Abstract
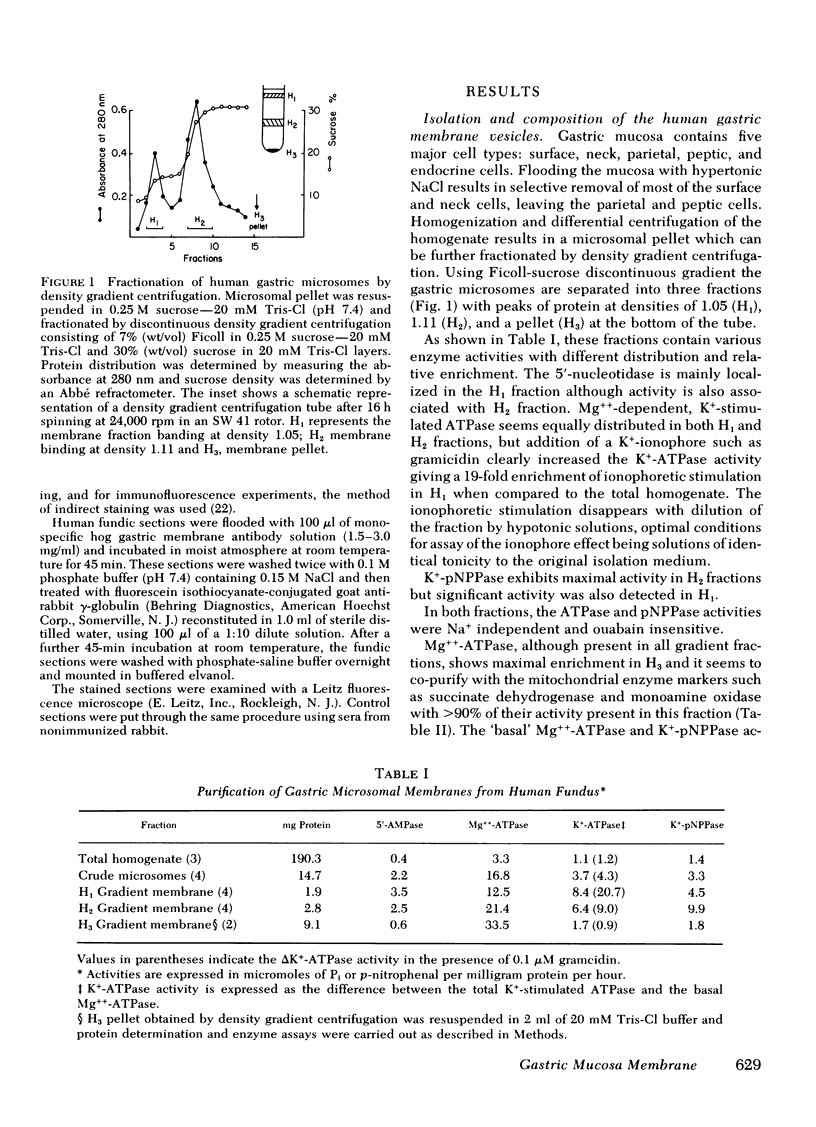
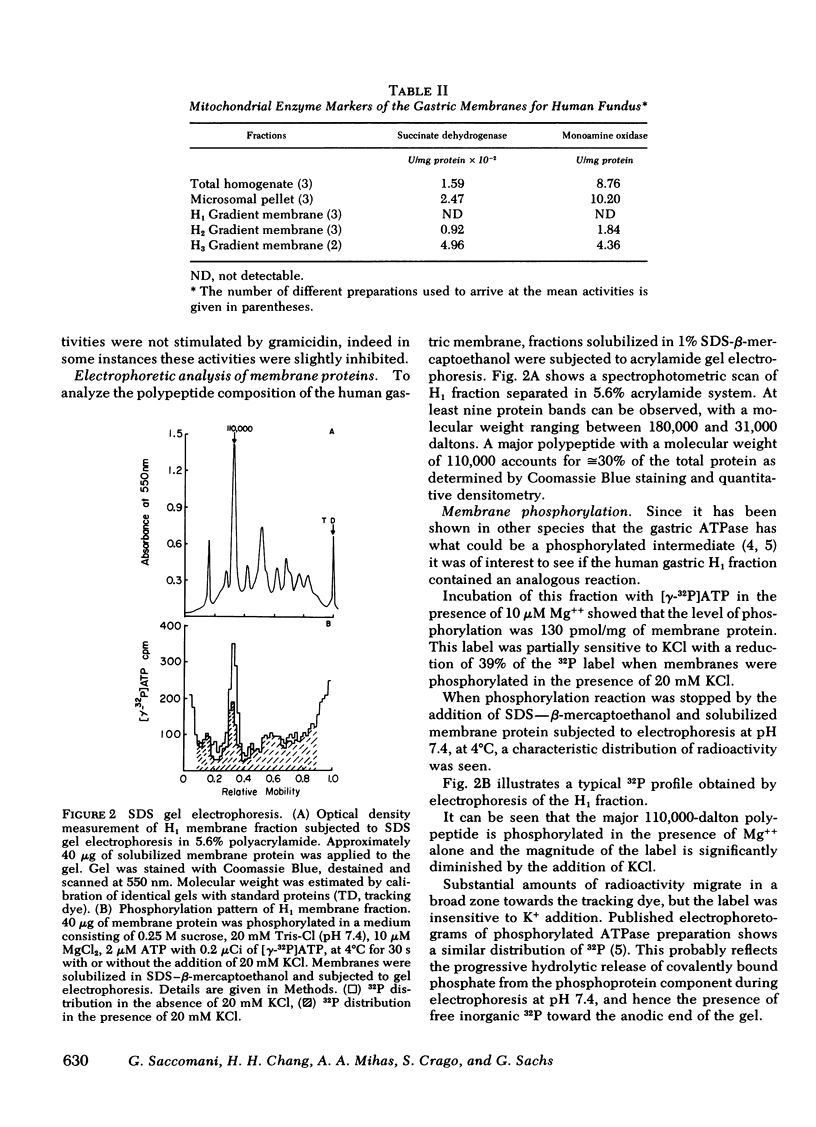
Isolation of a microsomal fraction from human gastric mucosa followed by density gradient centrifugation yielded a vesicular membrane preparation free of mitochondrial markers, containing a K+-activated, ouabain-insensitive ATPase with an activity of 20.7 mumol P1 released/mg protein per h. Sodium dodecyl sulfate gel electrophoresis showed that the human gastric membrane vesicles contained a major polypeptide of 110,000 daltons, which accounted for approximately or equal to 30% of the total protein stained and was phosphorylated by [gamma-32P]ATP and dephosphorylated in the presence of K+. Electron microscopy revealed the presence of vesicles with an average size of 0.13 micrometer in diameter. Addition of 0.65 microM ATP to this vesicular preparation resulted in the uptake of 17 nmol H+/mg protein which was dependent on the presence of K+. The gradient was dissipated by a combination of valinomycin and protonophore after consumption of the ATP. Incubation of fixed human fundic sections or human gastric biopsy with monospecific hog gastric membrane antibody followed by fluorescein-conjugated goat anti-rabbit gamma-globulin, showed fluorescent staining in the middle portion of the gastric glands. These data indicate that human stomach contains a H+ transport ATPase with characteristics similar to those established for lower species.
Full text
PDF

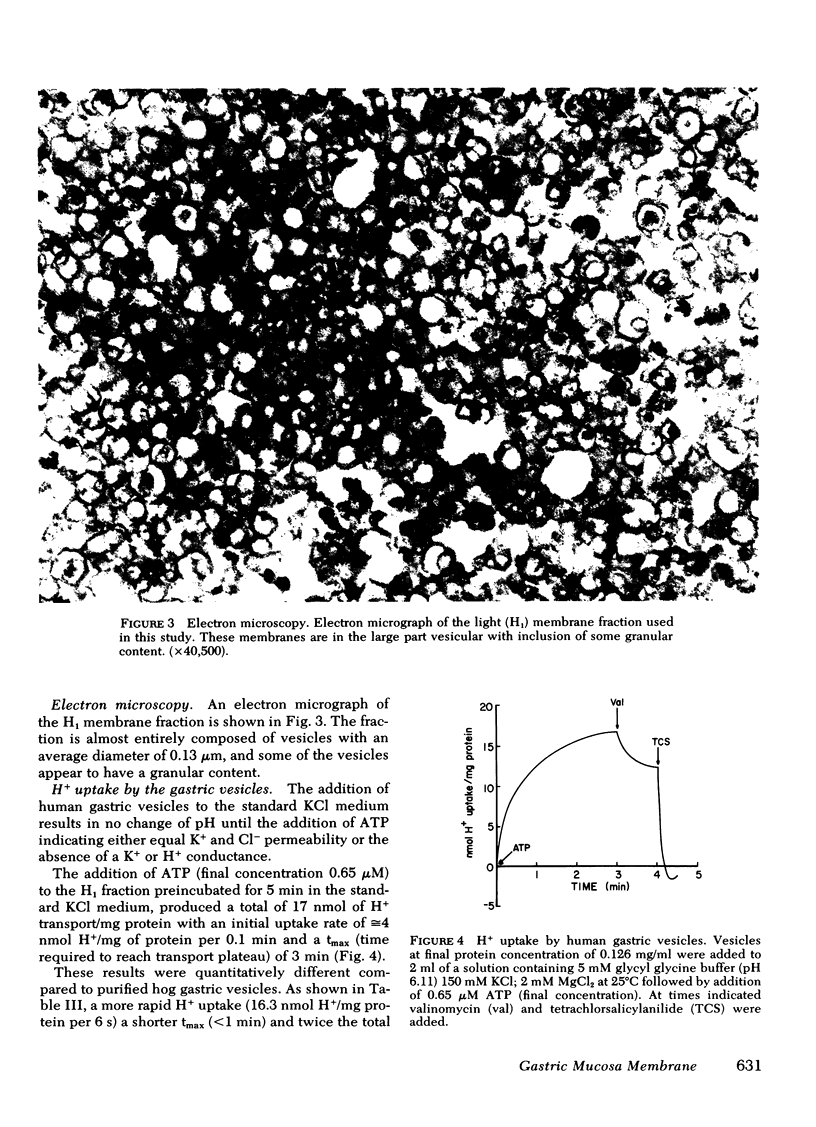
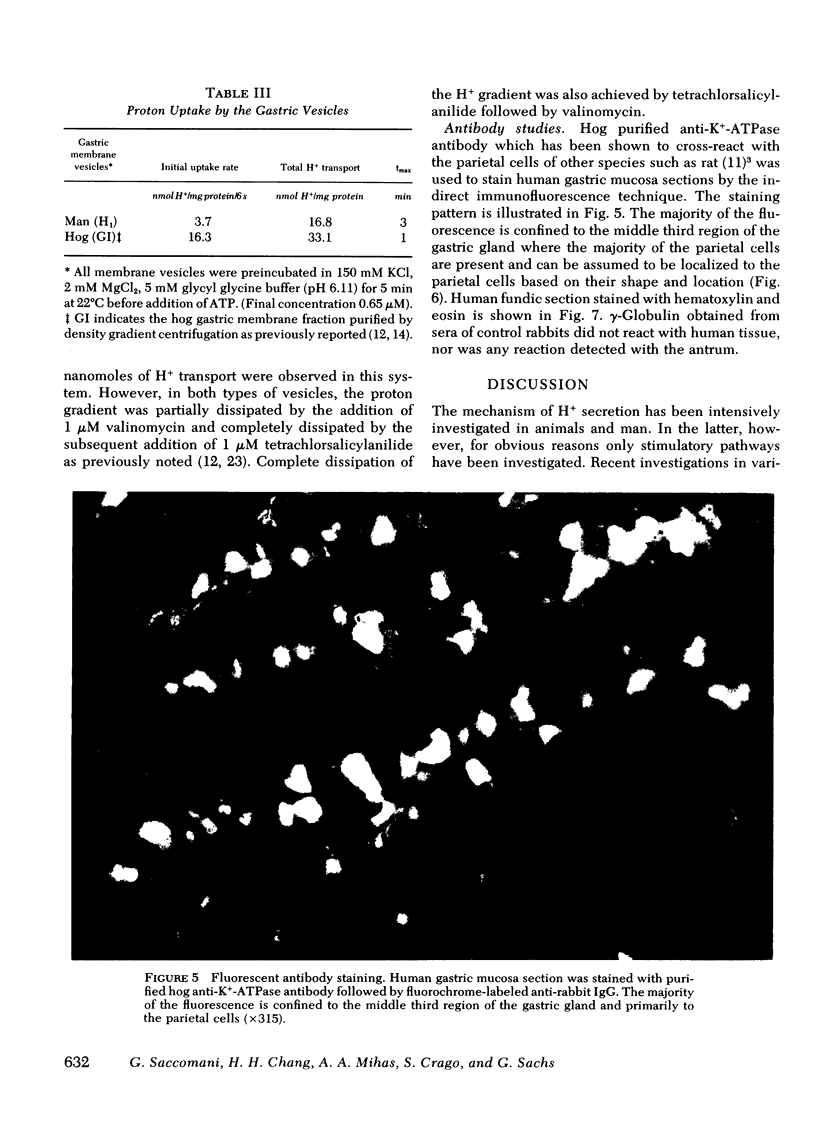



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglindh T. Effects of common inhibitors of gastric acid secretion on secretagogue-induced respiration and aminopyrine accumulation in isolated gastric glands. Biochim Biophys Acta. 1977 Jan 4;464(1):217–233. doi: 10.1016/0005-2736(77)90383-2. [DOI] [PubMed] [Google Scholar]
- Chang H., Saccomani G., Rabon E., Schackmann R., Sachs G. Proton transport by gastric membrane vesicles. Biochim Biophys Acta. 1977 Jan 21;464(2):313–327. doi: 10.1016/0005-2736(77)90006-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Forte G. M., Saltman P. K+-stimulated phosphatase of microsomes from gastric mucosa. J Cell Physiol. 1967 Jun;69(3):293–304. doi: 10.1002/jcp.1040690305. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Ganser A., Beesley R., Forte T. M. Unique enzymes of purified microsomes from pig fundic mucosa. K+-stimulated adenosine triphosphatase and K+-stimulated pNPPase. Gastroenterology. 1975 Jul;69(1):175–189. [PubMed] [Google Scholar]
- Forte J. G., Ray T. K., Poulter J. L. A method for preparing oxyntic cells from frog gastric mucosa. J Appl Physiol. 1972 May;32(5):714–717. doi: 10.1152/jappl.1972.32.5.714. [DOI] [PubMed] [Google Scholar]
- Forte J. G. Three components of Cl flux across isolated bullfrog gastric mucosa. Am J Physiol. 1969 Jan;216(1):167–174. doi: 10.1152/ajplegacy.1969.216.1.167. [DOI] [PubMed] [Google Scholar]
- Ganser A. L., Forte J. G. Ionophoretic stimulation of K+-ATPase of oxyntic cell microsomes. Biochem Biophys Res Commun. 1973 Sep 18;54(2):690–696. doi: 10.1016/0006-291x(73)91478-2. [DOI] [PubMed] [Google Scholar]
- HARRIS J. B., EDELMAN I. S. Transport of potassium by the gastric mucosa of the frog. Am J Physiol. 1960 Feb;198:280–284. doi: 10.1152/ajplegacy.1960.198.2.280. [DOI] [PubMed] [Google Scholar]
- Helander H. F. Stereological changes in rat parietal cells after vagotomy and antrectomy. Gastroenterology. 1976 Dec;71(6):1010–1018. [PubMed] [Google Scholar]
- Hirschowitz B. I., Sachs G. Insulin inhibition of gastric secretion: reversal by ribidium. Am J Physiol. 1967 Dec;213(6):1401–1405. doi: 10.1152/ajplegacy.1967.213.6.1401. [DOI] [PubMed] [Google Scholar]
- Kasbekar D. K., Durbin R. P. An adenosine triphosphatase from frog gastric mucosa. Biochim Biophys Acta. 1965 Sep 20;105(3):472–482. doi: 10.1016/s0926-6593(65)80232-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J., Simpson G., Scholes P. An ATPase from dog gastric mucosa: changes of outer pH in suspensions of membrane vesicles accompanying ATP hydrolysis. Biochem Biophys Res Commun. 1974 Sep 23;60(2):825–832. doi: 10.1016/0006-291x(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Rabon E., Chang H., Sachs G. Quantitation of hydrogen ion and potential gradients in gastric plasma membrane vesicles. Biochemistry. 1978 Aug 8;17(16):3345–3353. doi: 10.1021/bi00609a027. [DOI] [PubMed] [Google Scholar]
- Rabon E., Saccomani G., Kasbekar D. K., Sachs G. Transport characteristics of frog gastric membranes. Biochim Biophys Acta. 1979 Mar 8;551(2):432–447. doi: 10.1016/0005-2736(89)90018-7. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Forte J. G. Studies on the phosphorylated intermediates of a K+-stimulated ATPase from rabbit gastric mucosa. Biochim Biophys Acta. 1976 Sep 7;443(3):451–467. doi: 10.1016/0005-2736(76)90465-x. [DOI] [PubMed] [Google Scholar]
- Saccomani G., Shah G., Spenney J. G., Sachs G. Characterization of gastric mucosal membranes. VIII. The localization of peptides by iodination and phosphorylation. J Biol Chem. 1975 Jun 25;250(12):4802–4809. [PubMed] [Google Scholar]
- Saccomani G., Stewart H. B., Shaw D., Lewin M., Sachs G. Characterization of gastric mucosal membranes. IX. Fractionation and purification of K+-ATPase-containing vesicles by zonal centrifugation and free-flow electrophoresis technique. Biochim Biophys Acta. 1977 Mar 1;465(2):311–330. doi: 10.1016/0005-2736(77)90081-5. [DOI] [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Sachs G. H+ transport by a non-electrogenic gastric ATPase as a model for acid secretion. Rev Physiol Biochem Pharmacol. 1977;79:133–162. doi: 10.1007/BFb0037090. [DOI] [PubMed] [Google Scholar]
- Sachs G., Shoemaker R. L., Hirschowitz B. L. Effects of sodium removal on acid secretion by the frog gastric mucosa. Proc Soc Exp Biol Med. 1966 Oct;123(1):47–52. doi: 10.3181/00379727-123-31398. [DOI] [PubMed] [Google Scholar]
- Sachs G., Spenney J. G., Lewin M. H+ transport: regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978 Jan;58(1):106–173. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]
- Sedar A. W. Fine structure of the stimulated oxyntic cell. Fed Proc. 1965 Nov-Dec;24(6):1360–1367. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Stoichiometry of adenosine triphosphate-driven proton translocation in bovine heart submitochondrial particles. J Biol Chem. 1973 Aug 10;248(15):5395–5402. [PubMed] [Google Scholar]