Abstract
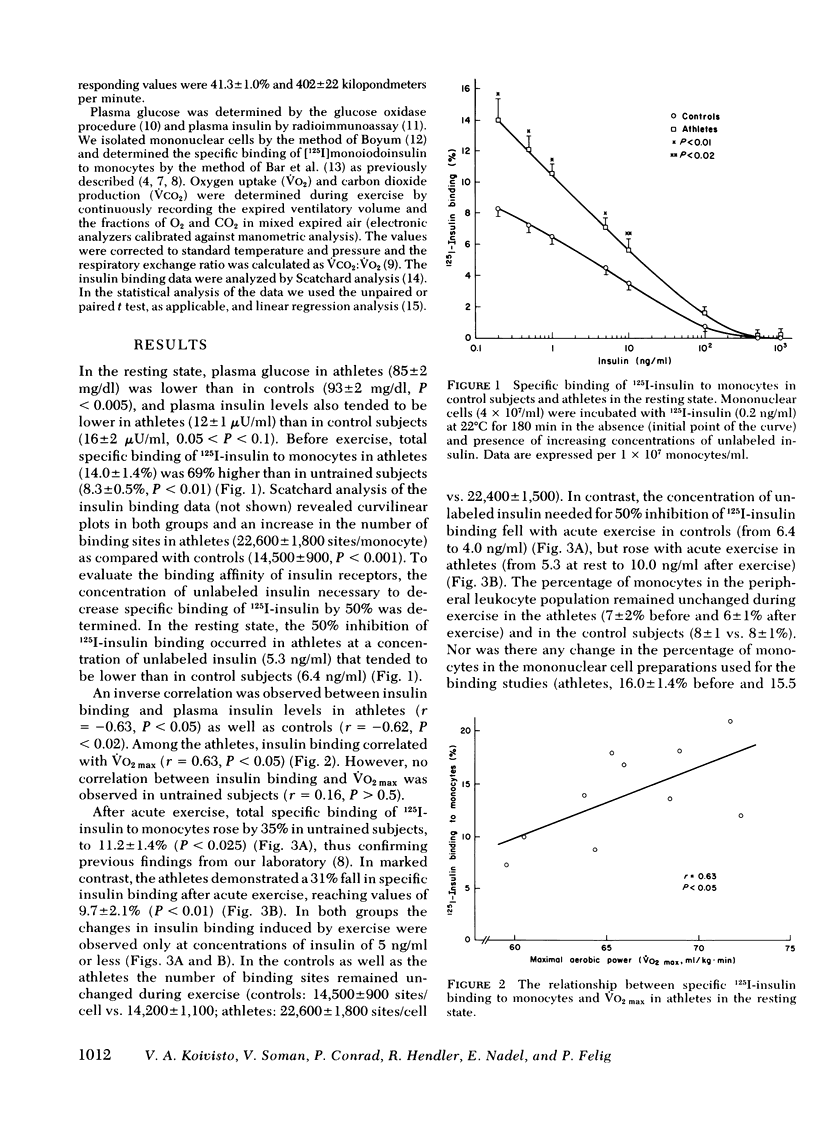
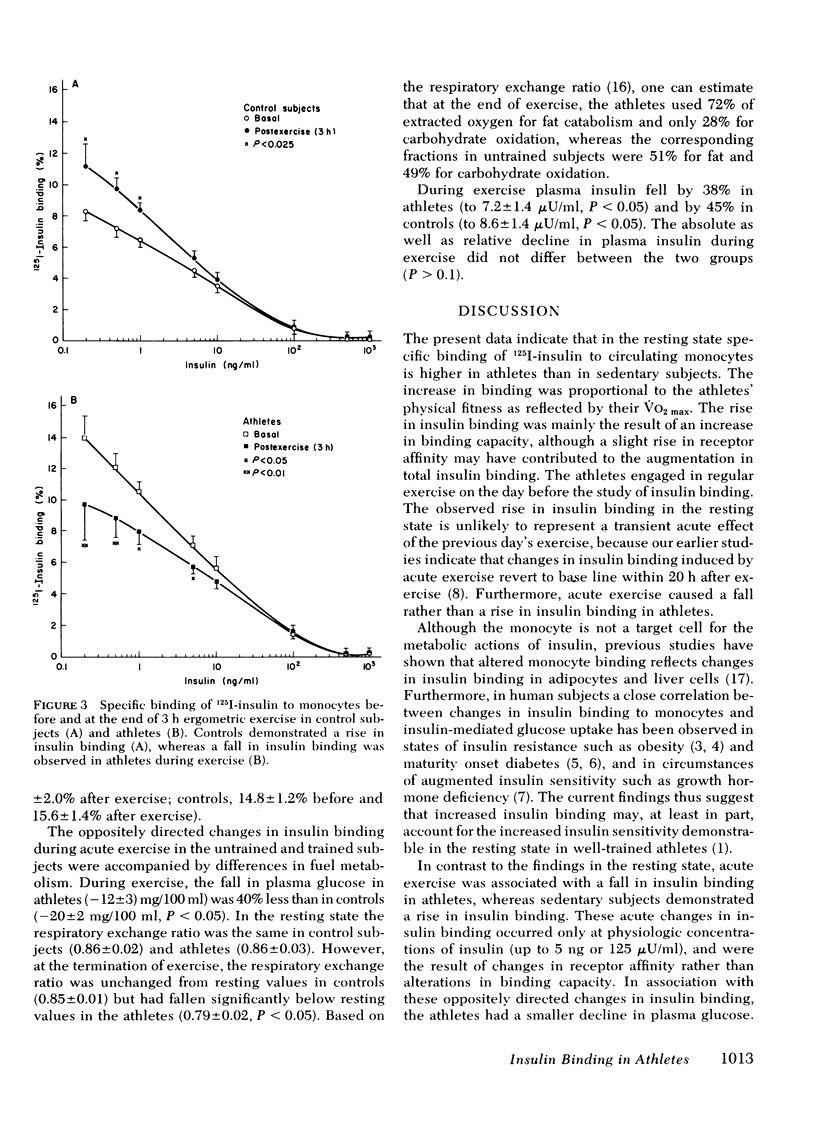
Insulin binding to monocytes was examined in trained athletes (long distance runners) and in sedentary control subjects in the resting state and after 3 h of exercise at 40% of maximal aerobic power. At rest, specific binding of 125-I-insulin to monocytes was 69% higher in athletes than in sedentary controls and correlated with maximal aerobic power. The increase in insulin binding was primarily due to an increase in binding capacity. During acute exercise, insulin binding fell by 31% in athletes but rose by 35% in controls. The athletes had a smaller decline in plasma glucose and a lower respiratory exchange ratio during exercise than did controls. We conclude that physical training increases insulin binding to monocytes in the resting state but results in a fall in insulin binding during acute exercise. Changes in insulin binding in athletes thus may account for augmented insulin sensitivity at rest as well as a greater shift from carbohydrate to fat usage during exercise than is observed in untrained controls.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Soman V., Sherwin R. S., Hendler R., Felig P. Insulin binding to monocytes and insulin action in human obesity, starvation, and refeeding. J Clin Invest. 1978 Jul;62(1):204–213. doi: 10.1172/JCI109108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Hultman E., Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967 Oct-Nov;71(2):129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Felig P. Effects of leg exercise on insulin absorption in diabetic patients. N Engl J Med. 1978 Jan 12;298(2):79–83. doi: 10.1056/NEJM197801122980205. [DOI] [PubMed] [Google Scholar]
- Lohmann D., Liebold F., Heilmann W., Senger H., Pohl A. Diminished insulin response in highly trained athletes. Metabolism. 1978 May;27(5):521–524. doi: 10.1016/0026-0495(78)90017-3. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nadel E. R., Pandolf K. B., Roberts M. F., Stolwijk J. A. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974 Oct;37(4):515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Roth J., Kahn C. R., Lesniak M. A., Gorden P., De Meyts P., Megyesi K., Neville D. M., Jr, Gavin J. R., 3rd, Soll A. H., Freychet P. Receptors for insulin, NSILA-s, and growth hormone: applications to disease states in man. Recent Prog Horm Res. 1975;31:95–139. doi: 10.1016/b978-0-12-571131-9.50007-4. [DOI] [PubMed] [Google Scholar]
- Soman V. R., Koivisto V. A., Grantham P., Felig P. Increased insulin binding to monocytes after acute exercise in normal man. J Clin Endocrinol Metab. 1978 Jul;47(1):216–219. doi: 10.1210/jcem-47-1-216. [DOI] [PubMed] [Google Scholar]
- Soman V., Tamborlane W., DeFronzo R., Genel M., Felig P. Insulin binding and insulin sensitivity in isolated growth hormone deficiency. N Engl J Med. 1978 Nov 9;299(19):1025–1030. doi: 10.1056/NEJM197811092991901. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R., Pek S., Kovacevic N., Wrenshall G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous exercise in dogs. J Clin Invest. 1976 Feb;57(2):245–255. doi: 10.1172/JCI108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]


