Abstract
Despite substantial recent advancements in psychiatric genetic research, progress in identifying the genetic basis of anxiety disorders has been limited. We review the candidate gene and genome-wide literatures in anxiety, which have made limited progress to date. We discuss several reasons for this hindered progress, including small samples sizes, heterogeneity, complicated comorbidity profiles, and blurred lines between normative and pathological anxiety. To address many of these challenges, we suggest a developmental, multivariate framework that can inform and enhance anxiety phenotypes for genetic research. We review the psychiatric and genetic epidemiological evidence that supports such a framework, including the early onset and chronic course of anxiety disorders, shared genetic risk factors among disorders both within and across time, and developmentally dynamic genetic influences. We propose three strategies for developmentally sensitive phenotyping: examination of early temperamental risk factors, use of latent factors to model underlying anxiety liability, and use of developmental trajectories as phenotypes. Expanding the range of phenotypic approaches will be important for advancing studies of the genetic architecture of anxiety disorders.
Many excellent papers in this Special Issue address the ways in which genetic and genomic sciences are informing our understanding of developmental psychopathology. Here, we take a complementary approach in which we explore the ways that developmental science can contribute to more informative genetic studies. We focus specifically on anxiety disorders and argue that a developmental approach to the phenotype will be particularly important, given the early onset and high prevalence of anxiety disorders in children.
We cover four main topics: the current state of the candidate gene and genome-wide association literatures in anxiety disorders; current obstacles to gene finding in anxiety genetics; the psychiatric and genetic epidemiology of anxiety disorders with a specific focus on data that can guide developmental, multivariate approaches to phenotype definition; and strategies for developmentally sensitive phenotyping that could be used in existing samples and in future study designs.
We note here a clarification of our use of the broad term anxiety disorders. DSM-IV-TR lists 13 separate anxiety disorders, but we will focus on the most commonly diagnosed, idiopathic anxiety disorders: generalized anxiety disorder (GAD), panic disorder (PD) with and without agoraphobia, agoraphobia without a history of PD, separation anxiety disorder, social phobia, and specific phobia. Although the DSM-IV-TR includes obsessive–compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) in the anxiety category, there is also evidence that these disorders have partly distinct etiologic and neurobiological underpinnings (Eley et al., 2003; Graybiel & Rauch, 2000; Heim & Nemeroff, 2009; Tambs et al., 2009). For this reason, this paper will not include OCD and PTSD, an approach that is consistent with the new DSM-5 proposal for the anxiety disorders category. We also acknowledge the strong phenotypic and genetic overlap between depression and many of the anxiety disorders (Brady & Kendall, 1992; Franic, Middeldorp, Dolan, Ligthart, & Boomsma, 2010; Kendler, Neale, Kessler, Heath, & Eaves, 1992; Middeldorp, Cath, Van Dyck, & Boomsma, 2005; Weissman et al., 2005), but for simplicity we focus here on the anxiety disorders themselves.
Anxiety disorders are among the most common forms of child, adolescent, and adult psychopathology (lifetime prevalence of 28.8%; Kessler, Berglund, et al., 2005; Merikangas, He, Brody, et al., 2010; Merikangas, He, Burstein, et al., 2010). These disorders not only affect a large number of individuals but also are chronic and disabling conditions resulting in considerable individual and societal cost. Anxiety disorders as a group had the largest burden of role disability among the common mental health conditions, exceeding the burden for mood disorders and substance abuse/dependence disorders in a large, national representative sample of adults (Merikangas et al., 2007). In addition, anxiety disorders typically emerge in childhood (Kessler, Berglund, et al., 2005; Merikangas, He, Burstein, et al., 2010) and can be impairing across development through disruption to family, peer, and academic functioning (Essau, Conradt, & Petermann, 2000; Ezpeleta, Keeler, Erkanli, Costello, & Angold, 2001). The economic burden of anxiety disorders is substantial (estimated at $63 billion in 1998 dollars; Greenberg et al., 1999). Beyond the costs for psychiatric treatment, there are also additional directs costs, such as repeated use of health care services for physical symptoms, and indirect costs, such as lost productivity at work (Greenberg et al., 1999). Improved prevention and treatment for anxiety disorders would be impactful both for individual quality of life and for societal productivity.
As with most psychiatric disorders, familial and genetic influences are among the best substantiated risk factors for anxiety disorders. Given this, the hope is that clarifying the genetic basis of these syndromes will point to more effective and specific interventions. Anxiety disorders are arguably in one of the strongest positions among the psychiatric disorders to execute successful translational work from genetic risk factors to novel treatments because of the uniquely strong neurobiological (Etkin, 2010; Johansen, Cain, Ostroff, & LeDoux, 2011; Pine, 2007; Shin & Liberzon, 2010) and animal modeling literature (Flint, Shifman, Munafo, & Mott, 2008) in fear and anxiety.
Review of Anxiety Genetics
Heritability
There is now accumulated evidence that anxiety disorders are familial and heritable (Hettema, Neale, & Kendler, 2001). The heritability estimates across each anxiety disorder and anxious traits are generally comparable in children, adolescents, and adults, with estimates clustering in the range of h2 values from ~0.25 to 0.60 (for reviews, see Franic et al., 2010; Gregory & Eley, 2007; Hettema et al., 2001). The variability across studies could be due to many potential factors, including parent report versus self-report, age of assessment, categorical versus dimensional categorizations, and specific anxiety disorder analyzed (Franic et al., 2010; Gregory & Eley, 2007). Despite these factors contributing to variability, there is also good consistency across studies that anxious traits and anxiety disorders are moderately heritable.
Candidate gene approaches
Like the psychiatric genetics field more generally, the candidate gene literature in anxiety genetics is large and complex, with few replicable associations (Duncan & Keller, 2011; Hewitt, 2012; Sullivan, 2007). To assess the current state of the literature, we conducted a systematic review of published candidate gene studies for the anxiety disorders or anxiety symptom measures (not including temperament or personality traits). Through Pubmed searches and manual inspection of meta-analyses and reviews, we identified over 350 anxiety candidate gene studies. Restricting the search to studies with a sample size of at least 200 cases (or nuclear families) yielded only 65 reports from which main effects could be extracted. Although this sample size threshold is quite low given current standards in complex disease genetics (Sullivan, 2010), it was chosen because of the small pool of well-powered anxiety genetic studies. We focus on the main effects because studies of Gene × Environment and Gene × Gene interactions have been largely underpowered (Duncan & Keller, 2011). We also focus on single nucleotide polymorphisms (SNPs) rather than haplotypes, which are difficult to compare across studies. An enumeration of the 65 studies is available from the first author upon request.
Our review of these 65 studies revealed that PD has been the most frequently studied single disorder (N = 30 studies), compared to GAD (N = 4), social anxiety disorder (N = 1), specific phobia (N = 1), and separation anxiety (N = 0). Anxiety symptom measures and/or a combined phenotype across anxiety disorders have also been frequently studied (N = 29 studies). Fifty-six different genes were investigated across the studies with many studies including more than one gene. The three most commonly studied genes were catechol-O-methyltransferase (COMT, 8 studies), solute carrier family 6, member 4 (SLC6A4, also known as serotonin transporter [5-HTT], 7 studies), and brain-derived neurotrophic factor (BDNF, 4 studies), each of which has been the focus of at least one meta-analysis for anxiety phenotypes, which will be discussed further below. Only 13 of these 56 genes (23%) had been studied in two independent reports with sample sizes above the 200 case (or family) minimum. Keeping in mind this lower bound, the average case sample size in this truncated sample was 387 cases (SD = 185, Mdn = 376) and 416 controls (SD = 224, Mdn = 351). Across studies, the largest case-control analysis examined SLC6A4 in 1,161 cases diagnosed with an anxiety disorder and/or depression and 1,051 controls (Wray et al., 2009). It is worth nothing that this study found no consistent evidence of association with the commonly investigated 5-HTT linked polymorphic region (5-HTTLPR) variant (Wray et al., 2009).
Given the high rate of false positives in genetic association studies, particularly when power is limited, we investigated whether there was any evidence for independently replicated association of a variant with a specific anxiety disorder at p < .05 (uncorrected). Studies considering a cross-disorder anxiety phenotype (e.g., case = GAD or PD) or anxiety symptoms were considered together. There was no restriction on the measures used to obtain these diagnoses or quantitative traits. Even when applying these liberal statistical and definitional criteria, there was not a single instance of replication. Although it is possible we have overlooked an instance of replication, our search makes clear that replicated association between a genetic variant and any anxiety disorder is at least rare when a sample size restriction of 200 cases is imposed. This observation is consistent with candidate gene findings in the larger psychiatric genetics literature (Duncan & Keller, 2011; Hewitt, 2012; Sullivan, 2007).
Three genetic variants have been studied frequently enough to be the subject of meta-analyses: the 5-HTTLPR polymorphism of SLC6A4 with panic (Blaya, Salum, Lima, Leistner-Segal, & Manfro, 2007), the Val/Met polymorphism of COMT with panic (Domschke, Deckert, O’Donovan M, & Glatt, 2007; Zintzaras & Sakelaridis, 2007), and the Val/Met polymorphism of BDNF with a cross-disorder anxiety phenotype (including phobias, GAD, PD, OCD, and PTSD; Frustaci, Pozzi, Gianfagna, Manzoli, & Boccia, 2008). Only one of these four meta-analyses reported a qualified positive result for COMT with panic (Domschke et al., 2007). Although the overall meta-analysis showed no significant effect, there was significant heterogeneity in the analysis, which was attributed to a female-specific effect and differential effects in Caucasian and Asian populations (Domschke et al., 2007). This meta-analysis included 6 case-control samples, each of which had fewer than 200 cases (total combined N = 557 cases and 763 controls; Domschke et al., 2007), which is why we did not observe the same effects in our review of the COMT literature. All of these meta-analyses have noted the tentative nature of the conclusions that can be drawn, whether positive or negative, because of the small number of case-control studies with small samples. Publication bias is also a crucial concern for the psychiatric genetics literature (Duncan & Keller, 2011).
In summary, keeping in mind the sample size requirements we imposed on the literature review, we did not identify any replication of candidate gene variants, even considering our liberal statistical criteria. This result is supported by four meta-analyses, none of which have reported a clear main effect. Our findings are also consistent with the observation that a priori candidate genes have not generally emerged as significant in genome-wide scans (e.g., Collins, Kim, Sklar, O’Donovan, & Sullivan, 2012). For this reason, we believe that efforts to assemble large anxiety samples for genome-wide investigations will be fruitful in focusing the field on novel, replicable genetic risk variants.
Genome-wide approaches
An alternative to the candidate gene approach is to utilize genome-wide scans, which examine genetic loci throughout the genome. Both linkage and association designs can be used for genome-wide scans. We focus here on genome-wide association results, which have been successful in identify risk loci for other psychiatric disorders. (For a review of linkage studies in anxiety, see Domschke & Reif, 2012; Maron, Hettema, & Shlik, 2010; Webb et al., 2012.)
Genome-wide association studies (GWAS)
To date, there have been two small-scale GWAS for anxiety disorders, both focusing on PD (Erhardt et al., 2011; Otowa et al., 2009). The study by Otowa et al. (2009) in 200 patients and 200 controls reported two genome-wide significant SNPs in the genes transmembrane protein 16B (TMEM16B) and plakophilin 1. However, a subsequent replication attempt in 558 cases and 566 controls did not support these findings (Otowa et al., 2010).
Erhardt et al. (2011) used a discovery sample of 216 cases and 222 controls along with several replication samples. The authors reported two SNPs in transmembrane (TMEM) protein 132D (TMEM132D) that were nominally associated with PD in three independent samples (combined sample = 909 cases and 915 controls), although the joint analysis p values did not exceed a genome-wide significance threshold (ps = 10−6). The authors went on to examine the biological relevance of these SNPs, demonstrating that the risk genotype was associated with higher TMEM132D mRNA expression in human postmortem frontal cortex. These results were supported by findings in a mouse model where anxiety-related behaviors were associated with a SNP in Tmem132d and with mRNA expression of Tmem132d in the anterior cingulate (Erhardt et al., 2011). The convergence of results indicating genetic association across samples, biological plausibility, and support from animal models is a compelling picture that would be further strengthened by genome-wide significant results in larger, independent samples.
In the broader psychiatric genetics literature, GWAS has been a key strategy for identifying replicable common genetic risk variants (Ripke et al., 2011; Sklar et al., 2011; Sullivan, 2012). This tangible progress has been invaluable for psychiatric genetics, but GWAS designs can have limitations that are important to consider. Because the sample size must be large, the phenotyping is often less precise than in smaller studies, sometimes relying on a few items from quantitative trait scales. In addition, GWAS studies typically have limited information on environmental exposures, which constrains testing of Gene × Environment interactions. Finally, by design, most GWAS studies only assay common genetic variants, which typically have modest effects and likely do not capture the full genetic architecture of complex disorders.
Genome-wide copy number variation (CNV) studies
CNVs are another form of genetic variation that can be investigated in genome-wide studies. CNVs are segments of DNA that range from 1 kilobase (kb) to millions of base pairs that are either deleted or duplicated. In autism and schizophrenia, large, rare CNVs have been shown to be etiologically important in a subset of cases (for a review, see Malhotra & Sebat, 2012).
To date, there has been only one genome-wide CNV study of anxiety disorders (Kawamura et al., 2011). This study focused on PD and included 535 cases and 1,520 controls of Japanese ancestry. The study did not detect an excess burden of rare CNVs, but the authors reported a Bonferroni-corrected significant association (p < .05) with common duplications in the 16p11.2 region. CNV detection is difficult in this pericentromeric region, so replication of the finding using multiple methods for CNV calling and laboratory validations will be important. The region is approximately 2 megabases away from a large, rare CNV in 16p11.2 that has been associated with autism and other neurodevelopmental disorders (Malhotra & Sebat, 2012).
In summary, genome-wide studies of anxiety disorders have been limited to date. However, given the successes achieved with these methods in other complex disorders, there is reason to be hopeful that larger studies will provide novel clues to the genetic basis of pathologic anxiety. We turn now to the major challenges facing the field of anxiety genetics and our recommendations to address some of these challenges.
Challenges in Gene Finding for Anxiety
There are four primary challenges that have hindered progress in gene finding for anxiety disorders or traits: (a) small sample sizes (b) etiological heterogeneity, (c) a complicated co-morbidity profile, and (d) blurred lines between normative and pathological anxiety.
Small sample sizes
A major catalyst for psychiatric genetics has been the Psychiatric GWAS Consortium (PGC), a collaborative effort to assemble large samples for GWAS in major depressive disorder (MDD), bipolar disorder, schizophrenia, attention-deficit/hyperactivity disorder, and autism (Sullivan, 2010). The anxiety disorders have not been part of the primary PGC efforts to date. A major lesson from this and other large-scale genome-wide studies has been the realization that sample sizes on the order of tens of thousands of individuals are necessary to identify and replicate genetic variants of modest effect size in complex, neurobehavioral phenotypes (i.e., Ripke et al., 2011; Sklar et al., 2011).
Even for smaller-scale candidate gene studies, it is clear that the typical sample sizes that have been examined in genetic studies of anxiety disorders (on average, a few hundred cases and controls) have been underpowered by at least an order of magnitude. If we take as an optimistic effect size, the largest odds ratios for genome-wide significant results observed in recent GWAS studies of schizophrenia and bipolar disorder (odds ratio = ~1.2; Ripke et al., 2011; Sklar et al., 2011), a liberal significance threshold of p< .05 and the most favorable minor allele frequency (MAF = 0.5), approximately 1,000 cases and 1,000 controls would be needed to achieve 80% power under an additive model (Gauderman & Morrison, 2006). Even under this most optimistic scenario, we identified only one of the hundreds of published case-control candidate gene studies (Wray et al., 2009) that was adequately powered. In the context of these power limitations, it is difficult to exclude Type I or Type II error in the existing candidate gene literature.
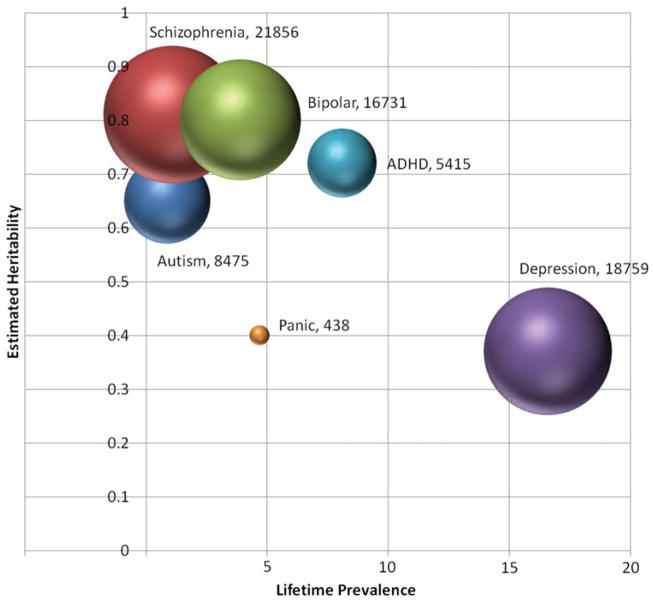
GWAS of anxiety have been similarly underpowered (~200 cases and 200 controls). Using the same optimistic effect size (odds ratio = ~1.2) and MAF (0.5) and a genome-wide significant threshold of p = 5 × 10−8, power analyses indicate that a minimum of 5,000 cases and 5,000 controls would be necessary to obtain 80% power. This estimate extends upward as the risk allele becomes less common (i.e., 7,000 cases/7,000 controls MAF = 0.2; 12,000 cases/12,000 controls MAF = 0.1; Gauderman & Morrison, 2006). As Figure 1 indicates, sample sizes of the magnitude necessary for successful genome-wide studies have not yet been reported for the anxiety disorders, although sample collection efforts are ongoing. The next generation of anxiety genetic studies will need to carefully consider power in light of plausible effect size estimates.
Figure 1.
The estimated heritability versus prevalence for disorders included in the Psychiatric GWAS Consortium (PGC) and panic disorder. Each bubble is centered at a point estimate for the lifetime prevalence and heritability of the disorder. The area of the bubbles is proportional to the largest discovery genome-wide association studies sample (cases and controls) reported to date (panic disorder: Erhardt et al., 2011; attention-deficit/hyperactivity disorder [ADHD]: Neale et al., 2010; depression: PGC, 2012; schizophrenia: Ripke et al., 2011; bipolar disorder: Sklar et al., 2011; autism: Wang et al., 2009). Total sample size is also given in the labels. (As an approximation, trios were counted as equivalent to a case.) [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Etiologic heterogeneity
The PGC analyses, especially those for MDD, can also serve as a cautionary tale for anxiety genetics. The PGC mega-analysis of MDD (> 18,000 cases/controls) yielded no genome-wide significant signals (PGC, 2012) in contrast to bipolar disorder and schizophrenia, which were more successful (Ripke et al., 2011; Sklar et al., 2011). One explanation for this disappointing result is that etiological heterogeneity is particularly important for depression and other disorders with high population prevalence and moderate heritability (PGC, 2012). This heterogeneity, which may include nongenetic phenocopies and etiologically distinct subtypes, can make gene finding particularly challenging. For example, consider the standard case-control genetic association study, which selects cases based on a cross-sectional assessment of lifetime diagnosis of an anxiety disorder in adulthood. Cases may show substantial etiologic heterogeneity owing to normative and transient peaks in anxiety over the lifespan and different developmental trajectories to the same anxiety disorder outcome (i.e., early vs. later onset). Such heterogeneity could substantially reduce power in a genetic association study.
Figure 1 compares the genetic landscape of the anxiety disorders to the disorder groups included in the PGC, as defined by approximate heritability and prevalence estimates. Thus far, replicable genetic findings have been identified only in highly heritable, lower prevalence disorders with the largest sample sizes. Anxiety disorders and MDD share a genetic landscape that may be particularly difficult for gene finding. In addition to large samples, the field may also need new approaches to phenotyping that can increase power for genetic association studies. For the anxiety disorders, a developmental, multivariate approach to phenotyping may be important for reducing heterogeneity.
Complicated comorbidity profile
Anxiety disorders are highly comorbid with each other in both child and adult samples (Costello, Egger, & Angold, 2005; Kessler, Chiu, Demler, Merikangas, & Walters, 2005). Although comorbidity is a general issue in psychiatry, it is compounded in anxiety, where there are 13 different disorders within the same anxiety class, in addition to cross-class comorbidities. Standard case-control genetic association studies typically focus on individuals who meet and do not meet criteria for one specific disorder. The complex co-morbidity of anxiety disorders raises challenging questions about the design and interpretation of studies. For example, should controls be screened only for the specific anxiety disorder being investigated or for all the anxiety disorders? Evidence that the various anxiety disorders share genetic underpinnings (discussed below) would argue for selecting controls free of any disorder in the class; however, the high prevalence of anxiety disorders may limit the feasibility of such a strategy. Moreover, if a significant association is found between a genetic variant and the disorder of interest, how does one ensure that the association is with the primary disorder and not a secondary, highly comorbid disorder? (Smoller, Lunetta, & Robins, 2000). As we discuss later, latent modeling approaches that extract the common phenotypic variance among anxiety disorders may be useful for modeling the multidimensional nature of the phenotype (see the Latent Modeling of the Multidimensional Anxiety Phenotype Section).
Blurred lines between normative and pathological anxiety
Anxiety is a universal human experience, and the line between pathological and normal anxiety is unclear. For case-control genetic association studies, an arbitrary diagnostic boundary must be drawn, creating the dilemma of how to handle individuals just above or below the threshold. Alternatively, quantitative trait approaches, which assume a continuous liability distribution (Plomin, Haworth, & Davis, 2009), can be used for constructing optimally informative latent phenotypes. If genetic variations that influence anxiety-related traits in nonclinical samples are continuous with those affecting pathologic anxiety, genetic studies could take advantage of existing population-based cohorts that have longitudinal anxiety information.
In summary, dissecting the genetic and phenotypic complexity of anxiety disorders will likely require larger, genome-wide study designs and innovative phenotypic techniques. In the rest of this paper, we will focus on new phenotypic approaches that are guided by the psychiatric and genetic epidemiology of anxiety disorders. We propose a developmental, multivariate framework that can incorporate quantitative traits, multidimensional phenotypes, and developmental trajectories, addressing many of the limitations just discussed. We turn now to a discussion of the psychiatric and genetic epidemiology of anxiety disorders, with a focus on implications for optimizing phenotype definition and incorporating a developmental perspective.
Psychiatric and Genetic Epidemiology of Anxiety Supports a Developmental, Multivariate Perspective
Early age of onset and chronic course
Anxiety disorders have an earlier age of onset than many other classes of psychopathology, including mood, psychotic, and substance use disorders. The median age of onset of anxiety disorders is reported to be 6 years in an adolescent population-based sample (Merikangas, He, Burstein, et al., 2010) and 11 years in an adult population-based sample (Kessler, Berglund, et al., 2005). The discrepancy is likely due to the inherent difficulties in retrospective reporting of onset.
Within the class of anxiety disorders, there are large variations in the median age of onset for specific types of anxiety. Separation anxiety and specific phobia are the earliest onset in childhood, followed by social phobia in early adolescence, and then PD/agoraphobia and GAD in late adolescence and early adulthood (Kessler, Berglund, et al., 2005). These age-of-onset patterns indicate a developmental shift in the expression of anxiety at different ages (for reviews, see Beesdo, Knappe, & Pine, 2009; Costello et al., 2005; see Figure 2b).
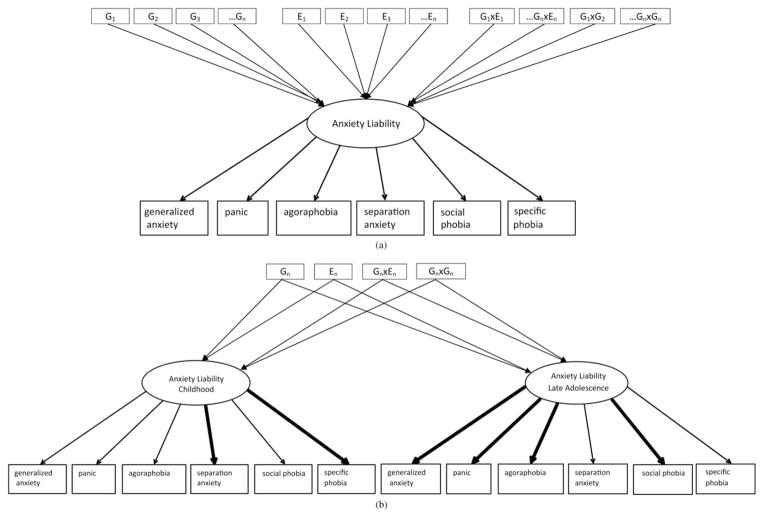
Figure 2.
(a) An illustration of a one-factor latent model of anxiety liability influenced by genetic variants (G), environmental factors (E), Gene× Environment interactions (G× E), and Gene × Gene interactions (G× G). Although multiple predictors are included to reflect the expected etiologic complexity of anxiety, this model could also be used to test single predictors, such as a single nucleotide polymorphism. (b) An illustration of a two-factor latent model of anxiety liability with developmental shifts in the factor loadings from childhood to late adolescence. In childhood, anxiety liability is predicted to be expressed as the earliest onset anxiety disorders: separation anxiety and specific phobia. In late adolescence, anxiety liability is predicted to be expressed as the later onset anxiety disorders: generalized anxiety, panic, agoraphobia, and social phobia. The thickness of the arrows indicates that the weighting of the variable is predicted to be stronger. The multiple genetic, environmental, G× E, and G× G predictors are condensed from (a) to simplify the figure but represent the same degree of etiologic complexity depicted in (a).
Anxiety disorders also tend to have a chronic course across development (for a review, see Hirshfeld-Becker, Micco, Simoes, & Henin, 2008). In large epidemiological samples, children and adolescents meeting criteria for an anxiety disorder were at high risk for meeting criteria as adults, with odds ratios generally in the range of 2.0–3.0 (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Gregory et al., 2007; Kim-Cohen et al., 2003; Newman et al., 1996; Pine, Cohen, Gurley, Brook, & Ma, 1998). There is evidence for both “homotypic continuity” (the future occurrence of the same disorder) and “heterotypic continuity” (the future occurrence of a different anxiety disorder; Gregory et al., 2007; Pine et al., 1998).
These developmental patterns have been largely neglected in anxiety phenotyping for genetic studies. All of the genome-wide studies and most of the candidate gene studies reviewed above have been conducted in cross-sectional, adult samples. Only 13% of the case-control studies have been in child or adolescent samples. Of these, only a handful have explicitly incorporated developmental trajectory information into the phenotype, a method discussed further below (see Ernst et al., 2011; Petersen et al., 2012). By neglecting the developmental nature of the anxiety phenotype, the field may be missing a critical opportunity for gene finding. New phenotypic models could incorporate early developmental time points and allow for changing symptomatology over time (see the Developmentally Sensitive Phenotypes for Anxiety Genetic Studies Section).
Genetic contributions to comorbidity
In the following two sections we highlight evidence that the strong cross-sectional and longitudinal relationship among the anxiety disorders is partially attributable to shared genetic risk factors. These findings can guide multivariate, longitudinal models of anxiety liability for genetic studies.
Genetically informative designs, such as the twin study, can be used to examine the genetic basis of co-occurring disorders or traits by examining the correlation between one disorder or trait in one twin (e.g., GAD) and another disorder or trait in the second twin (e.g., PD) for monozygotic and dizygotic twin pairs. A higher cross-trait correlation for monozygotic compared to dizygotic twin pairs provides evidence of shared genetic contributions to the two disorders or traits.
There have been several multivariate twin studies of childhood anxiety disorders and anxiety-related behaviors (Eley et al., 2003; Eley, Rijsdijk, Perrin, O’Connor, & Bolton, 2008; Hallett, Ronald, Rijsdijk, & Eley, 2009; Ogliari et al., 2010). The common thread uniting most of these studies is that there are common genetic risk factors underlying many of the childhood anxiety disorders and traits, although the magnitude of overlap differs depending on the age of the sample, measures used, and disorders or traits considered (for reviews see Franic et al., 2010; Gregory & Eley, 2007). Two studies have estimated the proportion of the phenotypic correlation that is due to shared genetic factors to be in the range of .30–.50 for anxiety-related behaviors in preschool and middle childhood (Eley et al., 2003; Hallett et al., 2009). One additional study, in a sample of broader age range (8–17 years), reported even higher estimates of .60–.99 for quantitative measures of generalized anxiety, social phobia, separation anxiety, and PD (Ogliari et al., 2010). The highest estimate of .99 was for social phobia and panic symptoms.
Multivariate twin studies in adults support the findings in child samples that the anxiety disorders reflect partly shared genetic influences (Hettema, Prescott, Myers, Neale, & Kendler, 2005; Kendler, Prescott, Myers, & Neale, 2003; Middeldorp et al., 2005; Mosing et al., 2009; Tambs et al., 2009). These studies provide some support for a two-factor internalizing model comprising two partly distinct genetic factors: anxious-misery (with loadings on depression, generalized anxiety, and panic, agoraphobia, social phobia) and fear (with loadings on specific phobias; Hettema et al., 2005; Kendler et al., 2003). At this point, the two-factor genetic model has not been explored in child samples, so its relevance to childhood anxiety remains to be determined.
Overall, these multivariate genetic results suggest that though the anxiety disorders may show phenotypic differentiation, even beginning at early developmental stages (Mian, Godoy, Briggs-Gowan, & Carter, 2011), many of the disorders share genetic influences. We concur with previous proposals that, for gene-finding efforts, it would be reasonable to focus on clusters of disorders with shared genetic risk factors rather than on a single individual anxiety disorder (Kendler et al., 2003). One methodological strategy would be to model a latent anxiety liability factor composed of anxiety disorders with substantial genetic overlap (see Figure 2 and the Latent Modeling of the Multidimensional Anxiety Phenotype Section).
Genetic contributions to the stability of anxiety
Longitudinal twin studies in which the same anxiety phenotypes are measured in both twins on two or more occasions can address questions about genetic and environmental contributions to the stability of anxiety over time. There have been several multivariate, longitudinal twin studies addressing this question in childhood and adolescence (Boomsma, van Beijsterveldt, Bartels, & Hudziak, 2007; Kendler, Gardner, Annas, & Lichtenstein, 2008; Kendler, Gardner, Annas, Neale, et al., 2008; Kendler, Gardner, & Lichtenstein, 2008; Roberson-Nay, Eaves, Hettema, Kendler, & Silberg, 2012; Trzaskowski, Zavos, Haworth, Plomin, & Eley, 2011). Depending on the measures included in the study, these twin studies can address genetic and environmental contributions to both homotypic and heterotypic continuity.
In one study explicitly addressing homotypic versus heterotypic continuity of anxiety-related behaviors (i.e., negative cognition, negative affect, fear, social anxiety) from ages 7 to 9 years, genetic influences on homotypic continuity were generally stronger than heterotopic continuity (Trzaskowski et al., 2011). Estimates of the proportion of homotypic continuity due to stable genetic factors ranged from .57 to .67. Genetic influences on heterotypic continuity were more varied, with estimates ranging from .28 (for fear at age 7 and negative affect at age 9) to .66 (for negative cognitions at age 7 and negative affect at age 9). This variability suggests that specific clusters of anxiety-related behaviors may be more genetically related over time than others, consistent with the two-factor genetic models in adults presented by Kendler et al. (2003) and Hettema et al. (2005). Additional evidence for genetic contributions to heterotypic continuity comes from a recent study reporting a shared genetic diathesis between childhood separation anxiety disorder and adult-onset panic attacks (Roberson-Nay et al., 2012).
Twin studies spanning larger age ranges have addressed mainly homotypic continuity. For example, Kendler, Gard-ner, and Lichtenstein (2008) examined a large twin sample assessed at ages 8–9, 13–14, 16–17, and 19–20 years with self- and parent-report measures of anxious/depressed symptoms. They found evidence for a “developmentally dynamic” pattern of genetic risk factors involving both “genetic attenuation” and “genetic innovation.” Genetic attenuation refers to the finding that genetic risk factors present at age 8–9 continued to contribute to anxious/depression symptoms over time but accounted for less of the variance, starting with 72% at age 8–9 and ending with 12% at age 19–20. Genetic innovation refers to the fact that strong new genetic effects emerged at each time point measured. Thus, both stable and developmentally dynamic genetic risk factors contribute to anxiety symptoms. A similar pattern was observed in the same twin sample using a phobia assessment (Kendler, Gardner, Annas, & Lichtenstein, 2008; Kendler, Gardner, Annas, Neale, et al., 2008). Among the phobias, social phobia showed the lowest degree of genetic continuity. The pattern of genetic effects on social phobia was distinguished by new and substantial genetic influences coming online in adolescence and early adulthood, compared to the pattern in the other specific phobias where the genetic innovations were more modest (Kendler, Gardner, Annas, & Lichtenstein, 2008). Once individuals reach adulthood, there is more evidence for genetic stability over time with only minor genetic innovations compared to childhood and adolescence (Gillespie et al., 2004; Nes, Roysamb, Reichborn-Kjennerud, Harris, & Tambs, 2007; Rijsdijk et al., 2003).
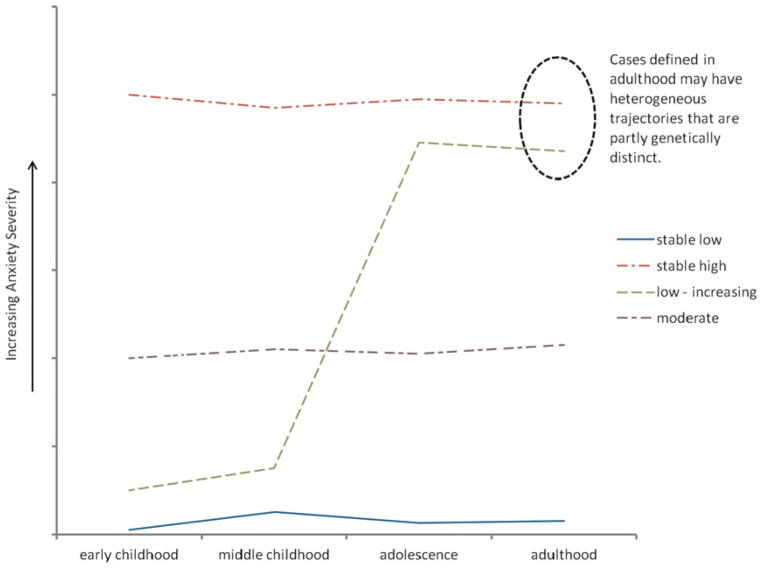
These studies highlight the value of incorporating a developmental perspective for genetic studies of anxiety. For example, the genetic epidemiology of social phobia suggests that genetic contributions to social phobia in adolescence and adulthood are substantially distinct. In this case, studies that collapse across developmental periods (such as the standard practice of assessing lifetime diagnoses in adulthood) will substantially increase the etiologic heterogeneity of the sample and diminish the power to detect genetic effects (see Figure 3). However, specific genetic risk factors may be more easily identified by focusing on a specific developmental period (e.g., adolescent vs. adult-onset social anxiety) or including longitudinal phenotypes that distinguish specific trajectories (see the Developmental Trajectories as Phenotypes Section).
Figure 3.
Examples of developmental trajectories that may have etiologic significance. Different pathways to adult disorder may reflect heterogeneous etiologies that are obscured in standard case-control studies of adults. The lines from top to bottom indicate stable high, moderate, low increasing, and stable low, respectively. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Developmentally Sensitive Phenotypes for Anxiety Genetic Studies
The genetic epidemiology of anxiety disorders has been well studied in sophisticated multivariate and developmental designs, but gene-finding efforts have not generally incorporated these approaches.
Given the evidence for (a) the early onset and chronic course of anxiety disorders, (b) shared genetic etiology of specific clusters of anxiety disorders, and (c) developmentally stable and dynamic genetic contributions to anxiety disorders, we propose three corresponding developmental strategies for phenotypic definition in genetic studies: (a) examination of early temperamental precursors of anxiety, (b) latent modeling of the multidimensional anxiety phenotype, and (c) examination of developmental trajectories. We explain these strategies further and then illustrate the approach with a representative application.
Early temperamental precursors of anxiety
There has been a rich tradition of developmental work characterizing temperamental risk factors for anxiety (e.g., Kagan & Snidman, 2004). Temperament describes a biologically based behavioral profile that is relatively stable across time and context in childhood (Nigg, 2006; Perez-Edgar & Fox, 2005; Rothbart, 2007). Multiple dimensions of temperament have been described in internalizing disorders, but much of the focus has been on behavioral inhibition (BI) to the unfamiliar (e.g., Biederman et al., 2001; Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Hirshfeld-Becker et al., 2007; Kagan, Snidman, Kahn, & Towsley, 2007; Rosenbaum et al., 2000).
BI is a stable, heritable temperamental profile that is associated with increased risk for later anxiety disorders, especially social anxiety (Biederman et al., 2001; Hirshfeld-Becker et al., 2007; Schwartz, Snidman, & Kagan, 1999). It is characterized by withdrawn and wary behaviors to novel situations and is measured with developmentally sensitive observational tasks (Kagan & Snidman, 2004) or parent reports of child temperament and shyness (e.g., Carter, Briggs-Gowan, Jones, & Little, 2003; Eley et al., 2003). There has been some debate about the extent to which BI is a separate construct from early manifestations of anxiety symptoms (Egger & Angold, 2006); however, there is an emerging consensus that the two concepts are related but distinguishable (Rapee, Schniering, & Hudson, 2009). One piece of evidence in support of this consensus is that even though BI has been associated with increased risk for later anxiety disorders, only about half of children with BI go on to develop an anxiety disorder (Degnan & Fox, 2007). While prior research has identified environmental and neurocognitive predictors of the transition from anxious temperament to anxiety disorder (Degnan, Almas, & Fox, 2010; Degnan & Fox, 2007), there has been much less research on how genetic variation influences this trajectory.
For genetic studies of anxiety susceptibility, BI is a particularly compelling phenotype because estimates of its heritability tend to be higher on average than estimates reported for child and adolescent anxiety disorders (DiLalla, Kagan, & Reznick, 1994; Goldsmith & Lemery, 2000; Plomin et al., 1993; Robinson, Kagan, Reznick, & Corley, 1992). Three twin studies utilizing observational measures of BI in toddlerhood found comparable heritability estimates h2 ranging from ~.40 to .55 and extending up to .70 in one study (DiLalla et al., 1994; Plomin et al., 1993; Robinson et al., 1992). Genetic studies also indicate overlap between genetic influences on inhibited temperament and other anxiety-related behaviors (Eley et al., 2003; Goldsmith & Lemery, 2000).
Several groups have pursued the strategy of examining anxiety-related temperament as an intermediate phenotype for anxiety disorders (Fox et al., 2005; Smoller et al., 2003, 2005, 2008). Intermediate phenotypes are traits that capture aspects of the underlying liability for a disorder but may be more closely related to the genetic risk factors than is the disorder itself (Gottesman & Gould, 2003; Kendler & Neale, 2010). The study highlighted below illustrates a strategy of examining multiple intermediate phenotypes, including temperament, personality, and neuroimaging profiles and capitalizing on genetic findings from experimental animal models (Smoller et al., 2008).
There are evolutionarily conserved fear responses in a wide range of species that recapitulate the behavioral and biological features of human fear (Flint, 2003). This cross-species correspondence makes genes implicated in animal models of anxiety compelling candidates for human studies. In 2004, regulator of G protein signaling 2 (Rgs2; Yalcin et al., 2004) was identified as a quantitative trait gene influencing anxious temperament through fine mapping of a well-replicated linkage signal for anxiety phenotypes in mice (Yalcin et al., 2004). The RGS2 protein is expressed in cortical and limbic brain regions and modulates G protein coupled receptor signaling in response to neurotransmitters such as serotonin and norepinephrine (Grafstein-Dunn, Young, Cockett, & Khawaja, 2001; Neubig & Siderovski, 2002). Rgs2 knockout mice exhibit increased anxiety and fear behavior, altered hippocampal synaptic plasticity, and elevated sympathetic tone (Oliveira-Dos-Santos et al., 2000; Yalcin et al., 2004).
Given the multiple lines of evidence in both human and animal models implicating Rgs2 in anxiety-related behaviors, Smoller et al. (2008) tested the association of human RGS2 with BI in a sample of children ages 2–6 years (N = 119 nuclear families) who underwent lab-based temperament assessments. Multiple variants in the RGS2 locus were associated with BI, including a SNP (rs4606) that is associated with reduced RGS2 expression in vitro (Semplicini et al., 2006).
To further explore this association between BI and RGS2, the authors also examined a personality phenotype closely related to BI in an independent adult sample (N = 744). Developmental studies have suggested that BI is a developmental precursor of introversion in adulthood (Caspi et al., 2003). The four markers showing the strongest association with childhood BI were also found to be associated with introversion among adults.
In a third adult sample (N = 55), the authors focused on brain phenotypes thought to mediate anxiety proneness: amygdala and insula reactivity during emotion processing (e.g., Killgore & Yurgelun-Todd, 2005; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002; Stein, Simmons, Feinstein, & Paulus, 2007). The alleles previously associated with BI and introverted personality were also associated with increased left amygdala and bilateral insular cortex activation in response to emotional faces.
The relevance of RGS2 to pathologic anxiety is supported by several studies that have reported association to a variety of anxiety disorders, including GAD, PD, and PTSD (Amstadter et al., 2009; Koenen et al., 2009; Leygraf et al., 2006; Mouri et al., 2009; Otowa et al., 2011), although the associated alleles have not been consistent across studies and the samples examined have been relatively small. Additional indirect evidence comes from a recent report that a SNP in mi-croRNA-22, an epigenetic regulator of RGS2, is associated with PD (Muinos-Gimeno et al., 2011).
The RGS2 story is one illustration of a strategy in which developmental precursors of anxiety, such as heritable temperamental traits, were used as intermediate phenotypes in genetic studies. A crucial next step in this approach is to test the association of identified genetic variants with the anxiety disorder of interest because it is possible that a genetic variant could be associated with temperament but not related anxiety disorders (Kendler & Neale, 2010). Studies attempting to demonstrate an association with the disorder of interest may require larger sample sizes than the original intermediate phenotype study because of the expected increase in phenotypic and etiologic heterogeneity in clinical samples.
Latent modeling of the multidimensional anxiety phenotype
Another approach to investigating complex, multidimensional anxiety phenotypes is to utilize statistical approaches that can model multiple outcomes. This could be done, for example, by constructing a latent phenotypic factor from the anxiety disorders known to share genetic underpinnings in the developmental period being considered (see Figure 2). The latent factor score could then be used in genetic analyses as a quantitative measure of “anxiety liability.” Although there is a rich tradition of latent modeling approaches in behavioral research, many of these methods have not been widely integrated into psychiatric genetic research (for examples, see McGrath et al., in press; Medland & Neale, 2010; Middeldorp et al., 2010). Here, we focus specifically on structural equation modeling (SEM) approaches (see Kline, 2005; Loehlin, 2004).
SEM provides several advantages for genetic research, including (a) reduction of measurement error, (b) the ability to test for genetic effects on means and covariance, and (c) developmental modeling. First, reduction of measurement error may increase power to detect genetic signals (Schulze & McMahon, 2004). Second, a latent phenotypic model can be used to test for genetic effects on the means of the latent factors as well as on the covariance structure among the anxiety disorders or traits that are modeled. The issue of covariance differences as a function of genotype is of particular relevance to neuropsychiatric phenotypes, where subgroups, or specific clusters of behavior, may be expected as a function of genotype (e.g., Craddock, O’Donovan, & Owen, 2006; Wessman et al., 2009). In other words, SEM models can test the hypothesis that subsets of anxiety disorders are more or less correlated as a function of genotype, allowing for the identification of genetically meaningful subgroups. For example, individuals with a specific genetic variant could be at increased risk for comorbid panic and agoraphobia compared to those without the variant. If this is the case, the correlation between panic and agoraphobia would be stronger among those with the risk variant. This application of SEM to investigate genetic differences in covariance has been vastly underexplored in psychiatry.
Third, SEM can take advantage of longitudinal data to extract stable traits over time and to explicitly model developmental trajectories. Here, we point out that SEM can incorporate developmental information by permitting changing weights on the diagnoses and dimensions contributing to a latent anxiety liability factor at different time points. For example, separation anxiety may be a stronger indicator of anxiety liability in early childhood compared to PD, which has a low prevalence at this development stage. However, in late adolescence, the opposite pattern may be expected. Figure 2b illustrates a potential phenotypic model for anxiety disorders in childhood and late adolescence, where stronger indicators are bolded. The genetic association analysis can then be conducted with the empirically derived, developmentally sensitive latent factors as the phenotype. This latent model approach diminishes the multiple-testing burden that would result if each anxiety diagnosis were tested individually.
In a study illustrating the incorporation of SEM in genetic analyses, Middeldorp et al. (2010) studied child/adolescent (N = 1,240) and adult (N = 1,943) participants who received repeated measures of anxious/depression symptoms. In both samples, a single latent anxious depression factor was modeled that incorporated multiple raters and time points. The heritability of the latent anxious depression factor was found to be higher (h2~.60–.70) than the individual anxious depression measures (h2~.40–.50), as expected based on the increased reliability of the latent factor. After multiple-testing correction, the authors did not find any significant associations with a set of candidate genes chosen based on previous literature (serotonergic and neurotrophic genes). Nevertheless, we concur with the authors that the latent modeling approach will be quite valuable for future genome-wide analyses because it can harness the reliable variance in anxiety measures across raters and time points (Middeldorp et al., 2010).
Developmental trajectories as phenotypes
Repeated measures can also be used to explicitly model developmental trajectories over time using SEM and other modeling approaches (Grimm, Ram, & Hamagami, 2011; McArdle, Nesselroade, Schinka, & Velicer, 2003; Muthen, 2001). Consistent with a developmental psychopathology orientation (Cicchetti & Toth, 2009), research on childhood internalizing disorders has explored developmental trajectories associated with psychopathology (e.g., Carter et al., 2010; Duchesne, Larose, Vitaro, & Tremblay, 2010; Letcher, Sanson, Smart, & Toumbourou, 2012). The idea of using developmental trajectories as phenotypes for psychiatric genetic studies is more novel (for examples, see McQueen et al., 2007; Petersen et al., 2012; Sakai et al., 2010), and new methods are emerging (Das et al., 2011; Kerner, North, & Fallin, 2009).
By examining phenotypic stability and change over time, trajectories are more informative than cross-sectional assessments that may be sensitive to normative anxiety patterns and transient environmental influences. For example, there are normative developmental peaks in anxiety, such as a period of separation distress and stranger wariness in toddlerhood and a period of increased concern regarding peer rejection in adolescence (Beesdo et al., 2009; Costello et al., 2005). Moreover, through the life span, bouts of anxiety in response to specific triggers can be normative responses. Thus, studies relying only on cross-sectional assessments of anxiety may capture variation that reflects transient factors rather than an underlying genetically influenced diathesis.
There are several approaches to incorporating developmental trajectories into genetic analyses. For example, using linear growth curve models, the rate of change of a phenotype over time (i.e., slope) can be estimated for each individual in an analysis. This slope parameter can then be used as a quantitative phenotype in genetic studies. The question being tested by such an analysis is whether there are genetic variants that are associated with a more accelerated increase in anxiety symptoms over time. Nonlinear growth curve models are more complex than linear models but may map more closely to developmental patterns in the data (Grimm et al., 2011). A different approach is to cluster individuals with similar developmental trajectories. Across phenotypic studies there have been diverse trajectories identified, but there is converging support for low, low-increasing, moderate, and stable high anxiety trajectories (Cote, Tremblay, Nagin, Zoccolillo, & Vitaro, 2002; Duchesne et al., 2010; Duchesne, Vitaro, Larose, & Tremblay, 2008; Feng, Shaw, & Silk, 2008; Letcher et al., 2012; Marmorstein et al., 2010; Figure 3). A variety of methods can be used to derive clusters (e.g., Muthen, 2002; Nagin, 1999), which can then be used as phenotypes for genetic studies.
In a recent illustration of this approach, Ernst et al. (2011) used data from a longitudinal cohort representative of the Quebec general population. Individuals were randomly selected for participation when they were in kindergarten. Annual ratings of anxiety traits from 6 to12 years old were used to cluster 640 individuals into five different developmental trajectories: high, moderately high, decreasing low, low, and very low. In early adulthood (21–23 years), the participants were reassessed with personality measures and a psychiatric diagnostic interview.
The study examined a functional 11-base pair deletion in tropomyosin-related kinase B (TRKB, also known as neurotrophic tyrosine kinase receptor type 2 gene), a receptor for BDNF that is involved in synaptic modeling, neurodevelopment, and cell signaling (Ernst et al., 2011), and has been implicated in mouse (Bergami et al., 2008) and human anxiety (Ernst et al., 2009). Results showed that children in the high and moderately high trajectory clusters were more likely to carry the deletion (4.1%, 4.0%, respectively) than those in the decreasing low, low, or very low clusters (0%, 0.6%, or 0%, respectively). In early adulthood, individuals carrying the deletion had higher trait anxiety scores and an approximately threefold increased odds of GAD and PD. The results suggest that individuals carrying a deletion in TRKB are at increased risk for anxiety pathology from childhood through early adulthood (Ernst et al., 2011). While replication is needed to validate this association, the study illustrates the utility of using developmental trajectories as phenotypes for genetic studies, a strategy that could be scaled to accommodate genome-wide association data.
Conclusions
Progress in anxiety genetics has lagged behind many of the other psychiatric disorders, in part because of a predominant focus on candidate genes and insufficient sample sizes. These limitations could be addressed by large-scale collaborations to assemble anxiety samples for GWAS and other genome-wide investigations. However, the phenotypic complexity of anxiety disorders or traits also presents real challenges for genetic studies that will not be automatically addressed by collaborative sample collections. Innovative phenotypic techniques may be necessary to maximize the impact of emerging genetic resources for anxiety. Fortunately, there is a rich literature on the psychiatric and genetic epidemiology of anxiety disorders that can guide more sophisticated phenotyping approaches. Data on the early onset and chronic course of anxiety, shared genetic risk factors among specific clusters of disorders, and developmentally dynamic genetic influences have yet to fully inform phenotypic models for genetic studies. These findings support a shift in thinking away from standard, single disorder, case-control studies in adults to a more developmental, multivariate perspective on study design and phenotyping. In this review, we have proposed three developmentally sensitive phenotyping approaches: (a) examination of early temperamental precursors of anxiety, (b) latent modeling of the multidimensional anxiety phenotype, and (c) examination of developmental trajectories. Given that large-scale anxiety genetic studies are currently being pursued, this is an opportune time to consider new phenotypic approaches.
Acknowledgments
The authors were supported by T32-MH16259 (to L.M.M.), T32-MH017119 (to E.B.R.), and K24-MH094614 (to J.W.S.). Special thanks to Alysa Doyle, Laramie Duncan, Erin Dunn, and Alisha Pollastri for helpful comments on the manuscript.
References
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proceedings of the National Academy of Sciences. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, et al. Further evidence of association between behavioral inhibition and social anxiety in children. American Journal of Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Blaya C, Salum GA, Lima MS, Leistner-Segal S, Manfro GG. Lack of association between the serotonin transporter promoter polymorphism (5-HTTLPR) and panic disorder: A systematic review and meta-analysis. Behavioral and Brain Functions. 2007;3:41. doi: 10.1186/1744-9081-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D, van Beijsterveldt CE, Bartels M, Hudziak JJ. Genetic and environmental influence on anxious/depression: A longitudinal study in 3- to 12-year-old children. In: Hudziak JJ, editor. Genetic and environmental influences on developmental psychopathology and wellness. Washington, DC: American Psychiatric Association; 2007. [Google Scholar]
- Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychological Bulletin. 1992;111:244–255. doi: 10.1037/0033-2909.111.2.244. [DOI] [PubMed] [Google Scholar]
- Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The Infant–Toddler Social and Emotional Assessment (ITSEA): Factor structure, reliability, and validity. Journal of Abnormal Child Psychology. 2003;31:495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- Carter AS, Godoy L, Wagmiller RL, Veliz P, Marakovitz S, Briggs-Gowan MJ. Internalizing trajectories in young boys and girls: The whole is not a simple sum of its parts. Journal of Abnormal Child Psychology. 2010;38:19–31. doi: 10.1007/s10802-009-9342-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children’s behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality. 2003;71:495–513. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Kim Y, Sklar P, O’Donovan MC, Sullivan PF. Hypothesis-driven candidate genes for schizophrenia compared to genome-wide association results. Psychological Medicine. 2012;42:607–616. doi: 10.1017/S0033291711001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and co-morbidity. Child and Adolescent Psychiatric Clinics of North America. 2005;14:631–648. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cote S, Tremblay RE, Nagin D, Zoccolillo M, Vitaro F. The development of impulsivity, fearfulness, and helpfulness during childhood: Patterns of consistency and change in the trajectories of boys and girls. Journal of Child Psychology and Psychiatry. 2002;43:609–618. doi: 10.1111/1469-7610.00050. [DOI] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophrenia Bulletin. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Li J, Wang Z, Tong C, Fu G, Li Y, et al. A dynamic model for genome-wide association studies. Human Genetics. 2011;129:629–639. doi: 10.1007/s00439-011-0960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51:497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- DiLalla LF, Kagan J, Reznick JS. Genetic etiology of behavioral inhibition among 2-year-old children. Infant Behavior & Development. 1994;17:405–412. [Google Scholar]
- Domschke K, Deckert J, O’Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: Ethnic heterogeneity and gender specificity. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144B:667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Domschke K, Reif A. Behavioral genetics of affective and anxiety disorders. Current Topics in Behavioral Neurosciences. 2012;12:463–502. doi: 10.1007/7854_2011_185. [DOI] [PubMed] [Google Scholar]
- Duchesne S, Larose S, Vitaro F, Tremblay RE. Trajectories of anxiety in a population sample of children: Clarifying the role of children’s behavioral characteristics and maternal parenting. Development and Psychopathology. 2010;22:361–373. doi: 10.1017/S0954579410000118. [DOI] [PubMed] [Google Scholar]
- Duchesne S, Vitaro F, Larose S, Tremblay RE. Trajectories of anxiety during elementary-school years and the prediction of high school noncompletion. Journal of Youth and Adolescence. 2008;37:1134–1146. [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in preschool children. Journal of Child Psychology and Psychiatry. 2003;44:945–960. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- Eley TC, Rijsdijk FV, Perrin S, O’Connor TG, Bolton D. A multivariate genetic analysis of specific phobia, separation anxiety and social phobia in early childhood. Journal of Abnormal Child Psychology. 2008;36:839–848. doi: 10.1007/s10802-008-9216-x. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Czibere L, Roeske D, Lucae S, Unschuld PG, Ripke S, et al. TMEM132D, a new candidate for anxiety phenotypes: Evidence from human and mouse studies. Molecular Psychiatry. 2011;16:647–663. doi: 10.1038/mp.2010.41. [DOI] [PubMed] [Google Scholar]
- Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Archives of General Psychiatry. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- Ernst C, Wanner B, Brezo J, Vitaro F, Tremblay R, Turecki G. A deletion in tropomyosin-related kinase B and the development of human anxiety. Biological Psychiatry. 2011;69:604–607. doi: 10.1016/j.biopsych.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Essau CA, Conradt J, Petermann F. Frequency, comorbidity, and psychosocial impairment of anxiety disorders in German adolescents. Journal of Anxiety Disorders. 2000;14:263–279. doi: 10.1016/s0887-6185(99)00039-0. [DOI] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: A neural circuit perspective. Current Topics in Behavioral Neurosciences. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Ezpeleta L, Keeler G, Erkanli A, Costello EJ, Angold A. Epidemiology of psychiatric disability in childhood and adolescence. Journal of Child Psychology and Psychiatry. 2001;42:901–914. doi: 10.1111/1469-7610.00786. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Silk JS. Developmental trajectories of anxiety symptoms among boys across early and middle childhood. Journal of Abnormal Psychology. 2008;117:32–47. doi: 10.1037/0021-843X.117.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J. Animal models of anxiety and their molecular dissection. Seminars in Cell and Developmental Biology. 2003;14:37–42. doi: 10.1016/s1084-9521(02)00170-2. [DOI] [PubMed] [Google Scholar]
- Flint J, Shifman S, Munafo M, Mott R. Genetic variants in major depression. Novartis Foundation Symposium. 2008;289:23–32. doi: 10.1002/9780470751251.ch3. discussion, 33–42, 87–93. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Franic S, Middeldorp CM, Dolan CV, Ligthart L, Boomsma DI. Childhood and adolescent anxiety and depression: Beyond heritability. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:820–829. doi: 10.1016/j.jaac.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Pozzi G, Gianfagna F, Manzoli L, Boccia S. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58:163–170. doi: 10.1159/000182892. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Morrison JM. QUANTO 1.2.4: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006 Retrieved from http://hydra.usc.edu/gxe/
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Research. 2004;7:39–53. doi: 10.1375/13690520460741435. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: A behavior–genetic perspective. Biological Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grafstein-Dunn E, Young KH, Cockett MI, Khawaja XZ. Regional distribution of regulators of G-protein signaling (RGS) 1, 2, 13, 14, 16, and GAIP messenger ribonucleic acids by in situ hybridization in rat brain. Brain Research. Molecular Brain Research. 2001;88:113–123. doi: 10.1016/s0169-328x(01)00038-9. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive–compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, Koenen K, Eley TC, Poulton R. Juvenile mental health histories of adults with anxiety disorders. American Journal of Psychiatry. 2007;164:301–308. doi: 10.1176/ajp.2007.164.2.301. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC. Genetic influences on anxiety in children: What we’ve learned and where we’re heading. Clinical Child & Family Psychology Review. 2007;10:199–212. doi: 10.1007/s10567-007-0022-8. [DOI] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Hamagami F. Nonlinear growth curves in developmental research. Child Development. 2011;82:1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Eley TC. Phenotypic and genetic differentiation of anxiety-related behaviors in middle childhood. Depression and Anxiety. 2009;26:316–324. doi: 10.1002/da.20539. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of posttraumatic stress disorder. CNS Spectrums. 2009;14(Suppl 1):13–24. [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hewitt JK. Editorial policy on candidate gene association and candidate gene-by-environment interaction studies of complex traits. Behavior Genetics. 2012;42:1–2. doi: 10.1007/s10519-011-9504-z. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: A five-year follow-up. Journal of Developmental and Behavioral Pediatrics. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Micco JA, Simoes NA, Henin A. High-risk studies and developmental antecedents of anxiety disorders. American Journal of Medical Genetics C: Seminars in Medical Genetics. 2008;148C:99–117. doi: 10.1002/ajmg.c.30170. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N. The long shadow of temperament. Cambridge, MA: Harvard University Press; 2004. [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72:1–75. doi: 10.1111/j.1540-5834.2007.00436.x. discussion, 76–91. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Otowa T, Koike A, Sugaya N, Yoshida E, Yasuda S, et al. A genome-wide CNV association study on panic disorder in a Japanese population. Journal of Human Genetics. 2011;56:852–856. doi: 10.1038/jhg.2011.117. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Lichtenstein P. The development of fears from early adolesence to young adulthood: A multivariate study. Psychological Medicine. 2008;38:1759–1769. doi: 10.1017/S0033291708002936. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A longitudinal twin study of fears from middle childhood to early adulthood: Evidence for a developmentally dynamic genome. Archives of General Psychiatry. 2008;65:421–429. doi: 10.1001/archpsyc.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: A conceptual analysis. Molecular Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Archives of General Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kerner B, North KE, Fallin MD. Use of longitudinal data in genetic studies in the genome-wide association studies era: Summary of Group 14. Genetic Epidemiology. 2009;33(Suppl 1):S93–S98. doi: 10.1002/gepi.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. NeuroReport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York: Guilford Press; 2005. [Google Scholar]
- Koenen KC, Amstadter AB, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depression and Anxiety. 2009;26:309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher P, Sanson A, Smart D, Toumbourou JW. Precursors and correlates of anxiety trajectories from late childhood to late adolescence. Journal of Clinical Child and Adolescent Psychology. 2012 doi: 10.1080/15374416.2012.680189. [DOI] [PubMed] [Google Scholar]
- Leygraf A, Hohoff C, Freitag C, Willis-Owen SA, Krakowitzky P, Fritze J, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? Journal of Neural Transmission. 2006;113:1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Latent variable models: An introduction to factor, path, and structural equation analysis. 4. Mahwah, NJ: Erlbaum; 2004. [Google Scholar]
- Malhotra D, Sebat J. CNVs: Harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein NR, White H, Chung T, Hipwell A, Stouthamer-Loeber M, Loeber R. Associations between first use of substances and change in internalizing symptoms among girls: Differences by symptom trajectory and substance use type. Journal of Clinical Child and Adolescent Psychology. 2010;39:545–558. doi: 10.1080/15374416.2010.486325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Hettema JM, Shlik J. Advances in molecular genetics of panic disorder. Molecular Psychiatry. 2010;15:681–701. doi: 10.1038/mp.2009.145. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR, Schinka JA, Velicer WF. Growth curve analysis in contemporary psychological research. New York: Wiley; 2003. [Google Scholar]
- McGrath LM, Mustanski B, Metzger A, Pine DS, Kistner-Griffin E, Cook EH, et al. A latent modeling approach to genotype–phenotype relationships: Maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Archives of Women’s Mental Health. doi: 10.1007/s00737-012-0286-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen MB, Bertram L, Lange C, Becker KD, Albert MS, Tanzi RE, et al. Exploring candidate gene associations with neuropsychological performance. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144B:987–991. doi: 10.1002/ajmg.b.30500. [DOI] [PubMed] [Google Scholar]
- Medland SE, Neale MC. An integrated phenomic approach to multivariate allelic association. European Journal of Human Genetics. 2010;18:233–239. doi: 10.1038/ejhg.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Ames M, Cui L, Stang PE, Ustun TB, Von Korff M, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Archives of General Psychiatry. 2007;64:1180–1188. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics. 2010;125:75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian ND, Godoy L, Briggs-Gowan MJ, Carter AS. Patterns of anxiety symptoms in toddlers and preschool-age children: Evidence of early differentiation. Journal of Anxiety Disorders. 2011 doi: 10.1016/j.janxdis.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The comorbidity of anxiety and depression in the perspective of genetic epidemiology: A review of twin and family studies. Psychological Medicine. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Slof-Op ‘t Landt MC, Medland SE, van Beijsterveldt CE, Bartels M, Willemsen G, et al. Anxiety and depression in children and adults: Influence of serotonergic and neurotrophic genes? Genes, Brain and Behavior. 2010;9:808–816. doi: 10.1111/j.1601-183X.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosing MA, Gordon SD, Medland SE, Statham DJ, Nelson EC, Heath AC, et al. Genetic and environmental influences on the comorbidity between depression, panic disorder, agoraphobia, and social phobia: A twin study. Depression and Anxiety. 2009;26:1004–1011. doi: 10.1002/da.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri K, Hishimoto A, Fukutake M, Nishiguchi N, Shirikawa O, Maeda K. Association study of RGS2 gene polymorphisms with panic disorder in Japanese. Kobe Journal of Medical Sciences. 2009;55:E116–E121. [PubMed] [Google Scholar]
- Muinos-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipila T, Maron E, et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biological Psychiatry. 2011;69:526–533. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Muthen BO. Second-generation structural equation modeling with a combination of categorical and continuous latent variables: New opportunities for latent class/latent growth modeling. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 291–322. [Google Scholar]
- Muthen BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29:81–117. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Neale BM, Medland S, Ripke S, Anney RJ, Asherson P, Buitelaar J, et al. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:906–920. doi: 10.1016/j.jaac.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes RB, Roysamb E, Reichborn-Kjennerud T, Harris JR, Tambs K. Symptoms of anxiety and depression in young adults: Genetic and environmental influences on stability and change. Twin Research and Human Genetics. 2007;10:450–461. doi: 10.1375/twin.10.3.450. [DOI] [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nature Reviews Drug Discovery. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: Prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. Journal of Consulting and Clinical Psychology. 1996;64:552–562. [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Ogliari A, Spatola CA, Pesenti-Gritti P, Medda E, Penna L, Stazi MA, et al. The role of genes and environment in shaping co-occurrence of DSM-IV defined anxiety dimensions among Italian twins aged 8–17. Journal of Anxiety Disorders. 2010;24:433–439. doi: 10.1016/j.janxdis.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proceedings of the National Academy of Sciences. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otowa T, Shimada T, Kawamura Y, Sugaya N, Yoshida E, Inoue K, et al. Association of RGS2 variants with panic disorder in a Japanese population. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2011;156B:430–434. doi: 10.1002/ajmg.b.31178. [DOI] [PubMed] [Google Scholar]
- Otowa T, Tanii H, Sugaya N, Yoshida E, Inoue K, Yasuda S, et al. Replication of a genome-wide association study of panic disorder in a Japanese population. Journal of Human Genetics. 2010;55:91–96. doi: 10.1038/jhg.2009.127. [DOI] [PubMed] [Google Scholar]
- Otowa T, Yoshida E, Sugaya N, Yasuda S, Nishimura Y, Inoue K, et al. Genome-wide association study of panic disorder in the Japanese population. Journal of Human Genetics. 2009;54:122–126. doi: 10.1038/jhg.2008.17. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Lansford JE, Pettit GS, et al. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents’ trajectories of anxious/depressed symptoms. Developmental Psychology. 2012 doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium. A mega-analysis of genome-wide association studies for major depressive disorder. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.21. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Plomin R, Emde RN, Braungart JM, Campos J, Corley R, Fulker DW, et al. Genetic change and continuity from fourteen to twenty months: The MacArthur Longitudinal Twin Study. Child Development. 1993;64:1354–1376. [PubMed] [Google Scholar]
- Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Schniering CA, Hudson JL. Anxiety disorders during childhood and adolescence: Origins and treatment. Annual Review of Clinical Psychology. 2009;5:311–341. doi: 10.1146/annurev.clinpsy.032408.153628. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Snieder H, Ormel J, Sham P, Goldberg DP, Spector TD. Genetic and environmental influences on psychological distress in the population: General Health Questionnaire analyses in UK twins. Psychological Medicine. 2003;33:793–801. doi: 10.1017/s0033291703007451. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, Eaves LJ, Hettema JM, Kendler KS, Silberg JL. Childhood separation anxiety disorder and adult-onset panic attacks share a common genetic diathesis. Depression and Anxiety. 2012;29:320–327. doi: 10.1002/da.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Kagan J, Reznick JS, Corley R. The heritability of inhibited and uninhibited behavior: A twin study. Developmental Psychology. 1992;28:1030–1037. [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld-Becker DR, Kagan J, Snidman N, Friedman D, et al. A controlled study of behavioral inhibition in children of parents with panic disorder and depression. American Journal of Psychiatry. 2000;157:2002–2010. doi: 10.1176/appi.ajp.157.12.2002. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Temperament, development, and personality. Current Directions in Psychological Science. 2007;16:207–212. [Google Scholar]
- Sakai JT, Boardman JD, Gelhorn HL, Smolen A, Corley RP, Huizinga D, et al. Using trajectory analyses to refine phenotype for genetic association: Conduct problems and the serotonin transporter (5HTTLPR) Psychiatric Genetics. 2010;20:199–206. doi: 10.1097/YPG.0b013e32833a20f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: Forward genetics and reverse phenotyping. Human Heredity. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Semplicini A, Lenzini L, Sartori M, Papparella I, Calo LA, Pagnin E, et al. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. Journal of Hypertension. 2006;24:1115–1124. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, Craddock N, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Lunetta KL, Robins J. Implications of comorbidity and ascertainment bias for identifying disease genes. American Journal of Medical Genetics. 2000;96:817–822. doi: 10.1002/1096-8628(20001204)96:6<817::aid-ajmg25>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, et al. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Archives of General Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biological Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biological Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Spurious genetic associations. Biological Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. The psychiatric GWAS consortium: Big science comes to psychiatry. Neuron. 2010;68:182–186. doi: 10.1016/j.neuron.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. Don’t give up on GWAS. Molecular Psychiatry. 2012;17:2–3. doi: 10.1038/mp.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambs K, Czajkowsky N, Roysamb E, Neale MC, Reichborn-Kjennerud T, Aggen SH, et al. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. British Journal of Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskowski M, Zavos HM, Haworth CM, Plomin R, Eley TC. Stable genetic influence on anxiety-related behaviours across middle childhood. Journal of Abnormal Child Psychology. 2011 doi: 10.1007/s10802-011-9545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BT, Guo AY, Maher BS, Zhao Z, van den Oord EJ, Kendler KS, et al. Meta-analyses of genome-wide linkage scans of anxiety-related phenotypes. European Journal of Human Genetics. 2012 doi: 10.1038/ejhg.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, et al. Families at high and low risk for depression: A 3-generation study. Archives of General Psychiatry. 2005;62:29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- Wessman J, Paunio T, Tuulio-Henriksson A, Koivisto M, Partonen T, Suvisaari J, et al. Mixture model clustering of phenotype features reveals evidence for association of DTNBP1 to a specific subtype of schizophrenia. Biological Psychiatry. 2009;66:990–996. doi: 10.1016/j.biopsych.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, et al. Accurate, large-scale genotyping of 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biological Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nature Genetics. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- Zintzaras E, Sakelaridis N. Is 472G/A catechol-O-methyl-transferase gene polymorphism related to panic disorder? Psychiatric Genetics. 2007;17:267–273. doi: 10.1097/YPG.0b013e3280d6472e. [DOI] [PubMed] [Google Scholar]