Abstract
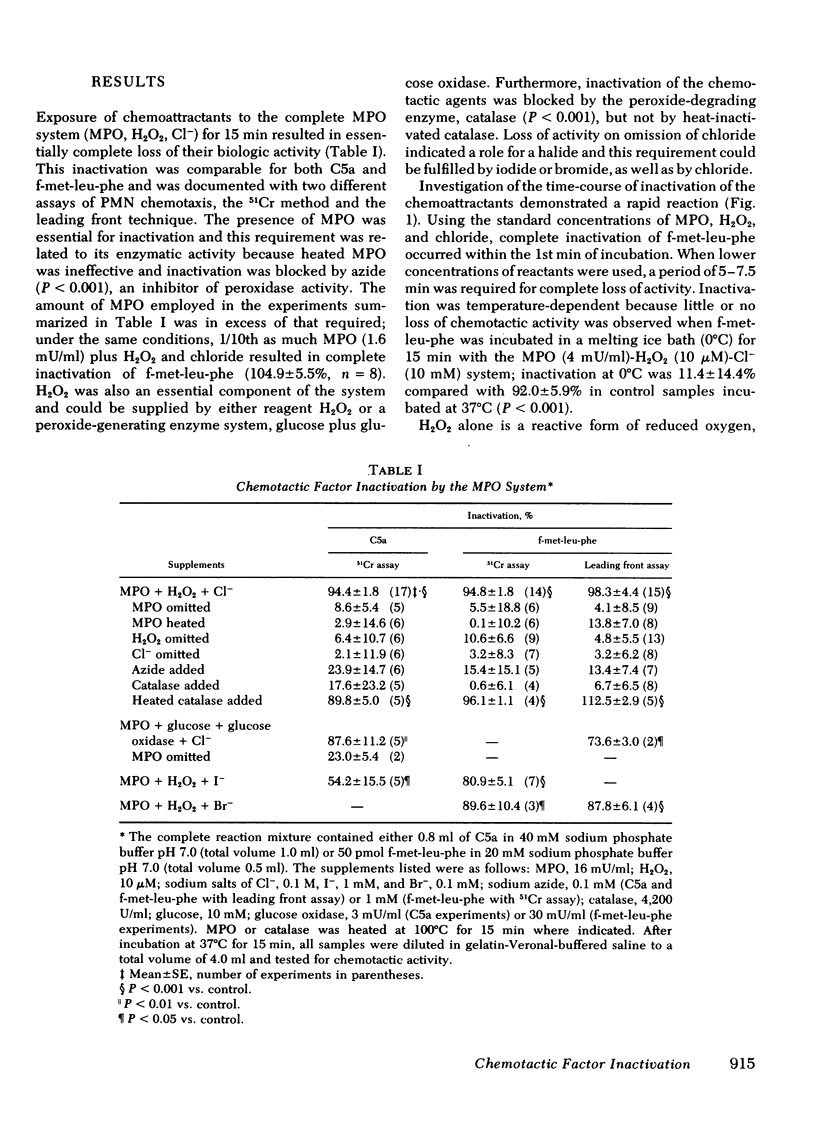
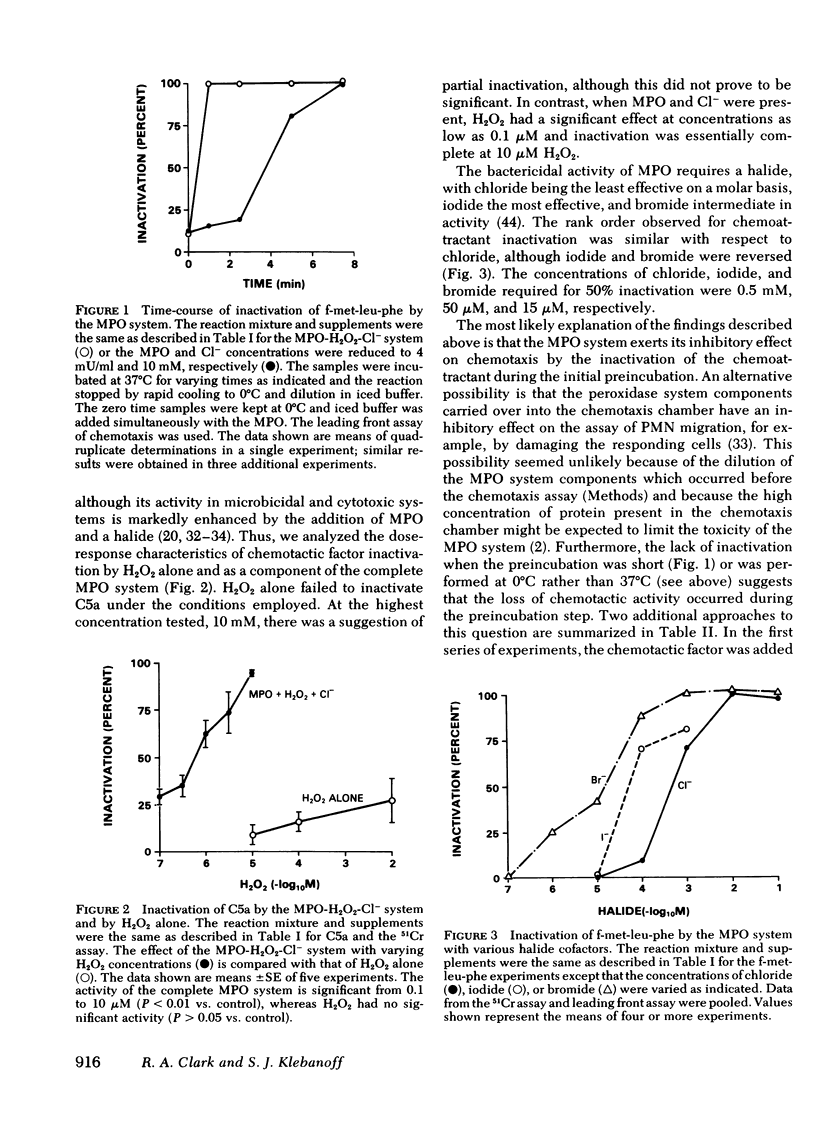
Polymorphonuclear leukocytes may modulate the acute inflammatory response by the secretion of enzymes capable of inactivating mediators of inflammation. The ability of the myeloperoxidase-H2O2-halide system of the neutrophil to inactivate chemoattractants was examined using both a radioassay and a morphologic assay of chemotaxis. Incubation of either a complement-derived agent, C5a, or a synthetic formyl-methionyl peptide chemoattractant with the myeloperoxidase system for 15 min at 37°C resulted in essentially complete loss of chemotactic activity. Inactivation was dependent on enzymatically active myeloperoxidase, H2O2 or a peroxide-generating enzyme system, and a halide cofactor. It was blocked by agents which inhibit peroxidase (azide) or degrade H2O2 (catalase). Inactivation of chemoattractants was time-dependent, reaching maximal levels within 1-5 min, and temperature-dependent with no significant inactivation occurring at 0°C. H2O2 alone had no significant inactivating ability at concentrations as high as 10 mM, whereas in the presence of myeloperoxidase and a halide, 0.1 μM H2O2 showed significant activity and 10 μM H2O2 caused complete inactivation. On a molar basis, the order of effectiveness of the halide cofactors was Br− > I− > Cl−, although only chloride was fully active at physiologic concentrations. Neutrophils stimulated by phagocytosis or by membraneperturbing agents secrete enzymatic constituents, including myeloperoxidase, and metabolic products such as H2O2. Thus, it is suggested that the myeloperoxidase system acting at an extracellular site serves as an inflammatory control mechanism by virtue of its ability to inactivate neutrophil chemoattractants.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Castle W. B. Oxidant injury of caucasian glucose-6-phosphate dehydrogenase-deficient red blood cells by phagocytosing leukocytes during infection. J Clin Invest. 1971 Dec;50(12):2466–2473. doi: 10.1172/JCI106747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F. Studies on chemotaxis. Effect of subcellular leukocyte fractions on neutrophils and macrophages. Int Arch Allergy Appl Immunol. 1970;39(2-3):247–271. [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase--H2O2--halide system: cytotoxic effect on human blood leukocytes. Blood. 1977 Jul;50(1):65–70. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979 Feb;63(2):177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Cornely H. P. Reversal of chemotaxis in vitro and chemotactic activity of leukocyte fractions. Proc Soc Exp Biol Med. 1966 Jul;122(3):831–835. doi: 10.3181/00379727-122-31263. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberg N. T., Schultz J. Evidence that myeloperoxidase is composed of isoenzymes. Arch Biochem Biophys. 1972 Feb;148(2):407–413. doi: 10.1016/0003-9861(72)90158-0. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Gigli I., Wasserman S., Austen K. F. A neutrophil immobilizing factor derived from human leukocytes. II. Specificity of action on polymorphonuclear leukocyte mobility. J Immunol. 1973 Sep;111(3):938–945. [PubMed] [Google Scholar]
- Goldstein I. M. Polymorphonuclear Leukocyte lysosomes and immune tissue injury. Prog Allergy. 1976;20:301–340. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974 Nov;113(5):1583–1588. [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Kaliner M. Mast cell granule peroxidase: location, secretion, and SRS-A inactivation. J Immunol. 1979 Apr;122(4):1322–1328. [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine P. H., Weinger R. S., Simon J., Scoon K. L., Krinsky N. I. Leukocyte-platelet interaction. Release of hydrogen peroxide by granulocytes as a modulator of platelet reactions. J Clin Invest. 1976 Apr;57(4):955–963. doi: 10.1172/JCI108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Phelps P. Polymorphonuclear leukocyte motility in vitro. 3. Possible release of a chemotactic substance after phagocytosis of urate crystals by polymorphonuclear leukocytes. Arthritis Rheum. 1969 Jun;12(3):197–204. doi: 10.1002/art.1780120306. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- Spilbert I., Gallacher A., Mehta J. M., Mandell B. Urate crystal-induced chemotactic factor: isolation and partial characterization. J Clin Invest. 1976 Oct;58(4):815–819. doi: 10.1172/JCI108533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G., Ward P. A. Two distinct chemotactic factor inactivators in human serum. J Immunol. 1975 Feb;114(2 Pt 2):843–847. [PubMed] [Google Scholar]
- Tynelius-Bratthall G., Lindhe J. Neutrophil chemotactic activity of rabbit neutrophils. Arch Oral Biol. 1974 Jan;19(1):97–101. doi: 10.1016/0003-9969(74)90232-5. [DOI] [PubMed] [Google Scholar]
- Venge P., Olsson I. Cationic proteins of human granulocytes. VI. Effects on the complement system and mediation of chemotactic activity. J Immunol. 1975 Dec;115(6):1505–1508. [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Ward P. A., Ozols J. Characterization of the protease activity in the chemotactic factor inactivator. J Clin Invest. 1976 Jul;58(1):123–129. doi: 10.1172/JCI108440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. II. Release of the C5-cleaving enzyme and inhibition of phagocytosis by rheumatoid factor. J Immunol. 1973 Dec;111(6):1777–1782. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. A functional differentiation of human neutrophil granules: generation of C5a by a specific (secondary) granule product and inactivation of C5a by azurophil (primary) granule products. J Immunol. 1977 Sep;119(3):1068–1076. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


