Abstract
The identification of the fundamental role of apoptosis in the growth balance and normal homeostasis against cell proliferation led to the recognition of its loss contributing to tumorigenesis. The mechanistic significance of reinstating apoptosis signaling towards selective targeting of malignant cells heavily exploits the caspase family of death-inducing molecules as a powerful therapeutic platform for the development of potent anticancer strategies. Some apoptosis inhibitors induce caspase expression and activity in preclinical models and clinical trials by targeting both the intrinsic and extrinsic apoptotic pathways and restoring the apoptotic capacity in human tumors. Furthermore, up-regulation of caspases emerges as a sensitizing mechanism for tumors exhibiting therapeutic resistance to radiation and adjuvant chemotherapy. This review provides a comprehensive discussion of the functional involvement of caspases in apoptosis control and the current understanding of reactivating caspase-mediated apoptosis signaling towards effective therapeutic modalities in cancer treatment.
Keywords: apoptin, apoptosis, caspase, prostate cancer, quinazolines, survivin
Introduction
Advances in targeted therapy for prostate cancer have resulted in the highest cancer survival rates among men (Siegel et al., 2012). Management of patients with localized prostate cancer with radical prostatectomy, radiation therapy and androgen deprivation therapy has met with partial success. A subset of patients refractory to androgen deprivation therapy will progress to castration-resistant prostate cancer (CRPC). Mechanisms implicated with progression to CRPC include extra-gonadal androgen synthesis (intra-tumoral, adrenal and peritumor adipose production) and modulation of several cell survival signaling axes (reviewed in Shore et al., 2012). Novel microtubule-targeting agents and androgen synthesis/signaling axis inhibitors, combined with the emergence of immunotherapy, may prove useful in the treatment of these advanced stage tumors (Sartor and Fitzpatrick, 2012). Although the investigation of candidate targets to prevent metastatic progression has provided valuable insights into the phenomenon of anoikis resistance, there is no treatment for metastatic prostate cancer that effectively increases survival (Sakamoto and Kyprianou, 2010; Hensley and Kyprianou, 2012).
At the dawn of 2013, it is clear that a ‘cure for cancer’ may be an abstract concept, with each tissue-specific tumor necessitating a unique therapeutic regimen to compliment the respective genetic and cell signaling pathways involved in tumorigenesis. However, a promising study by the Cancer Genome Atlas Network has identified common genetic mutations between tumors previously considered unrelated (Cancer Genome Atlas Network 2012). Therapeutic resistance creates an inherent need to target a more universal pathway shared by all solid tumors and hematopoietic malignancies. Reinstating apoptosis programming in malignant cells leads to selective tumor cell killing, and caspases as primary inducers of apoptosis provide an ideal platform from which to develop effective therapeutic strategies in human cancer. This review provides a comprehensive discussion of the physiological and oncogenic role of caspases and the current treatment modalities aimed at caspase-induced cell death.
Mechanisms of apoptosis
The intrinsic ability for genetically transformed cells to survive and proliferate despite the lack of growth stimuli, contact inhibition, anchorage dependence or immunoregulatory mechanisms has proven fundamental to our understanding of oncogenesis (Hanahan and Weinberg, 2011). In contrast to other cell death mechanisms, such as necrosis or cell-mediated cytotoxicity, apoptotic cells preserve the cellular milieu by maintaining the integrity of the plasma membrane, preventing inflammatory destruction of the surrounding tissue (Muñoz-Pinedo, 2012). The ability for cancer cells to overcome abundant pro-apoptotic signals provided by the tumor microenvironment establishes apoptosis as a critical target for cancer chemo-therapeutics (McKenzie and Kyprianou, 2006; Rennebeck et al., 2005; Sakamoto and Kyprianou, 2010). In addition to cancer, deregulation of apoptotic signaling contributes to the development of other human diseases, including neurological, cardiovascular and autoimmune diseases (Favaloro et al., 2012).
Caspase structure and function
The term ‘c-asp-ase’ is derived from the cysteine-dependent aspartate-specific protease activity shared by each of the members that catalyze cleavage at aspartate residues of target proteins via high-affinity cysteine residues (Alnemri et al., 1996). Protein cleavage by nucleophilic cysteine residues is a well conserved mechanism among proteases, but the specific affinity for aspartate is unique to caspases (Salvesen and Riedl, 2008). Inactivated caspases are in the quiescent zymogen procaspase form and activation of either of two pathways leads to cleavage (activation) and signal transduction. In vitro, apical caspases can be activated by dimerization, while the executioner caspases require proteolytic cleavage (Fuentes-Prior and Salvesen, 2004). These upstream caspases are similarly activated in vivo though density-dependent association with activation complexes, leading to conformational change and cleavage. Downstream caspases can only be activated by proteolytic cleavage of pro-domains by activated upstream caspases (Favaloro et al., 2012). Activated caspases result in intracellular proteolysis of cytoskeletal components and proteins vital for organelle integrity towards cell execution (apoptosis). The two main pathways signaling the execution of apoptosis are the extrinsic (death receptor-mediated) and intrinsic (mitochondrial) pathways (Figure 1).
Figure 1.
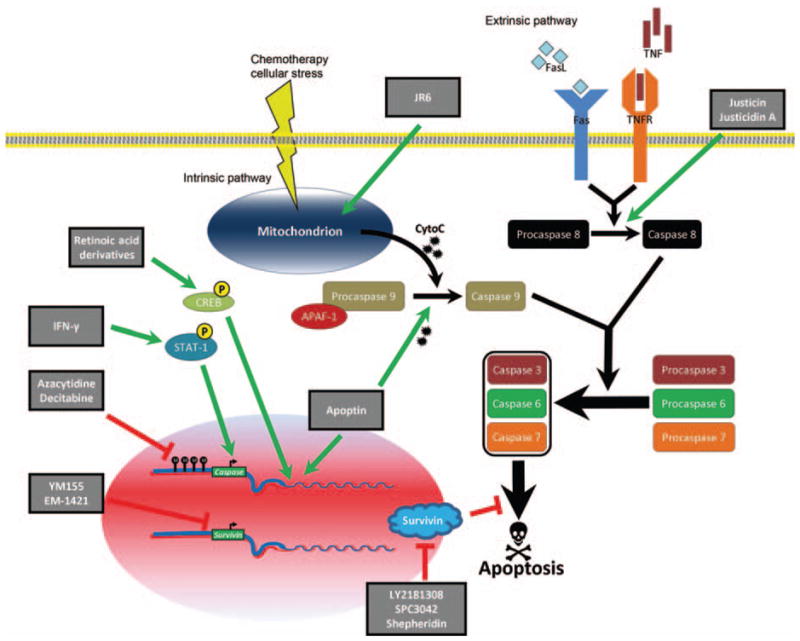
Therapeutic targeting of caspase activation signaling.
Ligand binding to death receptors (extrinsic pathway) results in death initiation signaling complex activation and signaling through initiator caspase 8. Numerous external signals cause the cytochrome c release from mitochondria (intrinsic pathway), resulting in apoptosome formation and signaling through initiator caspase 9. Initiator caspases amplify the apoptotic signal via cleavage of downstream, effector caspases that induce irreversible damage to DNA and vital cellular machinery. Apoptin’s subcellular localization might be driven by its phosphorylation status. Once in the nucleus, apoptin binds to several protein partners and DNA through a highly inconsistent secondary and tertiary structure. Novel therapeutic compounds, such as shepheridin, LY2181308 (an antisense oligonucleotide), EM-1421, and YM155, exhibit anti-tumor therapeutic efficacy in the clinical management of cancer patients by directly targeting survivin and/or inducing caspase expression and activity, ultimately resulting in apoptosis mediated via both intrinsic and extrinsic apoptotic pathways.
The extrinsic pathway is activated upon binding of a ligand to transmembrane death receptors belonging to the tumor necrosis factor (TNF) superfamily. Ligands involved in both inflammatory and cell death processes capable of extrinsic induction include TNF-α, Fas ligand (CD95L) and TNF-related apoptosis-inducing ligand (TRAIL). Upon ligand binding, death receptors undergo a conformational change, increasing affinity for intracellular adapter proteins and creating a receptor–protein complex, the death initiation signaling complex (DISC). The key DISC components are the initiator procaspases 8 and 10, which become activated though trans- and self-cleavage events. These initiator caspases cleave downstream effector caspases 3, 6 and 7 (Fiandalo and Kyprianou, 2012).
The intrinsic apoptosis cascade is initiated with the mitochondrial release of cytochrome c resulting from changes in mitochondrial outer membrane permeabilization in response to various cell stressors, including hypoxia (antiangiogenic therapy), reactive oxygen species (radiation therapy) or a paucity of available growth factors (Ocker and Höpfner, 2012). Permeabilization is increased with diminished membrane potential (Δψm) and ATP production. Intracellular stress activates proapoptotic B cell lymphoma 2 (Bcl2) family members Bad and Bim. These proteins function to inhibit antiapoptotic family members Bcl-2, Bcl-xL and Puma/Noxa, allowing Bax and Bak to dimerize and translocate to the mitochondrial outer membrane, causing the release of mitochondrial contents including cytochrome c (Ocker and Höpfner, 2012). Cytosolic Apaf-1 oligomerizes upon release of cyto-chrome c and recruits caspase 9, the apical caspase of the intrinsic pathway. The complex of Apaf-1, cytochrome c and caspase 9 is known as the apoptosome (Salvesen and Riedl, 2008). The functional redundancy between the intrinsic and extrinsic pathways is clearly illustrated by caspase 8 activation of the BH3-only protein Bid. Bid activates Bax and Bak, which are located on the mitochondrial membrane, to trigger the release of cytochrome c (Scaffidi et al., 1998). Cells undergoing apoptosis ensure an anti-inflammatory response by the phagocytosing cell, resulting in the quiescent removal of dead and dying cells (Martin et al., 2012). Specific ‘inflammatory caspases’ belong to the caspase 1 family and are expressed in hematopoietic lineages (Schroder and Tschopp, 2012). Caspases play a critical role in normal tissue homeostasis, primarily during patterning and tissue specialization throughout embryonic development as primitive tissues become replaced by differentiated, functionally-distinct tissue (Fuchs and Steller, 2011). Aberrant activation of caspases has been implicated in a number of conditions, including autoimmunity, transplant rejection and heart failure (Ocker and Höpfner, 2012). Manipulating caspase signaling has a therapeutic benefit on diverse diseases, such as cardiovascular, autoimmune and infectious disease (Favaloro et al., 2012).
Inhibitors of apoptosis
Inhibitors of apoptosis (IAP) comprise a family of intracellular proteins involved in dampening the caspase cascade. Eight members have been identified in the IAP family but only two, X-linked IAP (XIAP) and survivin, have the ability to directly inhibit caspases (Tamm et al., 1998 ; Salvesen and Duckett, 2002). XIAP negatively regulates caspases by competing for distinct binding domains. Binding to caspases 3 and 7 in their dimeric form sterically hinders the catalytic site, rendering the proteolytic activity of these effector caspases nonfunctional. Alternatively, when XIAP binds caspase 9, a cleavage event ultimately inhibits this initiator caspase. Survivin can directly inhibit caspase function and will be specifically discussed as a therapeutic target.
Novel caspase-targeting compounds
Derivatives of Justicia procumbens
The plant Justicia procumbens is a traditional Chinese herbal remedy for the treatment of fever, pharyngeal swelling and cancer; with whole-plant extract showing initial antitumor efficacy in lymphocytic leukemia and nasopharyngeal carcinoma (Fukamiya and Lee, 1986). Justicin has cytotoxic effects in a number of in vivo cancer models (He et al., 2012). A derivative, justicidin A, induces apoptosis in hepatocellular carcinoma via the activation of both intrinsic and extrinsic apoptosis pathways (Su et al., 2006). Through activation of caspase 8, justicidin A increases intracellular Bid, which modulates Δψm, causing the release of cytochrome c; but decreases (antiapoptotic) BCL-xL. Mitochondrial release of Smac/DIABLO activates caspases 9 and 3 to initiate the intrinsic pathway.
A novel compound from J. procumbens, 6′-hydroxy justicidin A (JR6), has promising apoptosis-induction efficacy in human bladder cancer cells (He et al., 2012). This compound shares a common molecular nucleus with the lead antitumor drug podophyllotoxin, a tubulin destabilizing agent that targets tumor vascularity (Liu et al., 2012). JR6 induces apoptosis via Δψm modulation and the creation of reactive oxygen species, resulting in caspase activation. Furthermore, JR6 decreases the activity of superoxide dismutase, increasing susceptibility to reactive oxygen species-mediated peroxidation injury.
Re-instating the expression and activation of caspase-8 in cancer therapy
Epigenetic regulation
Cleavage of circulating procaspases is the principal regulatory mechanism in apoptosis induction, although loss of caspase expression has been demonstrated in human cancers including neuroblastomas and small cell carcinomas (Teitz et al., 2000; Shivapurkar et al., 2002). Silencing caspase 8 in neuroblastomas occurs via CpG hypermethylation in the 5′ promoter region, as well as loss of heterozygosity allelic deletion. Homozygous deletion of caspase 8 is a factor contributing to the malignant potential of NCI-H82 cells, a small cell lung cancer model (Kohno et al., 1994). This recognition fueled additional studies that established a loss of caspase 8 expression in 27 out of 34 small cell carcinoma cell lines, with normal caspase 8 expression in 22 out of 22 non-small cell carcinoma cell lines (Shivapurkar et al., 2002). Moreover, functional loss of caspase 8 has been linked to therapeutic resistance to the death-receptor ligands TNFα, TRAIL and CD95 (Fulda et al., 2001). Thus, targeting caspase 8 expression may lead to a significant therapeutic effect, especially in tumors suffering from a gene dosage effect or hypermethylation of caspase 8 promoters. In this context, 5-aza-2′ deoxycytidine (decitabine) is a cytosine nucleoside analog that promotes demethylation by inhibiting DNA methyltransferase covalent binding. This compound increases caspase 8 promoter availability, allowing for the binding of SP1 and ETS-like transcription factors (Liedtke et al., 2005; Fulda, 2006). Decitabine-mediated restoration of caspase 8 has been demonstrated in breast cancer, neuroblastoma, medulloblastoma, Ewing ‘s sarcoma, lung carcinoma and gastric carcinoma (Hopkins -Donaldson et al., 2000; Eggert et al., 2001; Fulda et al., 2001; Grotzer et al., 2000 ; Wu et al., 2010; Geiger et al., 2012 ; Wang et al., 2012). Work pursuing a definitive caspase 8 promoter revealed that decitabine-mediated up-regulation occurs through genome-wide demethylation which up-regulates the expression of transactivators, rather than caspase 8 promoter-specific demethylation (Banelli et al., 2002). The parent compound, 5-azacytidine (azacytidine), incorporates into DNA with greater efficacy in the treatment of hematopoietic malignancies. Azacitidine induces differentiation in immature blasts (Taylor and Jones, 1982; Kaminskas et al., 2005) and is the only hypomethylating agent to prolong survival in high risk myelodysplastic syndromes (Fenaux et al., 2009; Lyons, 2012).
The emerging generation of histone deacetylase inhibitors provides an effective method for the therapeutic manipulation of caspases. By insuring adequate acetylation and transcriptional availability of the caspase 3 promoter, histone deacetylase inhibitors activate apoptosis and increase sensitization to TRAIL-, radiation- and chemotherapeutic-induced apoptosis in human cancer cells, including prostate, lung, Ewing’s sarcoma and medulloblastoma (Sonnemann et al., 2006a,b, 2007). A balance of promoter and coding DNA methylation maintains a homeostatic balance of transcriptional activity, since both hyper- and hypomethylation have been associated with oncogenesis (reviewed in Herman and Baylin, 2003; Ehrlich, 2009).
Interferon-γ
Interferon-γ (IFN-γ) has distinct molecular properties from other members of the interferon family, including differences in receptor affinity and tissue expression (Zaidi and Merlino, 2011). Loss of the IFN-γ signaling axis via a dominant negative INF-γR1 enhanced tumorigenesis in mice exposed to carcinogens, identifying the role of INF-γ in surveillance and tumor cell immunogenicity. Similar to caspase 8 expression, approximately 30% of melanoma and lung cancer cell lines harbor inactivating mutations in the IFN-γ signaling pathway (Kaplan et al., 1998). TRAIL-induced apoptosis has been well characterized in a variety of solid and liquid tumor cell lines (Wiley et al., 1995; Zhang et al., 1999a; Mitsiades et al., 2001). IFN-γ is capable of sensitizing caspase 8-deficient neuro-blastoma, medulloblastoma, and Ewing’s sarcoma cells to apoptosis induced by death-receptor ligand, cytotoxic drugs and radiotherapy in vitro (Fulda and Debatin, 2002 ; Tekautz et al., 2006). N-myc amplification and caspase 8 down-regulation in neuroblastoma is associated with TRAIL resistance and poor prognosis (Eggert et al., 2001). Reconstitution of caspase 8 expression sensitizes neuro-blastoma cells to TRAIL-mediated apoptosis, but a subset of cells with diminished TRAIL receptor (TR1/TR2) expression remain insensitive. Therapeutic regimens including IFN-γ aimed at increasing caspase 8 and TR expression have been effective in restoring apoptotic potential in cells previously insensitive when treated with death-receptor ligand (Yang et al., 2003). Sensitization in caspase 8 hypermethylated cell lines can occur through an epigenetic-independent mechanism (Fulda and Debatin, 2002). This may occur through a signal transducers and activators of transcription (STAT)-dependent pathway, by which INF-γ transcriptionally up-regulates molecules involved in apoptosis (Darnell et al., 1994; Ruiz-Ruiz et al., 2000). IFN-γ-mediated up-regulation of caspase 8 occurs by interferon-sensitive response elements located within the caspase 8 promoter. IFN-γ signaling results in phosphorylation of the intracellular kinases JAK-1/2, which subsequently phosphorylate and activate the transcription factor STAT-1. Homodimerization and nuclear localization of STAT-1 molecules results in the transcriptional modulation of interferon target genes at interferon-sensitive response elements (Casciano et al., 2004). Amplification of IFN-γ/STAT-1 signaling occurs via STAT-1 activation of interferon regulatory factor-1, a transactivator involved in the activation of additional IFN-γ responsive genes (Storm van’s Gravesande et al., 2002; Ruiz-Ruiz et al., 2004), and exposure of cells to IFN-γ results in increased signaling via up-regulation of interferon regulatory factor-1 and phosphoSTAT-1 (Casciano et al., 2004). The demethylating ability of decitabine and transcriptional activation by IFN-γ at the caspase 8 promoter increases caspase 8 expression and sensitizes cells to TRAIL-mediated apoptosis in neuroblastoma cells intrinsically lacking caspase 8 (Fulda and Debatin, 2006). Additionally, the expression of IFN-γ in tumor infiltrating T cells plays a critical role in bolstering the immune surveillance of oncogenically-transformed cells (Ye et al., 1995). Another STAT-dependent member of the interferon family, IFN-α, has significant antiviral therapeutic effect against hepatitis B and C. Caspase 8 induction sensitizes hepatocellular carcinoma cells to apoptosis upon TRAIL and IFN-α treatment (Liedtke et al., 2006).
Retinoic acid
Retinoids are vitamin A derivatives capable of inducing cellular differentiation in two pediatric tumors (reviewed in Masetti et al., 2012). Expression of the gene fusion product creating the retinoic acid receptor-α (t(15:17); PML/RAR-α) is involved in promyelocytic leukemia. All-trans RA induces differentiation of immature blasts and is capable of inducing nearly 90% remission. Retinoid signaling proceeds via binding of RA to the RAR to form a heterodimer with the retinoid X receptor, which acts as a transcription factor at RA response elements (RAREs; Jiang et al., 2008). RAREs are generally located in RA target gene promoters but can also be found in both introns and exons up- or downstream of these promoters (Jiang et al., 2005). RA induces differentiation, inhibits cell proliferation and results in decreased expression of N-myc in neuroblastoma cells (Thiele et al., 1985; Redfern et al., 1995; Matsuo and Thiele, 1998). RA also induces paracrine release of TRAIL in promyelocytic leukemia cells and up-regulates caspase 8 in neuroblastoma, medulloblastoma and small cell lung cancer cell lines (Ghobrial et al., 2005). This induction, consistent among treatment with RA and the RA derivatives 13-cis RA, 9-cis RA and 4HPR (fenretinide), occurs by up-regulation of phospho-CREB, which associates with the intronic regulatory elements of caspase 8. Induced, expression of caspase 8 sensitizes these cancer cells to TNF-α and other chemotherapeutics (Jiang et al., 2008). Increased CREB phosphorylation is independent of RAR association with RAREs (Aggarwal et al., 2006; Kim et al., 2007).
Chemically-induced apoptosis: death switches
A ‘death-switch’ has been employed by MacCorkle and colleagues to synthetically activate caspases selectively in cancer cells. This death switch approach utilizes a vector that is non-deleterious and quiescent in the uninduced state, but has the capability to trigger cell death (chemically-induced apoptosis) independent of tissue type when induced. Chimeric, conditional alleles of both caspase 1 and caspase 3 with an inducible amino terminal dimerization domain were engineered. Induction with a lipid-permeable chemical inducer of dimerization (CID, a FK06 analog) caused caspase dimerization and autolytic cleavage of zymogen to active protease, initiating the caspase cascade with caspase 1 and 3 vectors causing apoptosis in targeted cells.
Initial studies in which caspase 1 and caspase 3 replication-deficient adenoviral vectors were transduced into prostate tumors revealed focal extensive apoptosis devoid of cytotoxic damage to surrounding tissue with a reduction of tumor growth (Shariat et al., 2001). Furthermore, Xie et al. utilized a prostate-specific promoter, ARR2PB, to drive the expression of inducible caspase 9 in an adenoviral vector (ADV.ARR2PB-iCasp9). This synthetic prostate-specific promoter contains two androgen response regions situated upstream of the rat probasin promoter (Hensley and Kyprianou, 2011). High levels of intra-prostatic androgens drive the expression of inducible caspase 9, and subsequent activation with a CID resulted in significant apoptosis in prostate cancer xenografts (Xie et al., 2001). Initial contributions to the generation of chemical dimerization compounds (Clackson et al., 1998) and death-receptor ligands (Ogasawara et al., 1995; Beltinger et al., 1999) require further expansion.
Apoptin
Chicken embryos become increasingly susceptible to chicken anemia virus (CAV) as gestation progresses. CAV shows tropism for hematopoietic lineages and causes anemia and thrombocytopenia, leading to hemorrhage and death. However, the protective capacity of CAV in a specific viral-derived tumor in chickens has implicated its therapeutic potential in patients (Natesan et al., 2006). CAV has a single-stranded DNA genome and its structure and function are elegantly reviewed in Los et al., 2009. One of the proteins encoded by this polycistronic gene, VP3 or apoptin, contributes to the apoptosis-induction ability of CAV. Apoptin induces apoptosis in human cancer cells, in a tumor-cell specific action, without affecting the survival of adjacent normal tissue (Tavassoli et al., 2005 ; Backendorf et al., 2008). The physiological impact is dictated by its intracellular localization: apoptin localizes to the nucleus in tumor cells but remains cytosolic in non-transformed cells (Zhang et al., 2003). Apoptin is also induced by infection with tumor-associated viruses and ultraviolet radiation (Danen-Van Oorschot et al., 1997; Zhang et al., 1999). Apoptin has two domains capable of inducing apoptosis, each independently requiring nuclear localization signals to translocate, and these nuclear localization signals appear to be highly specific for transformed cells (Danen-Van Oorschot et al., 2003; Poon et al., 2005). Apoptin is heavily phosphorylated at threonine 108 in tumor cells, with negligible phosphorylation detected in normal cells (Rohn et al., 2002). It binds to protein partners and DNA (heterochromatin and nucleoli), although the affinity of apoptin for DNA does not functionally contribute to its role in apoptosis (Danen-Van Oorschot et al., 2003). Activation of caspases 3 and 7 and release of mitochondrial cytochrome c execute apoptotic response to apoptin (Burek et al., 2006). Apoptin also mediates apoptosis by causing DNA damage and interference with ribosomal biosynthesis (Zhang et al., 2003; Guelen et al., 2004; Teodoro et al., 2004). Intra-tumoral administration of apoptin, alone or in combination with other chemotherapeutics, impairs tumor growth (Pietersen et al., 1999; Maddika et al., 2006; Olijslagers et al., 2007).
Survivin inhibitors
Survivin is encoded by the BIRC5 gene and is the smallest protein in the IAP family (16 kDa). Pioneering work by Dario Altieri and colleagues established that survivin expression is highly up-regulated during embryogenesis to inhibit caspase function and promote cell survival, but is lost after birth with residual expression in hematolymphoid cells and the colonic epithelium (Adida et al., 1998 ; Church and Talbot, 2012). Analysis of the transcriptome from a variety of human cancers identified survivin as the fourth most up-regulated gene in cancer relative to normal tissue (Velculescu et al., 1999). Survivin is required for tumor cell survival and is overexpressed in most solid human cancers, including breast, esophageal, gastric, colorectal, pancreatic, non-small cell lung, ovarian and prostate tumors, as well as in neuroblastoma, gliomas and melanoma (Lladser et al., 2011; Church and Talbot, 2012). Although up-regulation in cancer-associated stroma enhances the potential of survivin inhibitors to impact the entire tumor microenvironment (Velculescu et al., 1999), the functional redundancy in the regulation of survivin expression still renders this player a ‘difficult’ target. Survivin is stimulated by an array of growth factors and cytokines and is down-regulated by (frequently mutated) tumor suppressors (Aoki et al., 2003; Jiang et al., 2004; Wang et al., 2008 ; Guha et al., 2009). Survivin is capable of directly binding to and inhibiting the protease ability of caspases 3 and 7 (Tamm et al., 1998). Apoptosis inhibition by survivin may proceed through molecular intermediates rather than direct caspase inhibition (Banks et al., 2000; Li et al., 2008). Loss of survivin results in the inhibition of cell proliferation, pushing the cell into a persistent prometaphase state, with chromosomal alignment defects and failure of cytokinesis (Altieri, 2006). Increased survivin is associated with loss of apoptosis, increased survival and enhanced angiogenesis (Kawasaki et al., 1998), as well as resistance to therapy (Church and Talbot, 2012). Therapeutic targeting of survivin is currently in various clinical trials, including antisense oligonucleotides, vaccination, inhibitors of transcription and immunotherapy (Altieri, 2008; Church and Talbot, 2012).
LY2181308 is an antisense oligonucleotide that significantly reduces survivin mRNA, inducing caspase 3 proteolytic activity, cell cycle arrest and inhibition of cytokinesis in human tumor cells (Figure 1). It also impairs cancer growth in human xenograft tumor models to adjuvant chemotherapy (Carrasco et al., 2011). The first clinical trials of LY2181308 revealed loss of survivin expression, resulting in restoration of intra-tumoral caspase-mediated apoptosis. Ongoing Phase II trials are pursuing the role of antisense oligonucleotide as adjuvant therapy in treating AML, CRPC and non-small cell lung carcinoma (Tanioka et al., 2011).
SPC3042 is an antisense oligonucleotide that increases stability and binding specificity to survivin transcript. Blocking survivin with SPC3042 induces apoptosis and sensitizes prostate cancer to microtubule-targeting (Hansen et al., 2008).
YM155 (sepantronium bromide) is an imidazolium-based small molecular inhibitor of survivin expression. First studied in CRPC cell lines and xenografts, YM155 was highly distributed intra-tumorally and continuous intravenous infusion led to a significant activation of caspases, resulting apoptosis and tumor regression without severe systemic toxicity. At the molecular level, YM155 results in reporter down-regulation of BIRC5 (Nakahara et al., 2007; Murakami et al., 2013). Phase I clinical trials revealed significant anti-tumor efficacy in a patient cohort with treatment-refractory lymphomas and prostate cancer, with Bcl-2 and survivin serving as independent negative prognostic factors (Tolcher et al., 2008; Satoh et al., 2009). The promise of therapeutic synergy emerges from evidence that YM155 sensitizes tumor cells to radiation and platinum- based chemotherapeutics by decreasing DNA repair mechanisms and up-regulating caspase-dependent apoptosis (Iwasa et al., 2008, 2010; Kumar et al., 2012). A Phase II clinical trial in advanced non small cell lung cancer revealed modest efficacy in response to YM155 (Giaccone et al., 2009), while therapeutic synergism between YM155 and taxanes (Nakahara et al., 2011; Yamanaka et al., 2011), rituximab (Kita et al., 2012) and gemcitabine (Yoon et al., 2012) has been established.
EM-1421 (terameprocol) is an additional inhibitor of BIRC5 transcriptional activity. EM-1421 induces growth arrest and apoptosis by inhibiting the expression of two Sp1-dependent genes, Cdc2/cyclin B and survivin (Chang et al., 2004). Furthermore, this compound interferes with HPV genomic expression and may prove efficacious as non-invasive treatment for HPV-associated cervical squamous intraepithelial neoplasia (Khanna et al., 2007). Studies in hematopoietic malignancies and gliomas support EM-1421 as a promising candidate in clinical trials (Fulciniti et al., 2011; Grossman et al., 2012).
A splice variant of survivin, survivin-2b, contains an HLA-24-restricted epitope that is recognized by CD8 cytotoxic T-cells (Hirohashi et al., 2002). Administration of survivin-2b peptide vaccine in patients with colorectal cancer resulted in a reduction of both serum tumor markers and tumor volume (Tsuruma et al., 2004), supporting the therapeutic impact of survivin targeting. Overexpression of survivin-2b in cancer cells correlates with reduced caspase 3 activity following doxorubicin or radiation (Jacob et al., 2012). The pro-apoptotic survivin-2b splice variant is down-regulated during tumor progression (Andersen et al., 2006).
Shepheridin is a 9 amino acid peptidomimetic that disrupts the binding interface between Hsp90 and survivin (Figure 1) and increases cancer cell caspase activity (Xiaojiang et al., 2010). It has a therapeutic effect in glioblastoma and leukemias (Gyurkocza et al., 2006; Siegelin et al., 2010).
Quinazoline-induced anoikis
Anoikis resistance (a mode of apoptosis upon insufficient cell–cell and cell–extracellular matrix interactions) confers therapeutic failure and metastasis. α1-Adrenoceptor antagonists are used for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (Lepor, 2007); the therapeutic effect of α-adrenoceptor antagonists, however, is not limited to smooth muscle relaxation but also to prostate epithelial and stromal cell apoptosis (Kyprianou et al., 1998; Kyprianou and Benning, 2000). This proceeds via anoikis induction, engaging transforming growth factor beta 1 signaling and exerting an anti-tumor effect (Benning and Kyprianou, 2002; Keledjian and Kyprianou, 2003; Partin et al., 2003). Pharmacological exploitation of quinazoline structure α1-antagonists led to novel derivatives that impair vascularity in prostate and renal cancer via caspase-8 mediated anoikis (Garrison et al., 2007; Sakamoto et al., 2011).
Conclusions
Regulation of apoptosis mediated by caspase expression and activity is critical in maintaining tissue homeostasis, and loss of this control via caspase deregulation promotes aberrant cell survival. Functional exploitation of caspases in the context of reinstating apoptosis mechanism and therapeutic sensitivity centers on tumor caspase-deficient cells, including the human breast carcinoma cell line MCF-7, which express neither caspase 10 (extrinsic pathway) nor the executioner, caspase 3 (Jänicke et al., 1998; Jänicke, 2009). Small molecule inhibitors act via intrinsic mitochondrial, extrinsic receptor pathways or endoplasmic reticulum stress pathways to elicit therapeutic apoptosis. Induction of caspases can be achieved by demethylating agents and transcriptional activators. Specific patterns of adaptation by tumor cells to the microenvironment can be exploited therapeutically, with more than 50% of tumor-derived mutations impacting the apoptotic machinery (Negrini et al., 2012; Gillies et al., 2012).
Emerging opportunities driven by powerful bioinformatics and imaging tools will allow for a precise prediction of differential sensitivity of tumors to apoptosis-based therapeutic approaches impairing death signaling effectors. The development of inducers of caspases via direct or indirect activation of the intrinsic or extrinsic apoptotic pathways will impact cancer treatment. Moreover, combination strategies of caspase ‘activators’ with IAP inhibitors and death-receptor ligands may sufficiently restore apoptosis and overcome therapeutic resistance and move towards a complete cure.
Acknowledgments
This study was supported by the James F. Hardymon Endowment and a University of Kentucky College of Medicine Clinical and Translational Training Fellowship. The authors acknowledge support by a grant from the National Institutes of Health, R01-GRANT00491815, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, UL1RR033173.
Contributor Information
Patrick Hensley, Department of Urology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA; and Department of Pathology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA.
Murli Mishra, Graduate Center for Toxicology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY340536, USA.
Natasha Kyprianou, Department of Urology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA; Department of Pathology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA; and Graduate Center for Toxicology, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA.
References
- Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnemri E, Livingston D, Nicholson D, Salvesen G, Thornberry N, Wong W, Yuan J. Human ICE/CED-3 Protease Nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Andersen MH, Soerensen RB, Becker JC, Thor Straten P. HLA-A24 and survivin: possibilities in therapeutic vaccination against cancer. J Transl Med. 2006;4:38. doi: 10.1186/1479-5876-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- Backendorf C, Visser AE, de Boer AG, Zimmerman R, Visser M, Voskamp P, Zhang YH, Noteborn M. Apoptin: therapeutic potential of an early sensor of carcinogenic transformation. Annu Rev Pharmacol Toxicol. 2008;48:143–169. doi: 10.1146/annurev.pharmtox.48.121806.154910. [DOI] [PubMed] [Google Scholar]
- Banelli B, Casciano I, Croce M, Di Vinci A, Gelvi I, Pagnan G, Brignole C, Allemanni G, Ferrini S, Ponzoni M, Romani M. Expression and methylation of CASP8 in neuroblastoma: identification of a promoter region. Nat Med. 2002;8:1333–1335. doi: 10.1038/nm1202-1333. [DOI] [PubMed] [Google Scholar]
- Banks DP, Plescia J, Altieri DC, Chen J, Rosenberg SH, Zhang H, Ng SC. Survivin does not inhibit caspase-3 activity. Blood. 2000;96:4002–4003. [PubMed] [Google Scholar]
- Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, Debatin KM. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci USA. 1999;96:8699–8704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning CM, Kyprianou N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res. 2002;62:597–602. [PubMed] [Google Scholar]
- Burek M, Maddika S, Burek CJ, Daniel PT, Schulze-Osthoff K, Los M. Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene. 2006;25:2213–2222. doi: 10.1038/sj.onc.1209258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco RA, Stamm NB, Marcusson E, Sandusky G, Iversen P, Patel BK. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther. 2011;10:221–232. doi: 10.1158/1535-7163.MCT-10-0756. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciano I, De Ambrosis A, Croce M, Pagnan G, Di Vinci A, Allemanni G, Banelli B, Ponzoni M, Romani M, Ferrini S. Expression of the caspase-8 gene in neuroblastoma cells is regulated through an essential interferon-sensitive response element (ISRE) Cell Death Differ. 2004;11:131–134. doi: 10.1038/sj.cdd.4401327. [DOI] [PubMed] [Google Scholar]
- Chang CC, Heller JD, Kuo J, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci USA. 2004;101:13239–13244. doi: 10.1073/pnas.0405407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012;14:120–128. doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci USA. 1998;95:10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen-Van Oorschot AA, Fischer DF, Grimbergen JM, Klein B, Zhuang S, Falkenburg JH, Backendorf C, Quax PH, Van der EbAJ, Noteborn MH. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci USA. 1997;94:5843–5847. doi: 10.1073/pnas.94.11.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen-Van Oorschot AA, Zhang YH, Leliveld SR, Rohn JL, Seelen MC, Bolk MW, Van Zon A, Erkeland SJ, Abrahams JP, Mumberg D, et al. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J Biol Chem. 2003;278:27729–27736. doi: 10.1074/jbc.M303114200. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt BR, Ho R, Ikegaki N, Brodeur GM. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 2001;61:1314–1319. [PubMed] [Google Scholar]
- Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY) 2012;4:330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165–175. [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamiya N, Lee KH. Antitumor agents, 81. Justicidin-A and diphyllin, two cytotoxic principles from Justicia procumbens. J Nat Prod. 1986;49:348–350. doi: 10.1021/np50044a030. [DOI] [PubMed] [Google Scholar]
- Fulciniti M, Amin S, Nanjappa P, Rodig S, Prabhala R, Li C, Minvielle S, Tai YT, Tassone P, Avet-Loiseau H, Hideshima T, et al. Significant biological role of sp1 transactivation in multiple myeloma. Clin Cancer Res. 2011;17:6500–6509. doi: 10.1158/1078-0432.CCR-11-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. 5-Aza-2′-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25:5125–5133. doi: 10.1038/sj.onc.1209518. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. IFNγ sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- Fulda S, Küfer MU, Meyer E, van Valen F, Dockhorn-Dworniczak B, Debatin KM. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–5877. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- Garrison JB, Shaw YJ, Chen CS, Kyprianou N. Novel quinazoline-based compounds impair prostate tumorigenesis by targeting tumor vascularity. Cancer Res. 2007;67:11344–11352. doi: 10.1158/0008-5472.CAN-07-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger K, Hagenbuchner J, Rupp M, Fiegl H, Sergi C, Meister B, Kiechl-Kohlendorfer U, Müller T, Ausserlechner MJ, Obexer P. FOXO3/FKHRL1 is activated by 5-aza-2-deoxy-cytidine and induces silenced caspase-8 in neuroblastoma. Mol Biol Cell. 2012;23:2226–2234. doi: 10.1091/mbc.E11-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A, Shamsili S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzer MA, Eggert A, Zuzak TJ, Janss AJ, Marwaha S, Wiewrodt BR, Ikegaki N, Brodeur GM, Phillips PC. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19:4604–4610. doi: 10.1038/sj.onc.1203816. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Ye X, Peereboom D, Rosenfeld MR, Mikkelsen T, Supko JG, Desideri S Adult Brain Tumor Consortium. Phase I study of terameprocol in patients with recurrent high-grade glioma. Neuro Oncol. 2012;14:511–517. doi: 10.1093/neuonc/nor230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelen L, Paterson H, Gäken J, Meyers M, Farzaneh F, Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–1165. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- Guha M, Plescia J, Leav I, Li J, Languino LR, Altieri DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009;69:4954–4958. doi: 10.1158/0008-5472.CAN-09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurkocza B, Plescia J, Raskett CM, Garlick DS, Lowry PA, Carter BZ, Andreeff M, Meli M, Colombo G, Altieri DC. Antileukemic activity of shepherdin and molecular diversity of hsp90 inhibitors. J Natl Cancer Inst. 2006;98:1068–1077. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA, Rosenbohm C, Wissenbach M, Orum H, Koch T. SPC3042: a proapoptotic survivin inhibitor. Mol Cancer Ther. 2008;7:2736–2745. doi: 10.1158/1535-7163.MCT-08-0161. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He XL, Zhang P, Dong XZ, Yang MH, Chen SL, Bi MG. JR6, a new compound isolated from Justicia procumbens, induces apoptosis in human bladder cancer EJ cells through caspase-dependent pathway. J Ethnopharmacol. 2012;144:284–292. doi: 10.1016/j.jep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Hensley PJ, Kyprianou N. Modeling prostate cancer in mice: limitations and opportunities. J Androl. 2012;33:133–144. doi: 10.2164/jandrol.111.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins-Donaldson S, Bodmer JL, Bourloud KB, Brognara CB, Tschopp J, Gross N. Loss of caspase-8 expression in highly malignant human neuroblastoma cells correlates with resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2000;60:4315–4319. [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hirohashi Y, Torigoe T, Maeda A, Nabeta Y, Kamiguchi K, Sato T, Yoda J, Ikeda H, Hirata K, Yamanaka N, et al. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res. 2002;8:1731–1739. [PubMed] [Google Scholar]
- Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–6504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Okamoto I, Takezawa K, Yamanaka K, Nakahara T, Kita A, Koutoku H, Sasamata M, Hatashita E, Yamada Y, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob NK, Cooley JV, Shirai K, Chakravarti A. Survivin splice variants are not essential for mitotic progression or inhibition of apoptosis induced by doxorubicin and radiation. Onco Targets Ther. 2012;5:7–20. doi: 10.2147/OTT.S28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke RU. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat. 2009;117:219–221. doi: 10.1007/s10549-008-0217-9. [DOI] [PubMed] [Google Scholar]
- Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Jiang SY, Wu MS, Chen LM, Hung MW, Lin HE, Chang GG, Chang TC. Identification and characterization of the retinoic acid response elements in the human RIG1 gene promoter. Biochem Biophys Res Commun. 2005;331:630–639. doi: 10.1016/j.bbrc.2005.03.214. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhu K, Grenet J, Lahti JM. Retinoic acid induces caspase-8 transcription via phospho-CREB and increases apoptotic responses to death stimuli in neuroblastoma cells. Biochim Biophys Acta. 2008;1783:1055–1067. doi: 10.1016/j.bbamcr.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Saavedra HI, Holloway MP, Leone G, Altura RA. Aberrant regulation of survivin by the RB/E2F family of proteins. J Biol Chem. 2004;279:40511–40520. doi: 10.1074/jbc.M404496200. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- Khanna N, Dalby R, Tan M, Arnold S, Stern J, Frazer N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol Oncol. 2007;107:554–562. doi: 10.1016/j.ygyno.2007.08.074. [DOI] [PubMed] [Google Scholar]
- Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10:176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon γ-dependent tumor surveillance system in immuno-competent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keledjian K, Kyprianou N. Anoikis induction by quinazoline based alpha 1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. J Urol. 2003;169:1150–1156. doi: 10.1097/01.ju.0000042453.12079.77. [DOI] [PubMed] [Google Scholar]
- Kim SW, Hong JS, Ryu SH, Chung WC, Yoon JH, Koo JS. Regulation of mucin gene expression by CREB via a nonclassical retinoic acid signaling pathway. Mol Cell Biol. 2007;27:6933–6947. doi: 10.1128/MCB.02385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita A, Mitsuoka K, Kaneko N, Nakata M, Yamanaka K, Jitsuoka M, Miyoshi S, Noda A, Mori M, Nakahara T, et al. Sepantronium bromide (YM155) enhances response of human B-cell non-Hodgkin lymphoma to rituximab. J Pharmacol Exp Ther. 2012;343:178–183. doi: 10.1124/jpet.112.195925. [DOI] [PubMed] [Google Scholar]
- Kohno T, Morishita K, Takano H, Shapiro DN, Yokota J. Homozygous deletion at chromosome 2q33 in human small-cell lung carcinoma identified by arbitrarily primed PCR genomic fingerprinting. Oncogene. 1994;9:103–108. [PubMed] [Google Scholar]
- Kumar B, Yadav A, Lang JC, Cipolla MJ, Schmitt AC, Arradaza N, Teknos TN, Kumar P. YM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levels. Mol Cancer Ther. 2012;11:1988–1998. doi: 10.1158/1535-7163.MCT-12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by α1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–4555. [PubMed] [Google Scholar]
- Kyprianou N, Litvak JP, Borkowski A, Alexander R, Jacobs SC. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J Urol. 1998;159:1810–1815. doi: 10.1016/S0022-5347(01)63162-8. [DOI] [PubMed] [Google Scholar]
- Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9:181–190. [PMC free article] [PubMed] [Google Scholar]
- Lladser A, Sanhueza C, Kiessling R, Quest AF. Is survivin the potential Achilles’ heel of cancer? Adv Cancer Res. 2011;111:1–37. doi: 10.1016/B978-0-12-385524-4.00001-5. [DOI] [PubMed] [Google Scholar]
- Li C, Wu Z, Liu M, Pazgier M, Lu W. Chemically synthesized human survivin does not inhibit caspase-3. Protein Sci. 2008;17:1624–1629. doi: 10.1110/ps.036145.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C, Gröger N, Manns MP, Trautwein C. Interferon-α enhances TRAIL-mediated apoptosis by up-regulating caspase-8 transcription in human hepatoma cells. J Hepatol. 2006;44:342–249. doi: 10.1016/j.jhep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Zschemisch NH, Cohrs A, Roskams T, Borlak J, Manns MP, Trautwein C. Silencing of caspase-8 in murine hepatocellular carcinomas is mediated via methylation of an essential promoter element. Gastroenterology. 2005;129:1602–1615. doi: 10.1053/j.gastro.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei D, Zhao Y, Cheng W, Lu Y, Ma Y, Li X, Han C, Wei Y, Cao H, et al. Synthesis and biological evaluation of a series of podophyllotoxins derivatives as a class of potent antitubulin agents. Bioorg Med Chem. 2012;20:6285–6295. doi: 10.1016/j.bmc.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Los M, Panigrahi S, Rashedi I, Mandal S, Stetefeld J, Essmann F, Schulze-Osthoff K. Apoptin, a tumor-selective killer. Biochim Biophys Acta. 2009;1793:1335–1342. doi: 10.1016/j.bbamcr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Lyons RM. Myelodysplastic syndromes: therapy and outlook. Am J Med. 2012;125:S18–S23. doi: 10.1016/j.amjmed.2012.04.018. [DOI] [PubMed] [Google Scholar]
- MacCorkle RA, Freeman KW, Spencer DM. Synthetic activation of caspases: artificial death switches. Proc Natl Acad Sci USA. 1998;95:3655–3660. doi: 10.1073/pnas.95.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Mendoza FJ, Hauff K, Zamzow CR, Paranjothy T, Los M. Cancer-selective therapy of the future: apoptin and its mechanism of action. Cancer Biol Ther. 2006;5:10–19. doi: 10.4161/cbt.5.1.2400. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Henry CM, Cullen SP. A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell. 2012;46:387–397. doi: 10.1016/j.molcel.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Masetti R, Biagi C, Zama D, Vendemini F, Martoni A, Morello W, Gasperini P, Pession A. Retinoids in pediatric onco-hematology: the model of acute promyelocytic leukemia and neuroblastoma. Adv Ther. 2012;29:747–762. doi: 10.1007/s12325-012-0047-3. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Thiele CJ. p27Kip1: a key mediator of retinoic acid induced growth arrest in the SMS-KCNR human neuroblastoma cell line. Oncogene. 1998;16:3337–3343. doi: 10.1038/sj.onc.1201830. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–2712. [PubMed] [Google Scholar]
- Murakami Y, Matsuya T, Kita A, Yamanaka K, Noda A, Mitsuoka K, Nakahara T, Miyoshi S, Nishimura S. Radiosynthesis, biodistribution and imaging of [(11) C]YM155, a novel survivin suppressant, in a human prostate tumor-xenograft mouse model. Nucl Med Biol. 2013;40:221–226. doi: 10.1016/j.nucmedbio.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Muñoz-Pinedo C. Signaling pathways that regulate life and cell death: evolution of apoptosis in the context of self-defense. Adv Exp Med Biol. 2012;738:124–143. doi: 10.1007/978-1-4614-1680-7_8. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I, Matsuhisa A, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Yamanaka K, Hatakeyama S, Kita A, Takeuchi M, Kinoyama I, Matsuhisa A, Nakano K, Shishido T, Koutoku H, et al. YM155, a novel survivin suppressant, enhances taxane-induced apoptosis and tumor regression in a human Calu 6 lung cancer xenograft model. Anticancer Drugs. 2011;22:454–462. doi: 10.1097/CAD.0b013e328344ac68. [DOI] [PubMed] [Google Scholar]
- Natesan S, Kataria JM, Dhama K, Bhardwaj N, Sylvester A. Anti-neoplastic effect of chicken anemia virus VP3 protein (apoptin) in Rous sarcoma virus-induced tumours in chicken. J Gen Virol. 2006;87:2933–2940. doi: 10.1099/vir.0.82085-0. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability – an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2012;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Ocker M, Höpfner M. Apoptosis-modulating drugs for improved cancer therapy. Eur Surg Res. 2012;48:111–120. doi: 10.1159/000336875. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J Exp Med. 1995;181:485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijslagers SJ, Zhang YH, Backendorf C, Noteborn MH. Additive cytotoxic effect of apoptin and chemotherapeutic agents paclitaxel and etoposide on human tumour cells. Basic Clin Pharmacol Toxicol. 2007;100:127–131. doi: 10.1111/j.1742-7843.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- Partin JV, Anglin IE, Kyprianou N. Quinazoline-based alpha 1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-β signalling and IκB α induction. Br J Cancer. 2003;88:1615–1621. doi: 10.1038/sj.bjc.6600961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen AM, van der EbMM, Rademaker HJ, van den Wollenberg DJ, Rabelink MJ, Kuppen PJ, van Dierendonck JH, van Ormondt H, Masman D, van de Velde CJ, et al. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 1999;6:882–892. doi: 10.1038/sj.gt.3300876. [DOI] [PubMed] [Google Scholar]
- Poon IK, Oro C, Dias MM, Zhang JP, Jans DA. A tumor cell-specific nuclear targeting signal within chicken anemia virus VP3/apoptin. J Virol. 2005;79:1339–1341. doi: 10.1128/JVI.79.2.1339-1341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern CP, Lovat PE, Malcolm AJ, Pearson AD. Gene expression and neuroblastoma cell differentiation in response to retinoic acid: differential effects of 9-cis and all-trans retinoic acid. Eur J Cancer. 1995;31A:486–494. doi: 10.1016/0959-8049(95)00066-r. [DOI] [PubMed] [Google Scholar]
- Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;65:11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn JL, Zhang YH, Aalbers RI, Otto N, Den Hertog J, Henriquez NV, Van De Velde CJ, Kuppen PJ, Mumberg D, Donner P, et al. A tumor-specific kinase activity regulates the viral death protein Apoptin. J Biol Chem. 2002;277:50820–50827. doi: 10.1074/jbc.M208557200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Muñoz-Pinedo C, López-Rivas A. Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res. 2000;60:5673–5680. [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Ruiz de Almodóvar C, Rodríguez A, Ortiz-Ferrón G, Redondo JM, López-and Rivas A. The up-regulation of human caspase-8 by interferon-γ in breast tumor cells requires the induction and action of the transcription factor interferon regulatory factor-1. J Biol Chem. 2004;279:19712–19720. doi: 10.1074/jbc.M313023200. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Kyprianou N. Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med. 2010;31:205–214. doi: 10.1016/j.mam.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Schwarze S, Kyprianou N. Anoikis disruption of focal adhesion-Akt signaling impairs renal cell carcinoma. Eur Urol. 2011;59:734–744. doi: 10.1016/j.eururo.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- Sartor AO, Fitzpatrick JM. Urologists and oncologists: adapting to a new treatment paradigm in castration-resistant prostate cancer (CRPC) BJU Int. 2012;110:328–335. doi: 10.1111/j.1464-410X.2011.10818.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2012;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Desai S, Song W, Khan T, Zhao J, Nguyen C, Foster BA, Greenberg N, Spencer DM, Slawin KM. Adenovirus-mediated transfer of inducible caspases: a novel “death switch” gene therapeutic approach to prostate cancer. Cancer Res. 2001;61:2562–2571. [PubMed] [Google Scholar]
- Shivapurkar N, Toyooka S, Eby MT, Huang CX, Sathyanarayana UG, Cunningham HT, Reddy JL, Brambilla E, Takahashi T, Minna JD, et al. Differential inactivation of caspase-8 in lung cancers. Cancer Biol Ther. 2002;1:65–69. doi: 10.4161/cbt.1.1.45. [DOI] [PubMed] [Google Scholar]
- Shore N, Mason M, de Reijke TM. New developments in castrate-resistant prostate cancer. BJU Int. 2012;109:22–32. doi: 10.1111/j.1464-410X.2012.11217.x. [DOI] [PubMed] [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Siegelin MD, Plescia J, Raskett CM, Gilbert CA, Ross AH, Altieri DC. Global targeting of subcellular heat shock protein-90 networks for therapy of glioblastoma. Mol Cancer Ther. 2010;9:1638–1646. doi: 10.1158/1535-7163.MCT-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnemann J, Hartwig M, Plath A, Saravana Kumar K, Müller C, Beck JF. Histone deacetylase inhibitors require caspase activity to induce apoptosis in lung and prostate carcinoma cells. Cancer Lett. 2006a;232:148–160. doi: 10.1016/j.canlet.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sonnemann J, Kumar KS, Heesch S, Müller C, Hartwig C, Maass M, Bader P, Beck JF. Histone deacetylase inhibitors induce cell death and enhance the susceptibility to ionizing radiation, etoposide, and TRAIL in medulloblastoma cells. Int J Oncol. 2006b;28:755–766. [PubMed] [Google Scholar]
- Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong le TT, Klier U, Bröker B, Völker U, Beck JF. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. J Cancer Res Clin Oncol. 2007;133:847–858. doi: 10.1007/s00432-007-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm van’s Gravesande K, Layne MD, Ye Q, Le L, Baron RM, Perrella MA, Santambrogio L, Silverman ES, Riese RJ. IFN regulatory factor-1 regulates IFN-γ-dependent cathepsin S expression. J Immunol. 2002;168:4488–4494. doi: 10.4049/jimmunol.168.9.4488. [DOI] [PubMed] [Google Scholar]
- Su CL, Huang LL, Huang LM, Lee JC, Lin CN, Won SJ. Caspase-8 acts as a key upstream executor of mitochondria during justicidin A-induced apoptosis in human hepatoma cells. FEBS Lett. 2006;580:3185–3191. doi: 10.1016/j.febslet.2006.04.085. [DOI] [PubMed] [Google Scholar]
- Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- Tanioka M, Nokihara H, Yamamoto N, Yamada Y, Yamada K, Goto Y, Fujimoto T, Sekiguchi R, Uenaka K, Callies S, et al. Phase I study of LY2181308, an antisense oligonucleotide against survivin, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:505–511. doi: 10.1007/s00280-010-1506-7. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, Guelen L, Luxon BA, Gäken J. Apoptin: specific killer of tumor cells? Apoptosis. 2005;10:717–724. doi: 10.1007/s10495-005-0930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SM, Jones PA. Changes in phenotypic expression in embryonic and adult cells treated with 5-azacytidine. J Cell Physiol. 1982;111:187–194. doi: 10.1002/jcp.1041110210. [DOI] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Tekautz TM, Zhu K, Grenet J, Kaushal D, Kidd VJ, Lahti JM. Evaluation of IFN-gamma effects on apoptosis and gene expression in neuroblastoma – preclinical studies. Biochim Biophys Acta. 2006;1763:1000–1010. doi: 10.1016/j.bbamcr.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Thiele CJ, Reynolds CP, Israel MA. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Teodoro JG, Heilman DW, Parker AE, Green MR. The viral protein Apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev. 2004;18:1952–1957. doi: 10.1101/gad.1198404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, et al. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen S, Xue M, Zhong J, Wang X, Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al. Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med. 2012;18:389–400. doi: 10.2119/molmed.2011.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Alvarez M, Slamon DJ, Koeffler P, Vadgama JV. Caspase 8 and maspin are downregulated in breast cancer cells due to CpG site promoter methylation. BMC Cancer. 2010;10:32. doi: 10.1186/1471-2407-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaojiang T, Jinsong Z, Jiansheng W, Chengen P, Guangxiao Y, Quanying W. Adeno-associated virus harboring fusion gene NT4-ant-shepherdin induce cell death in human lung cancer cells. Cancer Invest. 2010;28:465–471. doi: 10.3109/07357900903095706. [DOI] [PubMed] [Google Scholar]
- Xie X, Zhao X, Liu Y, Zhang J, Matusik RJ, Slawin KM, Spencer DM. Adenovirus-mediated tissue-targeted expression of a caspase-9-based artificial death switch for the treatment of prostate cancer. Cancer Res. 2001;61:6795–6804. [PubMed] [Google Scholar]
- Yamanaka K, Nakahara T, Yamauchi T, Kita A, Takeuchi M, Kiyonaga F, Kaneko N, Sasamata M. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17:5423–5431. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- Yang X, Merchant MS, Romero ME, Tsokos M, Wexler LH, Kontny U, Mackall CL, Thiele CJ. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63:1122–1129. [PubMed] [Google Scholar]
- Ye J, Ortaldo JR, Conlon K, Winkler-Pickett R, Young HA. Cellular and molecular mechanisms of IFN-γ production induced by IL-2 and IL-12 in a human NK cell line. J Leukoc Biol. 1995;58:225–233. doi: 10.1002/jlb.58.2.225. [DOI] [PubMed] [Google Scholar]
- Yoon DH, Shin JS, Jin DH, Hong SW, Jung KA, Kim SM, Hong YS, Kim KP, Lee JL, Suh C, et al. The survivin suppressant YM155 potentiates chemosensitivity to gemcitabine in the human pancreatic cancer cell line MiaPaCa-2. Anticancer Res. 2012;32:1681–1688. [PubMed] [Google Scholar]
- Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999a;59:2747–2753. [PubMed] [Google Scholar]
- Zhang YH, Abrahams PJ, van der EbAJ, Noteborn MH. The viral protein Apoptin induces apoptosis in UV-C-irradiated cells from individuals with various hereditary cancer-prone syndromes. Cancer Res. 1999b;59:3010–3015. [PubMed] [Google Scholar]
- Zhang YH, Leliveld SR, Kooistra K, Molenaar C, Rohn JL, Tanke HJ, Abrahams JP, Noteborn MH. Recombinant Apoptin multimers kill tumor cells but are nontoxic and epitope-shielded in a normal-cell-specific fashion. Exp Cell Res. 2003;289:36–46. doi: 10.1016/s0014-4827(03)00188-5. [DOI] [PubMed] [Google Scholar]


