containing substances, which could be used for the construction of more highly functionalized compounds. To that end we have studied an indole appended vinamidinium salt (8), an indole appended vinylogous amide (9) and an indole appended chloroenal (10) as building blocks for the regio controlled synthesis of pyrazoles and pyrroles. Since pyrazole11 and pyrrole12 ring systems are commonly found in medicinal agents as well, we believed it would be of value to develop appropriate synthetic methodology for such a purpose.
2. Results and Discussions
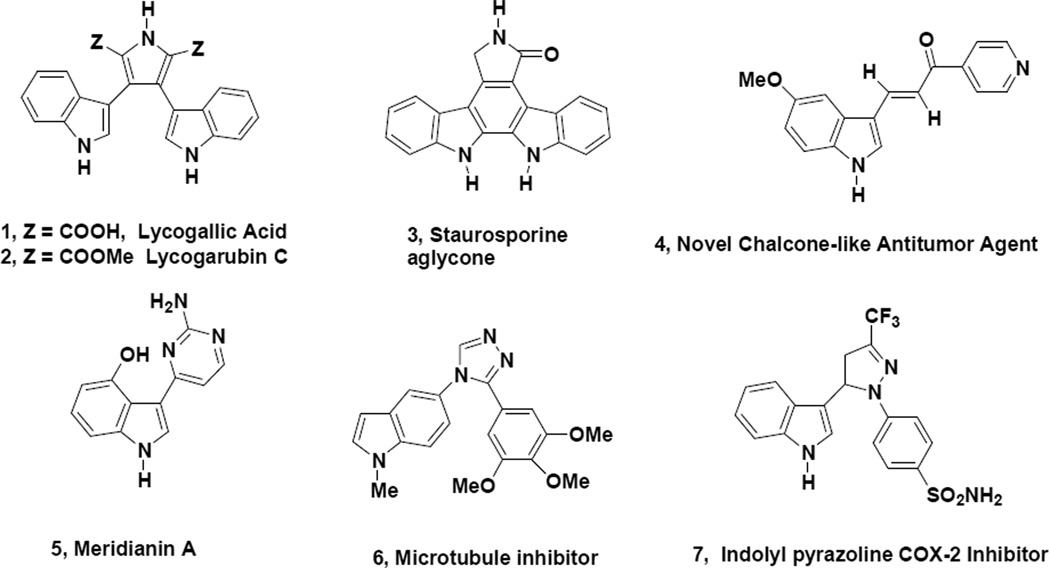
The synthesis of the 2-indolyl appended vinamidinium salt (the perchlorate version of 8) was reported in 1961 by Arnold13 but full characterization of this substance along with a detailed experimental procedure was not provided. We now report full details on the synthesis and characterization of the 2-indolyl appended vinamidinium hexfluorophosphate (8). Indole acetic acid is commercially available and this material is reacted under Vilmeier-Haack-Arnold conditions (Scheme 1) followed by quenching the reaction mixture with aqueous sodium hexfluorophosphate. The resulting salt (8) is somewhat tacky and is usually carefully dried under vacuum prior to being utilized in subsequent reactions.
Scheme 1.
Preparation of 2-Indole Apended Vinamidinium Salt
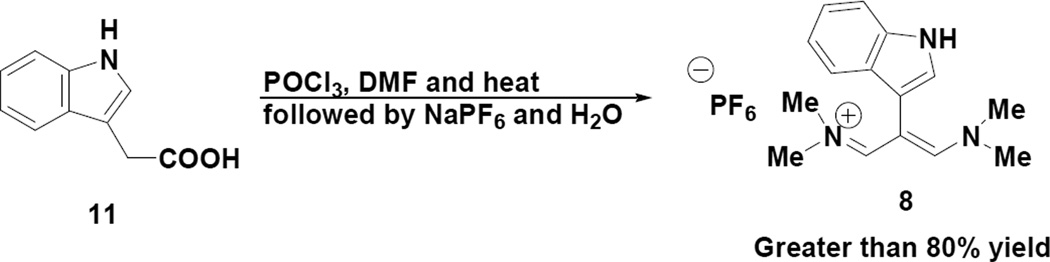
With this material (8) in hand we decided to initially look at its application to pyrazole synthesis (Scheme 2). One of the important aspects of using such a salt (8) for synthesis lies in the ability to control regiochemistry in the final product. Since the salt (8) is a symmetrically disposed molecule, the indole group would be expected to be located at the central carbon of the pyrazole ring. The following table provides a range of N-substituted pyrazoles, which were prepared by the indicated methodology.
Scheme 2.
Synthesis of 4-Indole Appended Pyrazoles
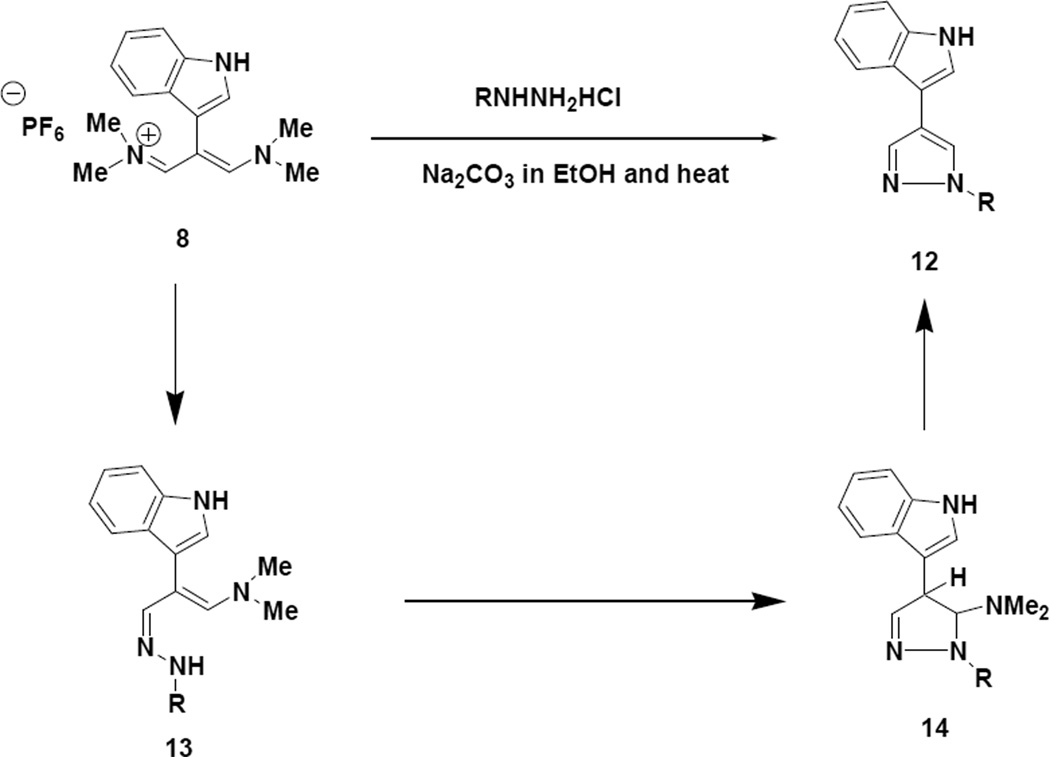
The crude reaction products are relatively clean and mild bases, such as the carbonates, can be used in these transformations. We envision the reaction proceeding as presented in Scheme 3.
Scheme 3.
Reaction Pathway to 4-Indole Appended Pyrazoles
Although the purified yields for the N-alkylpyrazoles were somewhat lower than for the N-arylpyrazoles, the reaction conditions appear to have considerable generality for the efficient preparation of 1,4-disusbtituted pyrazoles from the 2-indole appended vinamidinium salt (8).
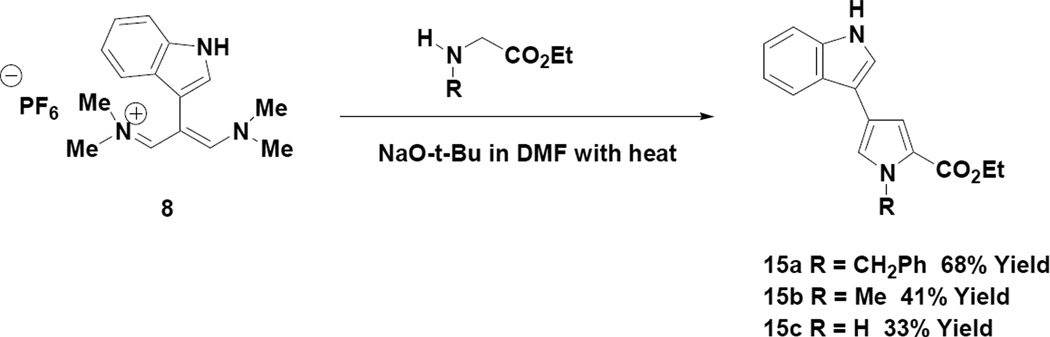
We next turned our attention to applying the indole appended vinamidinium salt (8) to the regioselective preparation of 2,4-disubstituted pyrroles9 (Scheme 4).
Scheme 4.
Conversion of Indole Vinamidinium Salt to 2,4-Disubstituted Pyrroles
From a mechanistic standpoint the reaction pathway to the 2,4-disubstituted pyrroles should resemble what is depicted in Scheme 3 with the exception that an enolate anion derived from the alpha carbon of the glycine derivatives would be the nucleophile, which is responsible for initiating the cyclization step. It should be noted that for the N-benzylglycine (15a) and N-methylglycine (15b) reactions, the intermediates are in fact azomethine ylids. The yields reported for the reactions depicted in Scheme 4 represent purified materials and may represent some physical loss during the purification process.
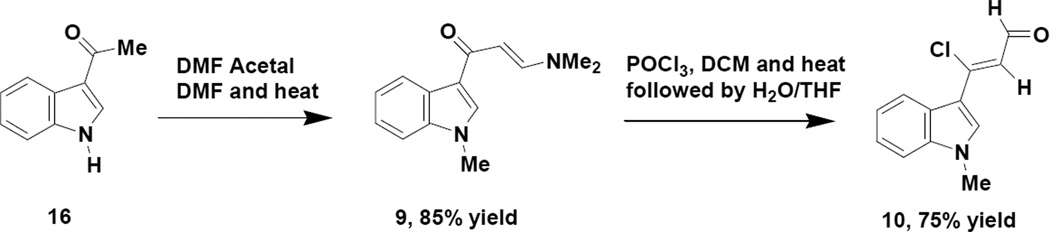
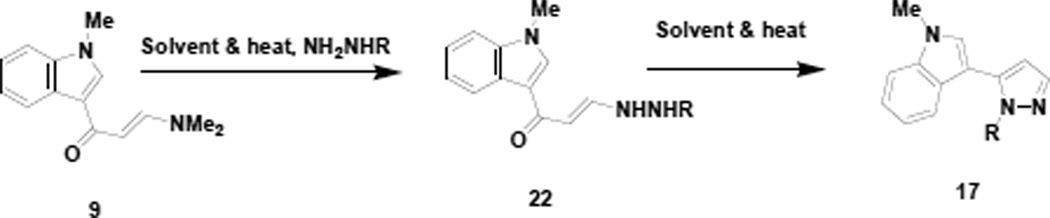
The ability to prepare indole appended pyrazoles and pyrroles that would be isomeric in a regiochemical sense to the compounds obtained from the vinamidium salt (8), could nicely compliment the chemistry already described and we subsequently decided to look at an indole appended vinylogous amide (9) and an indole appended chloroenal (10) for this reason. Scheme 5 represents the preparation of these materials (9 and 10).
Scheme 5.
Preparation of Indole Appended Vinylogous Amide and Chloroenal
Anizon6 and coworkers have previously described the preparation of the vinylogous amide (9) in their synthesis of indole appended pyrimidines, which are similar in structure to the Meridianin natural products. This material (9) is efficiently prepared from 3-acetylindole by reaction with DMF acetal in which case the nitrogen of the indole undergoes methylation as well. The crude vinylogous amide (9) is usually of very good purity and can be used in subsequent reactions without further purification. Treatment of vinylogous amide (9) with phosphorous oxychloride in dichloromethane with heating followed by room temperature treatment of the concentrated reaction mixture with a water/THF mixture produces the indole appended chloroenal (10) in good yield (75%). Our research group, as well as others14, have established the Z stereochemistry of such chloroenals and the indole appended chloroenal (10) exhibited spectral properties consistent with such an assignment. It should also be noted that Buchwald15 and Pellegatti have recently reported a continuous flow method for the preparation of the N-desmethyl analog of 10 by direct reaction of 3-acetylindole (16) with phosphorous oxychloride/DMF.
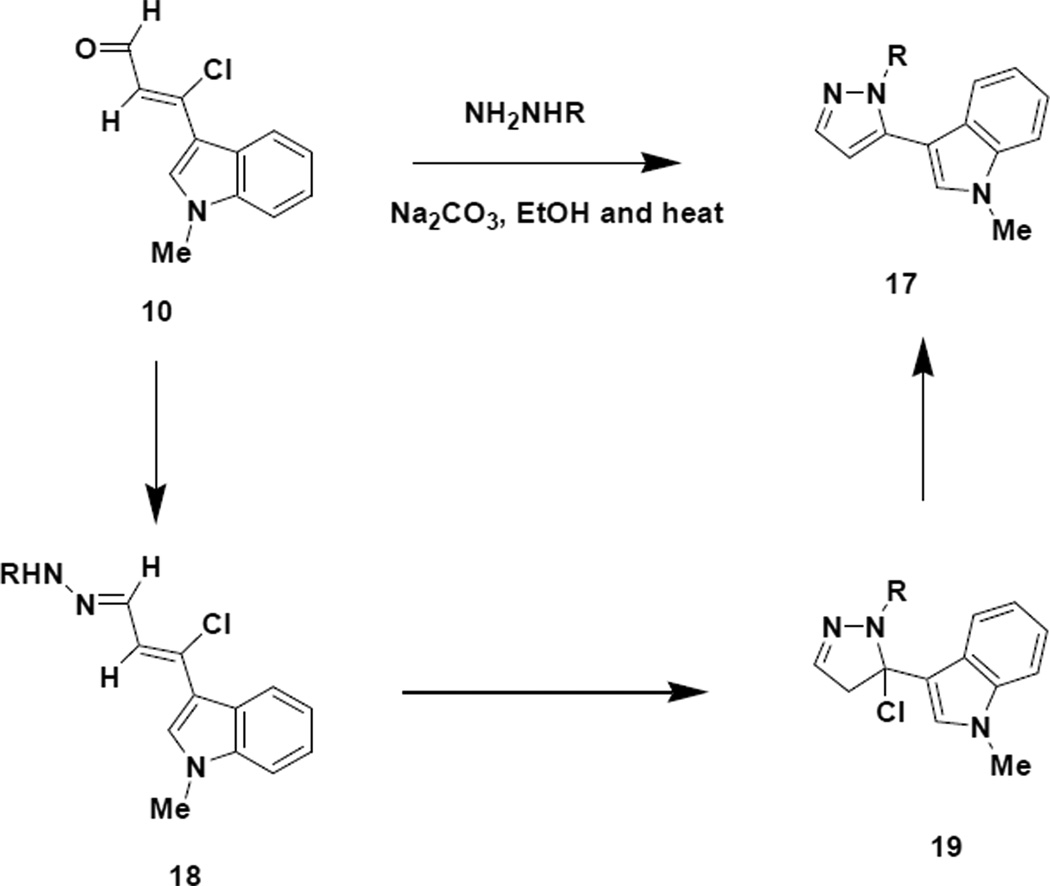
With both vinylogous amide (9) and chloroenal (10) in hand, we initiated studies to prepare the regioisomeric indole appended pyrazoles (17) as depicted in Scheme 6.
Scheme 6.
Preparation of 1,5-Disubstituted Pyrazoles
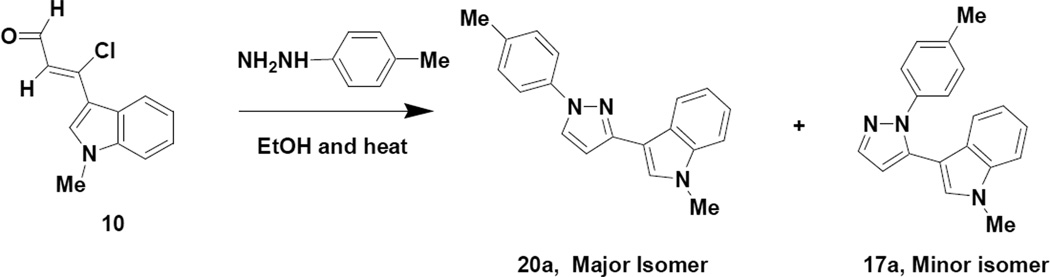
In order to obtain the 1,5-disubstituted regiochemistry, we propose that the hydrazone (18) of the chloroenal (10) is formed first followed by cyclization and loss of HCl to generate the aromatized product (17). NOESY NMR analysis of pyrazole 17a exhibited an interaction between the proton at the C-2 position of the indole ring with the proton at the C-2 position of the phenyl ring attached to the pyrazole nitrogen, thereby establishing the regiochemistry of the pyrazole being 1,5-disubstituted. Interestingly, when the reaction was repeated in the absence of base, two isomeric pyrazole products were indicated in the crude reaction mixture (Scheme 7). Our research group16 and others17 have reported that the chemical shift differential between ortho pyrazole hydrogens is increased in polar NMR solvents for the 1,3-isomer (chemical shift differential 1.45 Hz) relative to the 1,5-isomer (chemical shift differential 1.13 Hz). Applying this reasoning to our crude reaction mixture suggested an isomer ratio of 2:1 for the 1,3-isomer versus the 1,5-isomer, respectively. NOESY NMR analysis of the purified majority isomer (20a) indicated a NOESY interaction between the proton at the C-5 position of the pyrazole ring with the proton at C-2 position of the phenyl ring attached to the pyrazole nitrogen, thereby establishing the regiochemistry of the majority pyrazole product (20a) being 1,3-disubstituted. From a mechanistic standpoint this result suggests that, in the absence of base, initial reaction of the hydrazine occurs preferentially at the chlorovinyl carbon followed by cyclization at the aldehyde carbon.
Scheme 7.
Preparation of Isomeric Pyrazoles
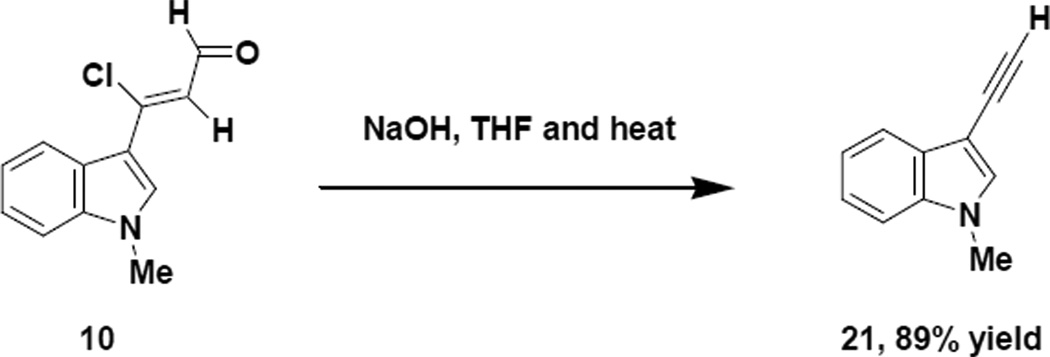
It is important to mention that most reactions conducted with the indole chloroenal (10) in the presence of base should be stirred at room temperature for at least 30 min to initiate the early steps in the reaction prior to providing heat to the reaction mixture. When the indole chloroenal (10) is subjected to heat and base in the absence of other reagents, a very efficient decarbonylative elimination takes place resulting in the clean formation of a 3-ethynylindole (21). Such a reaction can be of some utility as Boger18 has demonstrated in his synthesis of Lycogallic acid, which employs the use of 3-alkynylindoles.
As noted in Table 2, the purified yields of the 1,5-disubstituted-5-indole appended pyrazoles (17) were generally in the 50% range and were somewhat less than satisfying. We decided to look at the vinylogous amide (9) as a possible alternative precursor (Scheme 9) to the 1,5-disubstituted-5-indole appended pyrazoles (17). Using the vinylogous amide (9) would also have the advantages of shortening the reaction sequence by one step and removing the concern about an alternate elimination pathway (Scheme 8) in the case of the chloroenal (10). The results of this study are presented in Table 3.
Table 2.
Synthesis of 1,5-Disubstituted-5-Indole Appended Pyrazoles from Chloroenal
| Compound | R | % Yield (purified) |
|---|---|---|
| 17a | 4-Methylphenyl | 53 |
| 17b | 4-Chlorophenyl | 54 |
| 17c | 4-Bromophenyl | 31 |
| 17d | Phenyl | 52 |
| 17e | 4-Methoxyphenyl | 57 |
Scheme 9.
Conversion of Vinylogous Amide to 1,5-Disubstituted Pyrazole
Scheme 8.
Formation of 3-Ethynyl Indole
Table 3.
Synthesis of 1,5-Disubstituted-5-Indole Appended Pyrazoles from Vinylogous Amide
| Entry | Cpd. | R | % Yield (purified) | Solvent | Heat Source |
|---|---|---|---|---|---|
| 1 | 17a | 4-Methylphenyl | 82 | CH3CN | Microwave, 85°C for 2 hrs |
| 2 | 17b | 4-Chlorophenyl | 93 | CH3CN | Microwave, 85°C for 2 hrs |
| 3 | 17c | 4-Bromophenyl | 97 | CH3CN | Microwave, 85°C for 2 hrs |
| 4 | 17d | Phenyl | 52 | CH3CN | Microwave, 85°C for 2 hrs |
| 5 | 17e | 4-Methoxyphenyl | 84 | CH3CN | Microwave, 85°C for 2hrs |
| 6 | 17c | 4-Bromophenyl | 82 | HOAC | Microwave, 85°C for 2hrs |
| 7 | 17c | 4-Bromophenyl | 70 | EtOH | Microwave, 85°C for 2hrs |
| 8 | 17c | 4-Bromophenyl | 69 | CH3CN | Reflux for 4 hr |
| 9 | 17a | 4-Methylphenyl | 60 | HOAc | Reflux for 4 hr |
| 10 | 17b | 4-Chlorophenyl | 56 | HOAc | Reflux for 4 hr |
| 11 | 17c | 4-Bromophenyl | 82 | HOAc | Reflux for 4 hr |
| 12 | 17d | Phenyl | 51 | HOAc | Reflux for 4 hr |
| 13 | 17e | 4-Methoxyphenyl | 51 | HOAc | Reflux for 4 hr |
| 14 | 17f | H | 75 | HOAc | Reflux for 18 hr |
| 15 | 17g | Me | 59 (10:1 mix of the 1,5- to the 1,3-isomer by GC-MS) | HOAc | Reflux for 18 hr |
| 16 | 17h | Et | 69 (6:1 mix of the 1,5- to the 1,3-isomer by GC-MS) | HOAc | Reflux for 18 hr |
| 17 | 17i | t-Bu | 80 (32:1 mix of the 1,5- to the 1,3-isomer by GC-MS) | EtOH | Reflux for 18 hr |
| 18 | 17h | Et | 96 (78:1 mix of the 1,5- to the 1,3-isomer by GC-MS) | CH3CN | Reflux for 18 hr |
| 19 | 17i | t-Bu | 85 (22:1 mix of the 1,5- to the 1,3-isomer by GC-MS) | CH3CN | Reflux for 18 hr |
The entries 1–7 listed in Table 3 were conducted with the vinylogous amide (9) and the appropriate arylhydrazine in acetonitrile or acetic acid or ethanol with microwave heating. The crude reaction products were nearly analytically pure and after flash chromatography gave very good yields of the respective 1,5-disubstituted pyrazoles (17). When the reaction for an arylhydrazine was repeated with traditional heating in acetonitrile (entry 8), an acceptable, purified yield of the respective 1,5-disubstituted pyrazole was obtained. In addition to the conditions just described, we also evaluated arylhydrazines in acetic acid under microwave conditions (entry 6) along with arylhydrazines (entries 9–13) and alkylhydrazines (enties 15–16) in refluxing acetic acid. Both N-aryl- and N-alkylpyrazoles were formed in reasonable yields with acetic acid as the solvent and it was also noted that some of the 1,3-isomer was observed as a minor product for some of the N-alkyl analogs as determined by GC-MS analysis. The reaction with t-butyl hydrazine (entry 17) had to be performed in EtOH, since N-dealkylation of the pyrazole product was observed in acetic acid. When the ethylhydrazine (entry 18) and t-butylhydrazine reactions (entry 19) were repeated in refluxing acetonitrile, very good yields of the pyrazoles were obtained and excellent regiochemical selectivity was also observed. In an overall sense, the acetonitrile conditions appear to be superior for producing the 1,5-isomer selectively and in high yield.
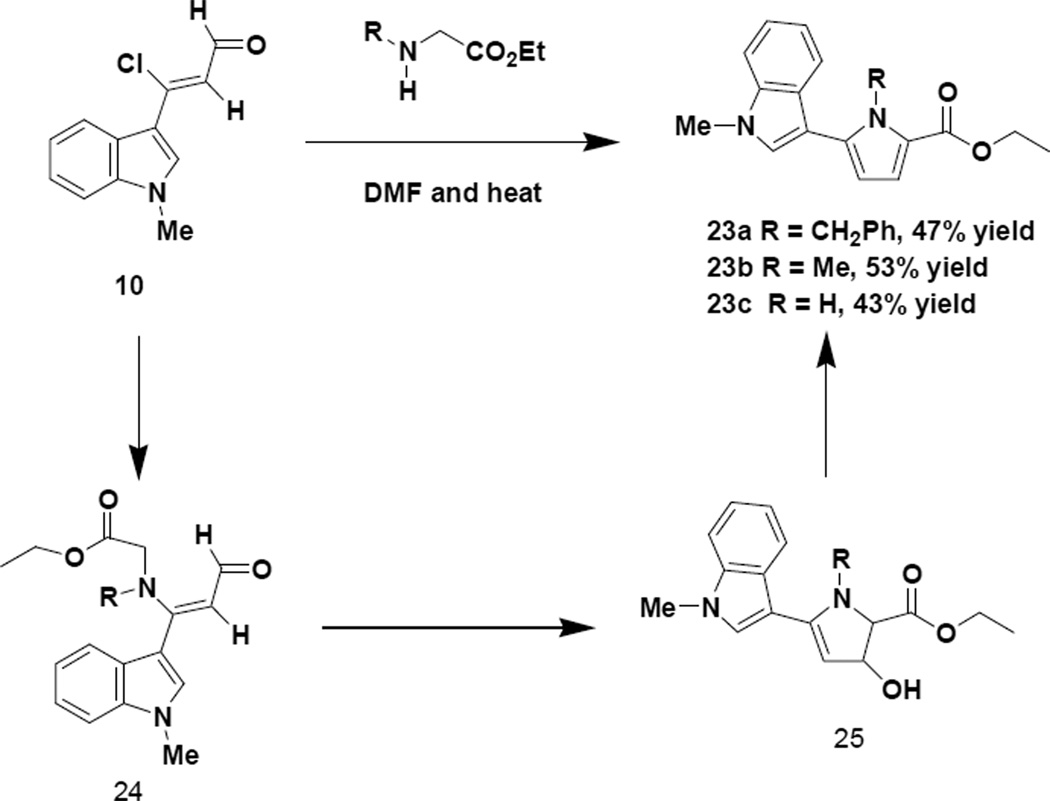
Our last set of studies involved reacting glycine ethyl ester and N-substituted derivatives with the indole chloroenal (10) in anticipation of obtaining a 2,5-disubstituted pyrrole9 (23) as opposed to a 2,4-disubstituted pyrrole (15), which had been produced with the indole vinamidinium salt (8). The suggested pathway for this process is given in Scheme 10.
Scheme 10.
Preparation of 2,5-Disubstituted Pyrroles
We believe that the first step in the process involves the displacement of the chlorine of the chloroenal (10) by the nitrogen of the amino acid ester to produce a vinylogous amide (24), which undergoes cyclization and dehydration to the resultant 2,5-disubstituted pyrrole. The regiochemistry of the 2,5-disubstituted pyrroles was determined by a NOESY NMR analysis of 23b, which contains a methyl group at the pyrrole nitrogen. An NOE was seen for the hydrogen at the 2 position of the indole with both the indole methyl group and also the pyrrole methyl group. An NOE was also seen for the methylene and methyl groups of the ester with the hydrogen at carbon 3 of the pyrrole ring.
3. Conclusions
Based on the results described herein, we believe that we have established that an indole appended vinamidinium salt (8), an indole appended vinylogous amide (9) and an indole appended chloroenal (10) represent useful and important building blocks for the regiospecific construction of the corresponding indole appended pyrazoles and pyrroles. Since the indole vinamidinium salt produces different regio isomers from the vinylogous amide and chloroenal, it is now possible to select a particular indole building block, which will furnish a predictable and selective regiochemical outcome. The fact that the indole appended pyrazoles and pyrroles can be assembled in a minimum number of steps from commercially available starting materials suggests that this chemistry could be very useful for SAR guided work in the course of optimizing pharmacodynamic or pharmacokinetic aspects of an indole containing drug candidate. Additionally, the indole appended chloroenal (10) can be used to prepare a 3-ethynylindole (21), which presents potential applications for Sonogashira type coupling reactions.
4. Experimental
4.1 General
All chemicals were used as received from the manufacturer (Aldrich Chemicals and Fisher Scientific). All solvents were dried over 4 angstrom molecular sieves prior to their use. NMR spectra were obtained on either a Bruker 300 MHz spectrometer, or a Bruker 500 MHz spectrometer in either CDCl3, d6-DMSO or d6-acetone solutions. IR spectra were recorded on a Nicolet Avatar 320 FT-IR spectrometer with an HATR attachment. Most high resolution mass spectra were obtained on a Bruker Biotof Q electrospray mass spectrometer at the University of Richmond. Some high resolution mass spectra were provided by the Merck Research Laboratories. Low resolution GC-MS spectra were obtained on a Shimadzu QP 5050 instrument. Melting points and boiling points are uncorrected. Chromatographic separations were carried out on a Biotage SP-1 instrument or a Biotage Isolera instrument (both equipped with a silica cartridge). Gradient elution with ethyl acetate/hexane was accomplished in both instances. The reaction products were normally eluted within the range of 4–8 column volumes of eluant with a gradient mixture of 60–80% ethyl acetate in hexane. TLC analyses were conducted on silica plates with hexane/ethyl acetate as the eluant. All purified reaction products gave TLC results, flash chromatograms, and 13C NMR spectra consistent with a sample purity of >95%.
4.1.1 2-(1-Indolyl)-1,3-bis(dimethylamino)trimethinium hexafluorophosphate (8)
To a 100 mL round bottom flask equipped with a magnetic stirring bar, thermometer, reflux condenser and addition funnel, was added 15 mL of dry DMF, which was immediately cooled to 0–5°C. Phosphorous oxychloride (5.250 g) was added dropwise via the addition funnel while the reaction temperature was controlled between 10–15 °C with an ice bath. The reaction mixture was then cooled to 0–5°C and indole-3-acetic acid (2.000 g) was added to the reaction mixture in small portions. After removal of the cooling bath, the reaction mixture was heated at 75–80°C for 3 hours while ensuring that the reaction temperature never exceeded 90°C. The reaction mixture was then cooled to 30°C and ~65g of ice was carefully added to the flask. After the ice had melted, the reaction mixture was poured into a solution of sodium hexafluorophosphate (6.180 g in 100 mL of water) with stirring in which case a dark solid precipitated. Due to the tacky nature of the solid, the reaction mixture was extracted with dichloromethane (2 × 80 mL) followed by drying the organic phase with anhydrous sodium sulfate. Removal of the drying agent via filtration and concentration of the organic phase in vacuo produced a dark solid (3.300 g, 75% yield), which exhibited the following physical properties: mp 196–198°C; 1H NMR (acetone-d6) δ 2.58 (s, 6H), 3.38 (s, 6H), 7.16 (t, J=7.8 Hz, 1H), 7.23 (t, J=7.8 Hz, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.46 (d, J = 7.8 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H) and 7.86 (s, 2H); 13C NMR (acetone-d6) 165.3, 136.2, 130.2, 127.0, 122.6, 120.5, 119.1, 112.0, 105.5, 97.3, 48.2 and 37.8; IR (neat) 1588 cm−1; HRMS (ES) m/z calcd for C15H20N3 242.1652, found 242.1582. It should be noted that the perchlorate derivative of this substance was previously prepared by Arnold13 but was not full characterized by NMR and MS techniques.
4.1.2 3-(1-p-Tolyl-1H-pyrazol-4-yl)-1H-indole (12a)
To a 100 mL round bottom flask equipped with a magnetic stirring bar and reflux condenser was added 0.500 g (1.29 mmol) of the indole trimethinium salt (8) along with 4-methylphenylhydrazine hydrochloride (0.307 g, 1.94 mmol) and 0.411 g (3.87 mmol) of sodium carbonate. Dry ethanol (30 mL) was then added to the flask and the resulting mixture was refluxed overnight. After cooling the reaction mixture to room temperature, the solvent was removed in vacuo and the residue was partitioned between chloroform (30 mL) and water (30 mL). The aqueous phase was extracted with additional chloroform (2 × 30 mL), the combined organic phases were dried over anhydrous magnesium sulfate, filtered and concentrated to give a dark solid. This material was subjected to flash chromatography on a Biotage SP-1 instrument with a silica column in which case 0.231 g (65% yield) of a red solid was obtained upon elution with six column volumes of a hexane/ethyl acetate gradient. This solid exhibited the following properties: mp 190–192°C; 1H NMR (acetone-d6) δ 2.40 (s, 3H), 7.15 (t, J=7.0 Hz, 1H), 7.20 (t, J=7.0 Hz, 1H), 7.33 (d, J = 9.0 Hz, 2H), 7.48 (d, J = 7.0 Hz, 1H),7.85 (d, J = 9.0 Hz, 2H), 7.67 (s, 1H), 7.96 (d, J = 7.0 Hz, 1H), 8.06 (s, 1H) and 8.63 (s, 1H); 13C NMR (acetone-d6) δ 138.7, 138.4, 137.0, 135.4, 129.8, 125.9, 122.4, 121.9, 121.6, 119.5, 118.7, 118.3, 111.6, 107.7, and 19.5; IR (neat) 3387 cm−1; HRMS (ES) m/z calcd for C18H16N3 274.1339, found 274.1356.
4.1.3 3-[1-(4-Methoxyphenyl)-1H-pyrazol-4-yl]-1H-indole (12b)
This material was prepared in a manner similar to 12a with the exception that 4-methoxyphenylhydrazine hydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a maroon solid was obtained in 61% yield after flash chromatographic purification. This solid exhibited the following properties: mp 166–168°C; 1H NMR (acetone-d6) δ 3.02 (s, 3H), 7.08 (d, J = 6.0 Hz, 2H), 7.17 (m, 2H), 7.49 (d, J = 7.2 Hz, 1H), 7.65 (d, J = 3.0 Hz, 1H), 7.87 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 7.2 Hz, 1H), 8.06 (s, 1H) and 8.56 (s, 1H); 13C NMR (acetone-d6) δ 158.1, 138.5, 137.0, 134.3, 125.9, 122.6, 121.8, 121.7, 119.9, 119.5, 118.5, 114.5, 111.6, 107.9 and 55.0; IR (neat) 3372 cm−1; HRMS (ES) m/z calcd for C18H16N3O 290.1288, found 290.1285.
4.1.4 3-(1-Phenyl-1H-pyrazol-4-yl)-1H-indole (12c)
This material was prepared in a manner similar to 12a with the exception that phenylhydrazine hydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a brown solid was obtained in 94% yield after flash chromatographic purification. This solid exhibited the following properties: mp 161–163°C; 1H NMR (acetone-d6) δ 7.14 (t, J=7.0 Hz, 1H), 7.20 (t, J=7.0 Hz, 1H), 7.32 (t, J=7.5 Hz, 1H), 7.48-7.55 (m, 3H), 7.69 (d, J = 2.0 Hz, 1H), 7.96-7.99 (m, 3H), 8.10 (s, 1H) and 8.69 (s, 1H); 13C NMR (acetone-d6) δ 140.5, 139.1, 137.1, 129.4, 125.9, 125.8, 122.6, 122.0, 121.7, 119.6, 119.5, 119.0, 118.3, 111.6 and 107.7; IR (neat) 3219 cm−1; HRMS (ES) m/z calcd for C17H14N3 260.1182, found 260.1206.
4.1.5 3-[1-(4-Bromophenyl)-1H-pyrazol-4-yl]-1H-indole (12d)
This material was prepared in a manner similar to 12a with the exception that 4-bromophenylhydrazine hydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a dark red solid was obtained in 60% yield after flash chromatographic purification. This solid exhibited the following properties: mp 194–196°C; 1H NMR (acetone-d6) δ 7.14 (t, J=8.0 Hz, 1H), 7.19 (t, J=8.0 Hz, 1H), 7.33 (d, J = 9.0 Hz, 2H), 7.47 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 5.0 Hz, 1H), 7.85 (d, J = 9.0 Hz, 2H), 7.96 (d, J = 8.0 Hz, 1H), 8.06 (s, 1H) and 8.63 (s, 1H); 13C NMR (acetone-d6) δ 139.7, 139.6, 137.1, 132.3, 125.8, 122.5, 122.2, 121.8, 120.1, 119.6, 119.5, 119.4, 118.3, 111.7 and 107.5; IR (neat) 3267 cm−1; HRMS (ES) m/z calcd for C17H13BrN3 338.0287, found 338.0271.
4.1.6 3-[1-(4-Chlorophenyl)-1H-pyrazol-4-yl]-1H-indole (12e)
This material was prepared in a manner similar to 12a with the exception that 4-chlorophenylhydrazine hydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a brown solid was obtained in 92% yield after flash chromatographic purification. This solid exhibited the following properties: mp 143–145°C; 1H NMR (acetone-d6) δ 7.12-7.23 (m, 2H), 7.50 (d, J = 9.0 Hz, 1H), 7.54 (d, J = 9 .0 Hz, 2H), 7.69 (d, J = 3.0 Hz, 1H), 7.95-8.01 (m, 3H), 8.12 (s, 1H) and 8.70 (s, 1H); 13C NMR (acetone-d6) δ 139.5, 139.3, 137.0, 130.5, 129.3, 125.8, 122.5, 122.0, 121.7, 119.7, 119.5, 119.4, 119.3, 111.6 and 107.5; IR (neat) 3302 cm−1; HRMS (ES) m/z calcd for C17H13ClN3 294.0793, found 294.0796.
4.1.7 3-[1-(4-Fluorophenyl)-1H-pyrazol-4-yl]-1H-indole (12f)
This material was prepared in a manner similar to 12a with the exception that 4-fluorophenylhydrazine hydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a brown solid was obtained in 87% yield after flash chromatographic purification. This solid exhibited the following properties: mp 148–150°C; 1H NMR (acetone-d6) δ 7.12-7.21 (m, 2H), 7.29 (t, J= 9.0 Hz, 2H), 7.49 (d, J = 9 .0 Hz, 1H), 7.67 (d, J = 3 .0 Hz, 1H), 7.96-8.011 (m, 3H), 8.11 (s, 1H) and 8.65 (s, 1H); 13C NMR (acetone-d6) δ 162.2 (d, J = 242.6 Hz), 139.1, 137.1, 125.8, 122.7, 121.8 (d, J = 19.5 Hz), 120.2 (d, J = 8.6 Hz), 119.5 (d, J =4.6 Hz),119.1, 116.1, 115.8, 111.6, 111.5 and 107.6; IR (neat) 3384 cm−1; HRMS (ES) m/z calcd for C17H13FN3 278.1088, found 278.1093.
4.1.8 3-(1-Methyl-1H-pyrazol-4-yl)-1H-indole (12g)
This material was prepared in a manner similar to 12a with the exception that methylhydrazine was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a solid was obtained in 65% yield after chromatographic purification. This solid exhibited the following properties: mp 121–122°C (dec.); 1H NMR (acetone-d6) δ 3.93 (s, 1H), 7.10 (t, J=7.5 Hz, 1H), 7.14 (t, J=7.5 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.51 (d, J = 2.4 Hz, 1H), 7.76 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H) and 7.93 (s, 1H); 13C NMR (acetone-d6) δ 137.2, 135.6, 126.7, 126.2, 121.7, 121.4, 121.3, 119.5, 116.8, 111.8, 108.7 and 38.3; HRMS (ES) m/z calcd for C12H12N3 198.1026, found 198.1067.
4.1.9 3-(1H-Pyrazol-4-yl)-1H-indole (12h)
This material was prepared in a manner similar to 12a with the exception that hydrazine dihydrochloride was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a solid was obtained in 44% yield after chromatographic purification. This solid exhibited the following properties: mp 175–180°C (dec.); 1H NMR (acetone-d6) δ 7.10 (t, J= 7.4 Hz, 1H), 7.16 (t, J=7.4 Hz, 1H), 7.45 (d, J = 7.6 Hz, 1H), 7.54 (s, 1H), 7.84 (d, J = 7.6 Hz, 1H) and 8.00 (s, 2H); 13C NMR (acetone-d6) δ 137.2, 126.3, 121.7, 121.6, 121.4, 119.6, 119.5, 115.9, 111.8, 111.7 and 108.8; HRMS (ES) m/z calcd for C11H10N3 184.0869, found 184.0621.
4.1.10 3-(1-Ethyl-1H-pyrazol-4-yl)-1H-indole (12i)
This material was prepared in a manner similar to 12a with the exception that ethylhydrazine oxalate was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a solid was obtained in 56% yield after chromatographic purification. This solid exhibited the following properties: mp 87–88°C; 1H NMR (acetone-d6) δ 1.48 (t, J= 7.2 Hz, 3H), 4.22 (q, J= 7.1 Hz, 2H), 7.11 (t, J= 7.4 Hz, 1H), 7.18 (t, J= 7.6 Hz, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.83 (m, 2H) and 7.98 (s, 1H); 13C NMR (acetone-d6) δ 137.2, 136.5, 126.2, 125.2, 121.8, 121.4, 119.5, 119.2, 116.5, 111.8, 108.8, 46.7 and 15.5; HRMS (ES) m/z calcd for C13H14N3 212.1182, found 212.1198.
4.1.11 3-(1-tert-Butyl-1H-pyrazol-4-yl)-1H-indole (12j)
This material was prepared in a manner similar to 12a with the exception that t-butylhydrazine oxalate was used in the reaction in place of 4-methylphenylhydrazine hydrochloride in which case a solid was obtained in 39% yield after chromatographic purification. This solid exhibited the following properties: mp 45–47°C; 1H NMR (acetone-d6) δ 1.64 (s, 9H), 7.08 (t, J= 7.4 Hz, 1H), 7.14 (t, J= 7.6 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.51 (d, J = 2.4 Hz, 1H), 7.81 (s, 1H), 7.84 (d, J = 7.6 Hz, 1H) and 8.06 (s, 1H); 13C NMR (acetone-d6) δ 137.2, 136.1, 126.2, 122.4, 121.7, 121.4, 119.6, 119.4, 111.7, 109.0, 58.2 and 29.0; HRMS (ES) m/z calcd for C15H18N3 240.1495, found 240.1508.
4.1.12 1-Benzyl-4-(1H-indol-3-yl)-1H-pyrrole-2-carboxylic acid ethyl ester (15a)
To a 100 mL round bottom flask equipped with a magnetic stirring bar, thermometer and reflux condenser was added sodium hydride (0.125g, 5.16 mmol), 20 mL of dry DMF and t-butanol (0.74 mL, 7.7 mmol). Once the reaction between the sodium hydride and the t-butanol was completed, N-benzylglycine ethyl ester (0.70 mL, 3.9 mmol) was added and the resultant mixture was stirred for several minutes. The indoletrimethinium salt (1) (0.500 g, 1.29 mmol) was then added to the reaction mixture in one portion and the resulting solution was heated at reflux overnight. After cooling the reaction mixture to room temperature, it was diluted with water (100 mL) and extracted with ethyl acetate (2 × 50 mL). The combined organic phases were washed with water (2 × 50 mL), brine (2 × 50 mL) and dried over anhydrous sodium sulfate. Removal of the drying agent by filtration and concentration of the filtrate in vacuo, resulted in a dark solid. This solid was subjected to flash chromatography on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient in which case a light yellow solid (0.302 g, 68% yield) was obtained. This solid exhibited the following properties: mp 105–107°C; 1H NMR (acetone-d6) δ 1.31 (t, J= 7.2 Hz, 3H), 4,25 (q, 2H), 5.71 (s, 2H), 7.11-7.19 (m, 2H), 7.25-7.33 (m, 6H), 7.46 (d, J = 7.2 Hz, 1H), 7.55 (m, 1H), 7.57 (d, J= 2.1 Hz, 1H) and 7.88 (d, J = 7.2 Hz, 1H); 13C NMR (acetone-d6) δ 160.8, 139.3, 137.1, 128.4, 127.2, 126.9, 125.9, 125.8, 122.1, 121.5, 121.4, 119.4, 119.3, 118.9, 115.7, 111.6, 110.5, 59.4, 51.5 and 13.9; IR (neat) 3382 and 1699 cm−1; HRMS (ES) m/z calcd for C22H21N2O2 345.1598, found 345.1521.
4.1.13 4-(1H-Indol-3-yl)-1-methyl-1H-pyrrole-2-carboxylic acid ethyl ester (15b)
This material was prepared in a manner similar to compound 15a with the exception that N-methylglycine ethyl ester hydrochloride was used in place of N-benzylglycine ethyl ester in which case a tan solid (41% yield) was obtained after flash chromatographic purification. This material exhibited the following properties: mp 105–107°C; 1H NMR (acetone-d6) δ 1.36 (t, J= 7.2 Hz, 3H), 4.00 (s, 3H), 4.30 (q, J= 7.2 Hz, 2H), 7.08-7.18 (m, 2H), 7.21 (d, J = 2.1 Hz, 1H), 7.37 (d, J = 1.8 Hz, 1H),7.44 (d, J = 9.0 Hz, 1H), 7.50 (d, J = 1.8 Hz, 1H) and 7.85 (d, J = 9.0 Hz, 1H); 13C NMR (acetone-d6) δ 160.9, 137.1, 126.3, 125.9, 122.5, 121.4, 121.2, 119.4, 119.2, 118.2, 115.0, 111.5, 110.7, 59.3, 36.0 and 13.9; IR (neat) 3391 and 1674 cm−1; HRMS (ES) m/z calcd for C16H17N2O2 269.1285, found 269.1184.
4.1.14 4-(1H-Indol-3-yl)-1H-pyrrole-2-carboxylic acid ethyl ester (15c)
This material was prepared in a manner similar to compound 15a with the exception that glycine ethyl ester hydrochloride was used in place of N-benzylglycine ethyl ester in which case a tan solid (33% yield) was obtained after flash chromatographic purification. This material exhibited the following properties: mp 135–136°C; 1H NMR (acetone-d6) δ 1.36 (t, J= 7.0 Hz, 3H), 4.32 (q, J= 7.0 Hz, 2H), 7.12 (t, J= 8.0 Hz, 1H), 7.17 (t, J= 8.0 Hz, 1H), 7.21 (m, 1H), 7.44 (m, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.55 (d, J = 2.5 Hz, 1H) and 7.86 (d, J = 8.0 Hz, 1H); 13C NMR 160.6, 137.1, 126.0, 123.0, 121.4, 121.3, 120.5, 119.7, 119.4, 119.3, 112.8, 111.5, 110.9, 59.5 and 13.9; IR (neat) 3386, 3310 and 1682 cm−1; HRMS (ES) m/z calcd for C15H15N2O2 255.1128, found 255.1127.
4.1.15 3-Dimethylamino-1-(1-methyl-1H-indol-3-yl)-propenone (9)
The preparation of this substance has been previously reported by Anizon6 and coworkers.
4.1.16 3-Chloro-3-(1-methyl-1H-indol-3-yl)-propenal (10)
To a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was added the indole vinylogous amide (9) (0.700 g, 3.06 mmol) followed by POCl3 (0.575 mL, 6.29 mmol) in dichloromethane (20 mL). The mixture was refluxed for 2 hours and the dichloromethane was removed in vacuo. The residue was dissolved in 50 mL of a 50:50 mixture of water:THF and stirred at room temperature for 24 hours. The reaction mixture was diluted with 50 mL of water, extracted with ethyl acetate (3 × 30 mL), washed with brine (30 mL), dried over anhydrous magnesium sulfate and concentrated in vacuo to yield 0.500 g (74.6% yield) of a solid. This solid was suitable for use without further purification but an analytical sample was prepared by flash chromatography on a Biotage Isolera instrument with a hexane:ethyl acetate gradient, which produced a dark solid and this material exhibited the following properties: mp: 103–106°C; 1H NMR (acetone-d6) δ 3.99 (s, 3H), 6.71 (d, J=7.0 Hz, 1H), 7.34 (t, J=6.5 Hz, 1H), 7.38 (t, J= 6.5 Hz, 1H), 7.69 (d, J= 6.5 Hz, 1H), 7.97 (d, J= 6.5 Hz, 1H), 8.15 (s, 1H), 10.20 (d, J=7.0 Hz, 1H); 13C NMR (acetone-d6) δ 189.8, 145.9, 138.7, 135.3, 124.5, 123.3, 122.2, 120.2, 119.4, 112.2, 111.1 and 32.8; IR (neat) 1656 cm−1; HRMS (ES) m/z calculated for C12H10NOCl 220.0524, found 220.0553. It should be noted that Buchwald15 and Pellegatti have recently reported the N-unsubstituted analog of this material using continuous flow methodology.
4.1.17 1-Methyl-3-(2-p-tolyl-2H-pyrazol-3-yl)-1H-indole (17a) Method A
To a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was added ethanol (40 mL), sodium carbonate (0.434 g, 4.10 mmol), 4-methylphenylhydrazine hydrochloride (0.260 g, 1.64 mmol) and the indole chloroenal (10) (0.300 g, 1.37 mmol). The resulting mixture was stirred for one hour at room temperature, heated at reflux overnight, cooled to room temperature and the solvent was removed in vacuo. The residue was partitioned between 30 mL of a 50:50 mixture of chloroform:water and the aqueous phase was extracted with additional chloroform (2 × 30 mL). The combined organic phases were dried over anhydrous magnesium sulfate and concentrated in vacuo to yield a solid, which was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant produced a dark solid (0.210 g, 53 % yield), which exhibited the following properties: mp: 122–125°C; 1H NMR (acetone-d6) δ 2.32 (s, 3H), 3.81 (s, 3H), 6.56 (d, J= 1.8 Hz, 1H), 7.03 (t, J= 7.2 Hz, 1H), 7.05 (s, 1H), 7.16 (d, J= 6.3 Hz, 2H), 7.21 (t, J= 7.2 Hz, 1H), 7.29 (d, J= 6.3 Hz, 2H), 7.39 (d, J= 7.2 Hz, 1H), 7.41 (d, J= 7.2 Hz, 1H) and 7.69 (d, J= 1.8 Hz, 1H); 13C NMR (acetone-d6) δ 139.7, 138.8, 137.0, 136.9, 136.8, 129.2, 128.6, 126.6, 124.7, 121.9, 119.8, 119.5, 109.7, 107.1, 104.9 , 32.1 and 20.1; HRMS (ES) m/z calcd for C19H18N3 288.1495, found 288.1689. NOESY NMR analysis of this compound indicated a NOESY interaction between the proton at the C-2 position of the indole ring with the proton at C-2 position of the phenyl ring, thereby establishing the regiochemistry of the pyrazole being 2,3-disubstituted.
4.1.18 1-Methyl-3-(1-p-tolyl-1H-pyrazol-3-yl)-1H-indole (20a) Method B
The above reaction conditions were repeated with the exception that no base was used in this trial. When the standard workup was applied to the reaction mixture, a dark solid was obtained in 97% yield. 1H NMR analysis of the crude product indicated a mixture of 1,2- and 1,3-isomers with regard to the location of the phenyl and indolyl groups around the pyrazole ring system. Our research group16 as well as others17 have reported that the chemical shift differential between ortho pyrazole hydrogens is increased in polar NMR solvents for the 1,3-isomer versus the 2,3-isomer and applying this reasoning to our crude reaction 1H NMR suggested an isomer ratio of 2:1 for the 1,3-isomer:2,3-isomer, respectively. The crude reaction mixture was then subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and hexane/ethyl acetate gradient. Elution with 4–5 column volumes of eluant produced a dark solid which exhibited the following properties: mp: 136–140°C; 1H NMR (acetone-d6) δ 2.39 (s, 3H), 3.91 (s, 3H), 6.82 (d, J= 2.6 Hz, 1H), 7.21 (t, J= 7.8 Hz, 1H), 7.23 (t, J= 7.8 Hz, 1H), 7.34 (d, J= 6.6 Hz, 2H), 7.43 (d, J= 7.8 Hz, 1H), 7.73 (s, 1H), 7.84 (d, J= 6.6 Hz, 2H), 8.27 (d, J= 2.6 Hz, 1H) and 8.42 (d, J= 7.8 Hz, 1H); 13C NMR (acetone-d6) δ 149.1, 138.4, 137.6, 135.0, 129.8, 127.6, 127.1, 126.0, 121.8, 121.7, 119.7, 117.9, 109.4, 109.1, 104.5, 32.1 and 19.9; HRMS (ES) m/z calcd for C19H18N3 288.1495, found 288.1504. NOESY NMR analysis of this compound indicated a NOESY interaction between the proton at the C-5 position of the pyrazole ring with the proton at C-2 position of the phenyl ring, thereby establishing the regiochemistry of the pyrazole being 1,3-disubstituted.
4.1.19 3-[2-(4-Chlorophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17b) Method A
This compound was prepared in an identical fashion to compound 17a (reaction with base) with the exception that 4-chlorophenylhydrazine hydrochloride was used in place of 4-methylphenylhydrazine hydrochloride in which case a 54% yield of a dark solid was obtained and this material exhibited the following properties: mp: 147–152°C; 1H NMR (acetone-d6) δ 3.86 (s, 3H), 6.58 (d, J= 1.5 Hz, 1H), 7.04 (t, J= 7.0 Hz, 1H), 7.19 (s, 1H), 7.23 (t, J= 7.0 Hz, 1H), 7.29 (d, J= 7.0 Hz, 1H), 7.37 (d, J= 6.5 Hz, 2H), 7.43-7.47 (m, 3H) and 7.74 (d, J= 1.5 Hz, 1H); 13C NMR (acetone-d6) δ 140.4, 139.8, 137.2, 137.1, 132.0, 128.9, 128.7, 126.4, 126.0, 122.1, 120.1, 119.4, 109.9, 107.9, 104.5 and 32.2; HRMS (ES) m/z calcd for C18H15ClN3 308.0949, found 308.1018.
4.1.20 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c) Method A
This compound was prepared in an identical fashion to compound 17a (reaction with base) with the exception that 4-bromophenylhydrazine hydrochloride was used in place of 4-methylphenylhydrazine hydrochloride in which case a 31% yield of a dark solid was obtained and this material exhibited the following properties: mp: 135–138°C; 1H NMR (acetone-d6) δ 3.86 (s, 3H), 6.58 (d, J= 1.5 Hz, 1H), 7.04 (t, J= 8.0 Hz, 1H), 7.20 (s, 1H), 7.23 (t, J= 8.0 Hz, 1H), 7.34 (d, J= 8.0 Hz, 1H), 7.38 (d, J= 6.5 Hz, 2H), 7.46 (d, J= 8.0 Hz, 1H), 7.51 (d, J= 6.5 Hz, 2H) and 7.74 (d, J= 1.5 Hz, 1H); 13C NMR (acetone-d6) δ 140.5, 140.3, 137.1, 137.0, 131.7, 128.9, 126.4, 126.3, 122.1, 120.0, 119.9, 119.4, 109.9, 107.9, 104.5 and 32.2; HRMS (ES) m/z calcd for C18H15BrN3 352.0444, found 352.0456.
4.1.21 1-Methyl-3-(2-phenyl-2H-pyrazol-3-yl)-1H-indole (17d) Method A
This compound was prepared in an identical fashion to compound 17a (reaction with base) with the exception that phenylhydrazine was used in place of 4-methylphenylhydrazine in which case a 52% yield of a dark solid was obtained and this material exhibited the following properties: mp: 135–139°C; 1H NMR (acetone-d6) δ 3.81 (s, 3H), 6.59 (d, J= 1.5 Hz, 1H), 7.02 (t, J= 7.5 Hz, 1H), 7.07 (s, 1H), 7.22 (t, J= 7.5 Hz, 1H), 7.30-7.37 (m, 4H), 7.42-7.44 (m, 3H) and 7.72 (d, J= 1.5 Hz, 1H); 13C NMR (acetone-d6) δ 141.2, 140.1, 137.1, 137.0, 128.7, 127.1, 126.5, 124.8, 122.0, 120.0, 119.5, 109.8, 107.4, 104.9 and 32.2; HRMS (ES) m/z calcd for C18H16N3 274.1339, found 274.1489.
4.1.22 3-[2-(4-Methoxyphenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17e) Method A
This compound was prepared in an identical fashion to compound 17a (reaction with base) with the exception that 4-methoxyphenylhydrazine hydrochloride was used in place of 4-methylphenylhydrazine hydrochloride in which case a 57% yield of a dark solid was obtained and this material exhibited the following properties: mp: 128–131°C; 1H NMR (acetone-d6) δ 3.81 (s, 3H), 3.80 (s, 3H), 6.57 (d, J= 2.0 Hz, 1H), 6.91 (d, J= 7.0 Hz, 2H), 7.02 (s, 1H), 7.05 (t, J= 8.0 Hz, 1H), 7.21 (t, J= 8.0 Hz, 1H), 7.31 (d, J= 7.0 Hz, 2H), 7.42 (d, J= 8.0 Hz, 1H) and 7.67 (d, J= 2.0 Hz, 1H); 13C NMR (acetone-d6) δ 158.9, 139.6, 137.1, 136.9, 134.2, 128.6, 126.6, 126.4, 122.0, 119.9, 119.5, 113.9, 109.8, 106.7, 104.9, 59.7 and 32.3; HRMS (ES) m/z calcd for C19H18N3O 304.1444, found 304.1525.
4.1.23 3-Ethynyl-1-methyl-1H-indole (21)
To a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was added sodium hydroxide (0.920g, 22.9 mmol), THF (40 mL) and the indole chloroenal (10) (1.26g, 5.74 mmol). The resulting mixture was refluxed overnight, cooled to room temperature and diluted with water (50 mL). The reaction mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic phases were washed with brine (2 × 25 mL), dried with anhydrous sodium sulfate, filtered and concentrated in vacuo to yield a viscous oil (0.790g, 89% yield), which exhibited the following properties: bp: 104–105°C @ 0.67 torr; 1H NMR (CDCl3) δ 3.23 (s, 1H), 3.81 (s, 3H), 7.23 (t, J= 7.0 Hz, 1H), 7.32 (t, J= 7.0 Hz, 1H), 7.33 (s, 1H), 7.35 (d, J= 7.0 Hz, 1H) and 7.76 (d, J= 7.0 Hz, 1H); 13C NMR (acetone-d6) δ 136.3, 133.5, 129.3, 122.4, 120.1, 119.3, 110.0, 79.1, 77.4 and 32.2; IR (neat) 2918 and 2362 cm−1; HRMS (ES) m/z calcd for C11H10N 156.0808, found 156.0792.
4.1.24 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c) Method C
Into a 20 mL microwave reactor tube equipped with a magnetic stir bar was placed acetonitrile (8 mL), 4-bromophenylhydrazine hydrochloride (0.590g, 2.63 mmol) and the indole vinylogous amide (9) (0.400g, 1.75 mmol). The reaction tube was sealed with a crimping cap and heated in a Biotage Initiator microwave reactor system at 85°C for two hours. After the microwave heating was completed, the crimping cap was removed and the reaction mixture was concentrated in vacuo. The residue was taken up in a water (40 mL) and ethyl acetate (30 mL) mixture and, after separation of the phases, the aqueous phase was extracted with additional ethyl acetate (2 × 30 mL). The combined organic phases were washed with brine and dried over anhydrous magnesium sulfate followed by filtration and concentration in vacuo. The resultant solid was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant produced a dark solid (0.600g, 97% yield), which was identical to compound 17c by proton NMR and tlc comparison.
4.1.25 1-Methyl-3-(1-p-tolyl-1H-pyrazol-3-yl)-1H-indole (17a) Method C
This compound was prepared in an identical fashion to the previous example with the exception that 4-methylphenylhydrazine hydrochloride was used in place of 4-bromophenylhydrazine hydrochloride in which case an 82% yield of a dark solid was obtained and this material was identical to compound 17a by proton NMR and tlc comparison.
4.1.26 3-[2-(4-Chlorophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17b) Method C
This compound was prepared in an identical fashion to the previous example with the exception that 4-chlorophenylhydrazine hydrochloride was used in place of 4-methylphenylhydrazine hydrochloride in which case an 93% yield of a dark solid was obtained and this material was identical to compound 17b by proton NMR and tlc comparison.
4.1.27 1-Methyl-3-(2-phenyl-2H-pyrazol-3-yl)-1H-indole (17d) Method C
This compound was prepared in an identical fashion to the previous example with the exception that phenylhydrazine hydrochloride was used in place of 4-chlorophenylhydrazine hydrochloride in which case a 52% yield of a dark solid was obtained and this material was identical to compound 17d by proton NMR and tlc comparison.
4.1.28 3-[2-(4-Methoxyphenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17e) Method C
This compound was prepared in an identical fashion to the previous example with the exception that 4-methoxyphenylhydrazine hydrochloride was used in place of phenylhydrazine hydrochloride in which case an 84% yield of a dark solid was obtained and this material was identical to compound 17e by proton NMR and tlc comparison.
4.1.29 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c) Method C
This compound was prepared in an identical fashion to the previous example with the exception that glacial acetic acid was used as the solvent and 4-bromophenylhydrazine hydrochloride was used in place of the 4-methoxyphenylhydrazine hydrochloride in which case an 82% yield of a dark solid was obtained and this material was identical to compound 17c by proton NMR and tlc comparison.
4.1.30 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c) Method C
This compound was prepared in an identical fashion to the previous example with the exception that anhydrous ethanol was used as the solvent in which case a 100% crude yield of a dark solid was obtained and this material was identical to compound 17c by proton NMR and tlc comparison.
4.1.31 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c): Method D
To a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was added acetonitrile (30 mL), 4-bromophenylhydrazine hydrochloride (0.590g, 2.63 mmol) and the indole vinylogous amide (9) (0.400g, 1.75 mmol). The mixture was stirred and heated at reflux for 4 hours and the reaction mixture was concentrated in vacuo after cooling to room temperature. The reaction mixture was diluted with ethyl acetate (30 mL) and water (30 mL), the phases were separated and the aqueous phase was extracted with additional ethyl acetate (2 × 30 mL).The combined organic phases were washed with brine, dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo to yield a solid. This material was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant produced a dark solid (0.424g, 69% yield), which was identical to compound 17c by proton NMR and tlc comparison.
4.1.32 3-[2-(4-Bromophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17c) Method D
To a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was added glacial acetic acid (30 mL), 4-bromophenylhydrazine hydrochloride (0.590g, 2.63 mmol) and the indole vinylogous amide (3) (0.400g, 1.75 mmol). The mixture was stirred and heated at reflux for 4 hours, cooled to room temperature and diluted with 50 mL of water. The reaction mixture was carefully neutralized with aqueous sodium bicarbonate solution and extracted with ethyl acetate (3 × 30 mL).The combined organic phases were washed with brine, dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo to yield a solid. This material was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant produced a dark solid (0.506g, 82% yield), which was identical to compound 17c by proton NMR and tlc comparison.
4.1.33 1-Methyl-3-(1-p-tolyl-1H-pyrazol-3-yl)-1H-indole (17a) Method D
This compound was prepared in an identical fashion to the previous example with the exception that 4-methylphenylhydrazine hydrochloride was used in place of 4-bromophenylhydrazine hydrochloride in which case a 60% yield of a dark solid was obtained and this material was identical to compound 17a by proton NMR and tlc comparison.
4.1.34 3-[2-(4-Chlorophenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17b) Method D
This compound was prepared in an identical fashion to the previous example with the exception that 4-chlorophenylhydrazine hydrochloride was used in place of 4-methylphenylhydrazine hydrochloride in which case a 56% yield of a dark solid was obtained and this material was identical to compound 17b by proton NMR and tlc comparison.
4.1.35 1-Methyl-3-(2-phenyl-2H-pyrazol-3-yl)-1H-indole (17d) Method D
This compound was prepared in an identical fashion to the previous example with the exception that phenylhydrazine hydrochloride was used in place of 4-chlorophenylhydrazine hydrochloride in which case a 51% yield of a dark solid was obtained and this material was identical to compound 17d by proton NMR and tlc comparison.
4.1.36 3-[2-(4-Methoxyphenyl)-2H-pyrazol-3-yl]-1-methyl-1H-indole (17e) Method D
This compound was prepared in an identical fashion to the previous example with the exception that 4-methoxyphenylhydrazine hydrochloride was used in place of phenylhydrazine hydrochloride in which case a 51% yield of a dark solid was obtained and this material was identical to compound 17e by proton NMR and tlc comparison.
4.1.37 1-Methyl-3-(2H-pyrazol-3-yl)-1H-indole (17f) Method D
This compound was prepared in an identical fashion to the previous example with the exception that hydrazine dihydrochloride was used in place of 4-methoxyphenylhydrazine hydrochloride in which case a 75% yield of a dark solid was obtained and this material exhibited the following properties: mp: 119–120°C; 1H NMR (acetone-d6) δ 3.83 (s, 3H), 6.61 (d, J= 2.0 Hz, 1H), 7.15 (t, J= 7.4 Hz, 1H), 7.30 (t, J= 7.4 Hz, 1H), 7.41 (d, J= 8.0 Hz, 1H), 7.61 (s, 1H), 7.68 (d, J= 2.0 Hz, 1H) and 8.17 (d, J= 8.0 Hz, 1H); 13C NMR (acetone-d6) δ 142.8, 137.3, 134.9, 127.4, 126.1, 122.4, 120.4, 120.3, 110.0, 107.0, 102.7 and 32.9; HRMS (ES) m/z calcd for C12H12N3 198.1026, found 198.1024.
4.1.38 1-Methyl-3-(2-methyl-2H-pyrazol-3-yl)-1H-indole (17g) Method D
This compound was prepared in an identical fashion to the previous example with the exception that methylhydrazine was used in place of hydrazine dihydrochloride in which case a 59% yield of a dark oil was obtained and this material exhibited the following properties: bp: 205–215°C @ 0.75 torr; 1H NMR (acetone-d6) δ 3.86 (s, 3H), 3,93 (s, 3H), 6.40 (d, J= 1.6 Hz, 1H), 7.16 (t, J= 7.4 Hz, 1H), 7.27 (t, J= 7.6 Hz, 1H), 7.45 (d, J= 1.6 Hz, 1H), 7.50 (d, J= 8.0 Hz, 1H), 7.54 (s, 1H) and 7.62 (d, J= 7.6 Hz, 1H); 13C NMR (acetone-d6) δ 138.1, 137.2, 136.9, 128.7, 127.1, 122.3, 120.2, 119.6, 110.1, 105.3, 104.9, 37.1 and 32.5; HRMS (ES) m/z calcd for C13H13N3 212.1182, found 212.1179.
4.1.39 3-(2-Ethyl-2H-pyrazol-3-yl)-1-methyl-1H-indole (17h) Method D
This compound was prepared in an identical fashion to the previous example with the exception that ethylhydrazine oxalate was used in place of methylhydrazine in which case a 69% yield of a dark oil was obtained and this material exhibited the following properties: bp: 181–190°C @ 0.50 torr; 1H NMR (acetone-d6) δ 1.35 (t, J= 7.2 Hz, 3H), 3.91 (s, 3H), 4,22 (q, J= 7.2 Hz, 2H), 6.36 (d, J= 2.0 Hz, 1H), 7.15 (t, J= 7.4 Hz, 1H), 7.27 (d, J= 7.6 Hz, 1H), 7.47 (s, 1H), 7.52-7.49 (m, 2H) and 7.57 (d, J= 7.6 Hz, 1H); 13C NMR (acetone-d6) δ 138.4, 137.3, 136.1, 128.5, 127.4, 122.3, 120.2, 119.5, 110.1, 105.7, 104.8, 44.2, 32.4 and 15.4; HRMS (ES) m/z calcd for C14H16N3 226.1339, found 226.1336.
4.1.40 3-(2-Ethyl-2H-pyrazol-3-yl)-1-methyl-1H-indole (17h) Method D
This compound was prepared in an identical fashion to the previous example with the exception that acetonitrile was used as the solvent in which case a 96% yield of a dark oil was obtained and this material was identical to compound 17h by proton NMR and GC-MS comparison.
4.1.41 3-(2-tert-Butyl-2H-pyrazol-3-yl)-1-methyl-1H-indole (17i) Method D
This compound was prepared in an identical fashion to the previous example with the exception that t-butylhydrazine hydrochloride was used in place of ethylhydrazine oxalate and ethanol was used as the solvent in which case an 80% yield of a dark solid was obtained and this material exhibited the following properties: mp: 118–121°C; 1H NMR (acetone-d6) δ 1.46 (s, 9H), 3.91 (s, 3H), 6.15 (d, J= 1.6 Hz, 1H), 7.09 (t, J= 7.4 Hz, 1H), 7.20-7.27 (m, 3H), 7.34 (s, 1H) and 7.46 (d, J= 8.0 Hz, 1H); 13C NMR (acetone-d6) δ 139.4, 136.2, 130.0, 129.5, 123.2, 122.5, 122.0, 120.0, 117.7, 110.8, 110.0, 60.5, 32.4 and 30.4; HRMS (ES) m/z calcd for C16H20N3 254.1652, found 254.1652.
4.1.41 3-(2-tert-Butyl-2H-pyrazol-3-yl)-1-methyl-1H-indole (17i) Method D
This compound was prepared in an identical fashion to the previous example with the exception that acetonitrile was used as the solvent in which case an 85% yield of a dark solid was obtained and this material was identical to compound 17i by proton NMR and GC-MS comparison.
4.1.42 1-Benzyl-5-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2-carboxylic acid ethyl ester (23a)
Into a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was placed indole chloroenal (10) (0.250g, 1.14 mmol), 30 mL of dry DMF and benzyl glycine ethyl ester (0.53 mL, 2.9 mmol). The reaction mixture was stirred at room temperature for one hour after which it was heated to reflux overnight. The reaction mixture was allowed to cool to room temperature, diluted with 100 mL of water and extracted with ethyl acetate (3 × 25 mL). The combined organic layers were washed with water (2 × 25 mL) and brine (1 × 15 mL) and dried over anhydrous sodium sulfate. The drying agent was removed by filtration and the filtrate was concentrated in vacuo to give an orange liquid (0.410 g). This material was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant produced an orange liquid (0.192g, 47% yield), which exhibited the following properties: bp: 58–59°C @ 0.13 torr; 1H NMR (acetone-d6) δ 1.26 (t, J= 7.0 Hz, 3H), 3.83 (s, 3H), 4.18 (q, J= 7.0 Hz, 2H), 5.75 (s, 2H), 6.39 (d, J= 4.0 Hz, 1H), 6.88 (d, J= 8.5 Hz, 2H), 7.13-7.22 (m, 6H), 7.24 (t, J= 7.0 Hz, 1H), 7.45 (d, J= 7.0 Hz, 1H) and 7.61 (d, J= 7.0 Hz, 1H); 13C NMR (acetone-d6) δ 160.6, 140.0, 137.0, 135.6, 128.6, 128.2, 127.6, 126.6, 125.1, 122.6, 122.1, 120.0, 119.3, 118.5, 109.8, 109.7, 105.9, 59.1, 48.7, 32.1 and 13.8; IR (neat) 1690 cm−1; HRMS (ES) m/z calcd for C23H23N2O2 359.1754, found 359.1773.
4.1.43 1-Methyl-5-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2-carboxylic acid ethyl ester (23b)
Into a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was placed indole chloroenal (10) (0.500g, 2.28 mmol), 30 mL of dry DMF and sarcosine ethyl ester hydrochloride (0.700g, 4.55 mmol). The reaction mixture was stirred at room temperature for one hour after which it was heated to reflux overnight. The reaction mixture was allowed to cool to room temperature, diluted with 100 mL of water and extracted with ethyl acetate (3 × 25 mL). The combined organic layers were washed with water (2 × 25 mL) and brine (1 × 15 mL) and dried over anhydrous sodium sulfate. The drying agent was removed by filtration and the filtrate was concentrated in vacuo to give a crude residue (0.600g). The crude residue (diluted with ethyl acetate) was placed on the surface of a silica plug and eluted with 50 mL of hexane followed by 50 mL of 50:50 mix of hexane:ethyl acetate. The two fractiosn were combined to give 0.340g (53% yield) of a yellow liquid, which exhibited the following properties: bp: 169–172°C @ 1.50 torr; 1H NMR (acetone-d6) δ 1.35 (t, J= 7.5 Hz, 3H), 3.92 (s, 3H), 3.95 (s, 3H), 4.28 (q, J= 7.5 Hz, 2H), 6.27 (d, J= 4.0 Hz, 1H), 7.03 (d, J= 4.0 Hz, 1H), 7.16 (t, J= 7.0 Hz, 1H), 7.28 (t, J= 7.0 Hz, 1H), 7.48 (s, 1H), 7.50 (d, J= 7.0 Hz, 1H) and 7.59 (d, J= 7.0 Hz, 1H); 13C NMR (acetone-d6) δ 160.9, 137.0, 135.4, 129.0, 127.4, 122.8, 122.0, 119.9, 119.5, 117.4, 109.8, 108.7, 106.1, 59.1, 33.6, 32.2 and 13.9; IR 1702 (neat) cm−1; HRMS (ES) m/z calcd for C17H19N2O2 283.1441, found 283.1438. The regiochemistry of the 2,5-disubstituted pyrrole was determined by a NOESY NMR analysis. An NOE was seen for the hydrogen at the 2 position of the indole with both the indole methyl group and also the pyrrole methyl group. An NOE was also seen for the methylene and methyl groups of the ester with the hydrogen at carbon 3 of the pyrrole ring.
4.1.44 5-(1-Methyl-1H-indol-3-yl)-1H-pyrrole-2-carboxylic acid ethyl ester (23c)
Into a 100 mL round bottom flask equipped with a magnetic stir bar and reflux condensor was placed indole chloroenal (10) (0.350g, 1.59 mmol), 30 mL of dry DMF and glycine ester hydrochloride (0.77 g, 5.56 mmol). The reaction mixture was stirred at room temperature for one hour after which it was heated to reflux overnight. The reaction mixture was allowed to cool, diluted with 100 mL of water and extracted with ethyl acetate (3 × 25 mL). The combined organic layers were washed with water (2 × 25 mL), brine (1 × 15 mL) and dried over anhydrous sodium sulfate. Removal of the drying agent by filtration and concentration of the filtrate in vacuo gave a crude residue (0.316 g) of a dark solid. This material was subjected to flash chromatographic purification on a Biotage SP-1 instrument with a silica column and a hexane/ethyl acetate gradient. Elution with 5–6 column volumes of eluant gave 0.137g (43% yield), which exhibited the following properties: mp: 148–150°C; 1H NMR (acetone-d6) δ 1.33 (t, J= 7.5 Hz, 3H), 3.89 (s, 3H), 4.28 (q, J= 7.5 Hz, 2H), 6.57 (d, J= 4.0 Hz, 1H), 6.95 (d, J= 4.0 Hz, 1H), 7.18 (t, J= 7.0 Hz, 1H), 7.26 (t, J= 7.0 Hz, 1H),7.46 (d, J= 7.0 Hz, 1H), 7.78 (s, 1H) and 7.91 (d, J= 7.0 Hz, 1H); 13C NMR 160.5, 137.4, 132.7, 126.9, 125.8, 121.9, 121.5, 119.9, 119.7, 116.4, 109.8, 107.5, 106.8, 59.3, 32.2 and 14.0; IR (neat) 1678 cm−1; HRMS (ES) m/z calcd for C16H17N2O2 269.1285, found 269.1301.
Figure 2.
Indole Appended Building Blocks
Table 1.
Synthesis of 4-Indolyl Appended Pyrazoles
| Compound | R | % Yield (purified) |
|---|---|---|
| 12a | 4-Methylphenyl | 65 |
| 12b | 4-Methoxyphenyl | 61 |
| 12c | Phenyl | 94 |
| 12d | 4-Bromophenyl | 60 |
| 12e | 4-Chlorophenyl | 92 |
| 12f | 4-Fluorophenyl | 87 |
| 12g | Methyl | 65 |
| 12h | H | 44 |
| 12i | Ethyl | 56 |
| 12j | t-Butyl | 39 |
Acknowledgements
We gratefully thank the National Institutes of Health (grant no. R15-CA67236) for support of this research. Thanks also to the Floyd D. and Elisabeth S. Gottwald Endowment and to the University of Richmond Faculty Research Grant program for support to JTG. We are exceedingly grateful to Mr. Dave Patteson formerly of Biotage Inc. for the generous donation of an SP-1 flash chromatography system, which was used in the majority of sample purifications. We are also very appreciative of Advion Inc. for the generous donation of a CMS electrospray mass spectrometer. Previous grants from the MRI program of the National Science Foundation for the purchase of a 500 MHz NMR spectrometer (CHE-0116492) and a high resolution electrospray mass spectrometer (CHE-0320669) are also gratefully acknowledged. We also appreciate assistance from Christopher Warme and Wendy Zhong at the Merck Research Laboratories in obtaining HRMS data for some of our compounds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Humphrey G, Kuethe J. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 2.Horton D, Bourne G, Smythe M. Chem. Rev. 2003;103:893. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 3.Frode R, Hinze C, Josten I, Schmidt B, Steffan B, Steglich W. Tetrahedron Lett. 1994;35:1689. [Google Scholar]
- 4.Nishizawa T, Gruschow S, Jayamaha D, Nishizawa-Harada C, Sherman D. J. Am Chem. Soc. 2006;128:724. doi: 10.1021/ja056749x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson M, Overmeyer J, Young A, Erhardt P, Maltese W. J. Med. Chem. 2012;55:1940. doi: 10.1021/jm201006x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossignol E, Youssef A, Moreau P, Prudhomme M, Anizon F. Tetrahedron. 2007;63:10169. [Google Scholar]
- 7.Arora S, Wang X, Keenan S, Andaya C, Zhang Q, Peng Y, Welsh W. Cancer Res. 2009;69:1910. doi: 10.1158/0008-5472.CAN-08-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy M, Billa V, Pallela V, Mallireddigari M, Boominathan R, Gabriel J, Reddy P. Bioorg. Med. Chem. 2008;16:3907. doi: 10.1016/j.bmc.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 9.See for example; Gupton J, Yu R, Krolikowski D, Riesinger S, Sikorski J. J. Org. Chem. 1990;55:4735. Gupton J, Krolikowski D, Yu R, Vu P, Sikorski J, Dahl M, Jones C. J. Org. Chem. 1992;57:5480.
- 10.Li H, Boonnak N, Padwa A. J. Org. Chem. 2011;76:9488. doi: 10.1021/jo201955c. [DOI] [PubMed] [Google Scholar]
- 11.Thaher B, Arnsmann M, Totzke F, Ehlert J, Kubbutat M, Schachtele C, Zimmermann M, Koch P, Boeckler F, Laufer S. J. Med. Chem. 2012;55:961. doi: 10.1021/jm201391u. [DOI] [PubMed] [Google Scholar]
- 12.Pfefferkorn J, Bowles D, Kissel W, Boyles D, Choi C, Larsen S, Song Y, Sun K, Miller S, Trivedi B. Tetrahedron. 2007;63:8124. [Google Scholar]
- 13.Arnold Z. Coll. Czech. Chem. Commun. 1961;26:2852. [Google Scholar]
- 14.Prim D, Fuss A, Kirsch G, Silva A. J. Chem. Soc. Perkin Trans 2. 1999:1175. [Google Scholar]
- 15.Pellegatti L, Buchwald S. Org. Process Res. Dev. 2012;16:1442. [Google Scholar]
- 16.Gupton J, Clough S, Miller R, Norwood B, Hickenboth C, Chertudi I, Cutro S, Petrich S, Hicks F, Wilkinson D, Sikorski J. Tetrahedron. 2002;58:5467. [Google Scholar]
- 17.Cohen-Fernandes P, Erkelens C, van Endenberg C, Verhoeven J, Habraken C. J. Org. Chem. 1979;44:4156. [Google Scholar]
- 18.Oakdale J, Boger D. Org. Lett. 2010;12:1132. doi: 10.1021/ol100146b. [DOI] [PMC free article] [PubMed] [Google Scholar]