Abstract
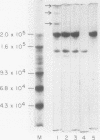
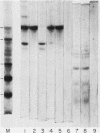
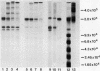
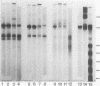
Soluble fibronectin is found in body fluids and media of adherent cultured cells and binds to fibrin and collagen. Insoluble fibronectin is found in tissue stroma and in extracellular matrices of cultured cells. Fibronectin is a substrate for Factor XIIIa (plasma transglutaminase) and can be cross-linked by Factor XIIIa to itself and the the alpha-chain of fibrin. We used sodium dodecyl sulfate-polyacrylamide gel electrophoresis to investigate Factor XIIIa-mediated crosslinking of fibronectin to collagen. At O degrees or 37 degrees C, fibronectin could be cross-linked to iodinated cyanogen bromide fragment 7 of the alpha 1(I) chain. At 22 degrees or 37 degrees C, fibronectin could be cross-linked to isolated alpha 1(I) chains of type I collagen. Fibronectin could also be crosslinked to types I and III collagen, but only at 37 degrees C. alpha 1(I)-CB7, alpha 1(I) collagen chains, type I collagen, type III collagen, and fibrin all blocked cross-linking between 125I-alpha 1 (I)-CB7 and fibronectin. alpha 1(I)-CB7 blocked cross-linking between fibronectin and fibrin. These results indicate that the determinants of fibronectin-fibrin and fibronectin-collagen binding and cross-linking are similar. Cross-linking of fibronectin to collagen likely occurs in vivo and may be important for normal wound healing, collagen fibrillogenesis, and embryogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Birckbichler P. J., Patterson M. K., Jr Cellular transglutaminase, growth, and transformation. Ann N Y Acad Sci. 1978 Jun 20;312:354–365. doi: 10.1111/j.1749-6632.1978.tb16813.x. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Ash J. F. Cell surface-associated structural proteins in connective tissue cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2480–2484. doi: 10.1073/pnas.74.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Murray A., Segal R. A., Bushnell A., Walsh M. L. Studies on intercellular LETS glycoprotein matrices. Cell. 1978 Jun;14(2):377–391. doi: 10.1016/0092-8674(78)90123-x. [DOI] [PubMed] [Google Scholar]
- Cottrell B. A., Doolittle R. F. The amino acid sequence of the carboxy-terminal 142 amino acids of the alpha-chain of human fibrinogen. Thromb Res. 1978 Jun;12(6):1135–1146. doi: 10.1016/0049-3848(78)90068-3. [DOI] [PubMed] [Google Scholar]
- Crouch E., Balian G., Holbrook K., Duksin D., Bornstein P. Amniotic fluid fibronectin. Characterization and synthesis by cells in culture. J Cell Biol. 1978 Sep;78(3):701–715. doi: 10.1083/jcb.78.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Adelmann B. C., Timpl R. Identification of the sites in collagen alpha-chains that bind serum anti-gelatin factor (cold-insoluble globulin). Biochem J. 1978 Jan 1;169(1):55–59. doi: 10.1042/bj1690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Jilek F., Adelmann B. C., Hörmann H. Similarity of antigelatin factor and cold insoluble globulin. Biochim Biophys Acta. 1978 Mar 28;533(1):227–237. doi: 10.1016/0005-2795(78)90566-4. [DOI] [PubMed] [Google Scholar]
- Duckert F. Documentation of the plasma factor XIII deficiency in man. Ann N Y Acad Sci. 1972 Dec 8;202:190–199. doi: 10.1111/j.1749-6632.1972.tb16331.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Sullivan C. E., Piez K. A. The resolution of Ascaris cuticle collagen into three chain types. Biochemistry. 1976 Apr 6;15(7):1435–1439. doi: 10.1021/bi00652a013. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper K. E., Adelmann B. C., Gentner G., Gay S. Recongnition by guinea-pig peritoneal exudate cells of conformationally different states of the collagen molecule. Immunology. 1976 Feb;30(2):249–259. [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Suzuki K., Hashimoto S. Bovine plasma cold-insoluble globulin: gross structure and function. Ann N Y Acad Sci. 1978 Jun 20;312:56–73. doi: 10.1111/j.1749-6632.1978.tb16793.x. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin (fibronectin), IV[1-35 affinity to soluble collagen of various types. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):247–250. doi: 10.1515/bchm.1978.359.1.247. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin, II[1,2]. Cyanogen bromide and plasminolysis fragments containing a label introduced by transamidation. Hoppe Seylers Z Physiol Chem. 1977 Sep;358(9):1165–1168. [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J. Polymerization of a major surface-associated glycoprotein, fibronectin, in cultured fibroblasts. FEBS Lett. 1976 Dec 1;71(2):325–329. doi: 10.1016/0014-5793(76)80962-3. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J. Binding of cell attachment protein to collagen: effect of chemical modifications. Ann N Y Acad Sci. 1978 Jun 20;312:436–438. doi: 10.1111/j.1749-6632.1978.tb16829.x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Lembach K. J. Induction of human fibroblast proliferation by epidermal growth factor (EGF): enhancement by an EGF-binding arginine esterase and by ascorbate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):183–187. doi: 10.1073/pnas.73.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F., Henschen A. Amino acid sequence of human fibrin. Preliminary note on the completion of the C-terminal part of the alpha-chain sequence. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1451–1455. [PubMed] [Google Scholar]
- Matsuda M., Yoshida N., Aoki N., Wakabayashi K. Distribution of cold-insoluble globulin in plasma and tissues. Ann N Y Acad Sci. 1978 Jun 20;312:74–92. doi: 10.1111/j.1749-6632.1978.tb16794.x. [DOI] [PubMed] [Google Scholar]
- Micko S., Schlaepfer W. W. Metachromasy of peripheral nerve collagen on polyacrylamide gels stained with Coomassie brilliant blue R-250. Anal Biochem. 1978 Aug 1;88(2):566–572. doi: 10.1016/0003-2697(78)90457-8. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F. Labeling of a major fibroblast surface protein (fibronectin) catalyzed by blood coagulation factor XIIa. Biochim Biophys Acta. 1977 Mar 28;491(1):205–210. doi: 10.1016/0005-2795(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Vaheri A. Thrombin stimulates the production and release of a major surface-associated glycoprotein (fibronectin) in cultures of human fibroblasts. Exp Cell Res. 1978 Mar 15;112(2):323–334. doi: 10.1016/0014-4827(78)90215-x. [DOI] [PubMed] [Google Scholar]
- Nyman D., Duckert F. Proceedings: Factor XIII, fibrin and collagen. Thromb Diath Haemorrh. 1975 Nov 15;34(2):551–551. [PubMed] [Google Scholar]
- Soria A., Soria C., Boulard C. Fibrin stabilizing factor (F XIII) and collagen polymerization. Experientia. 1975 Nov 15;31(11):1355–1357. doi: 10.1007/BF01945824. [DOI] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Sun T. T., Chen L. B., Buchanan J. M. Thrombin potentiates the mitogenic response of cultured fibroblasts to serum and other growth promoting agents. J Cell Physiol. 1977 Aug;92(2):233–239. doi: 10.1002/jcp.1040920211. [DOI] [PubMed] [Google Scholar]