Abstract
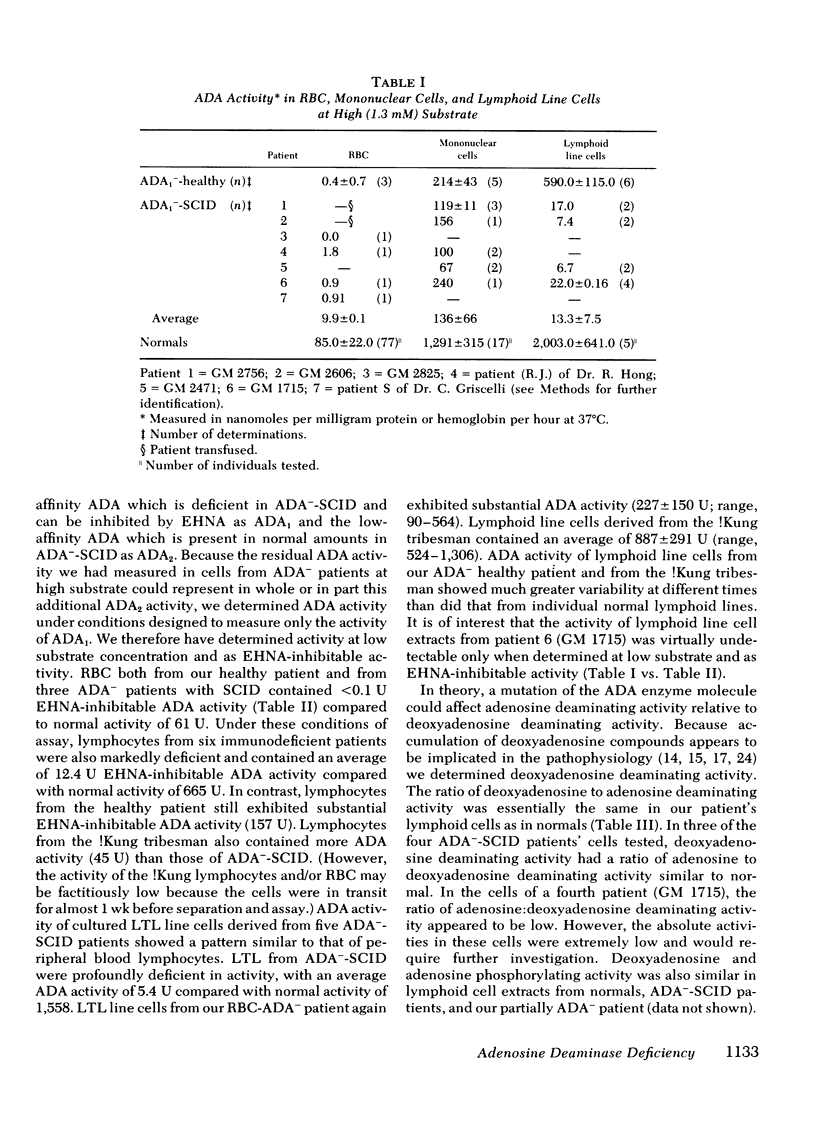
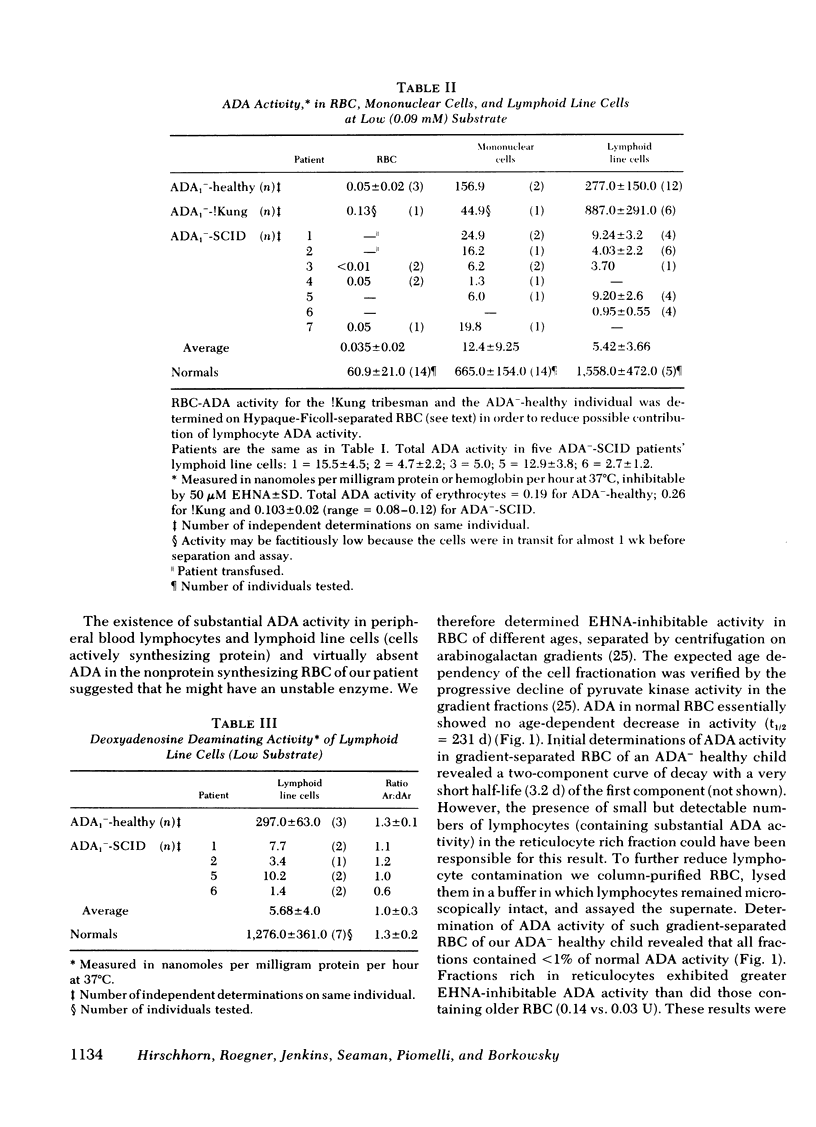
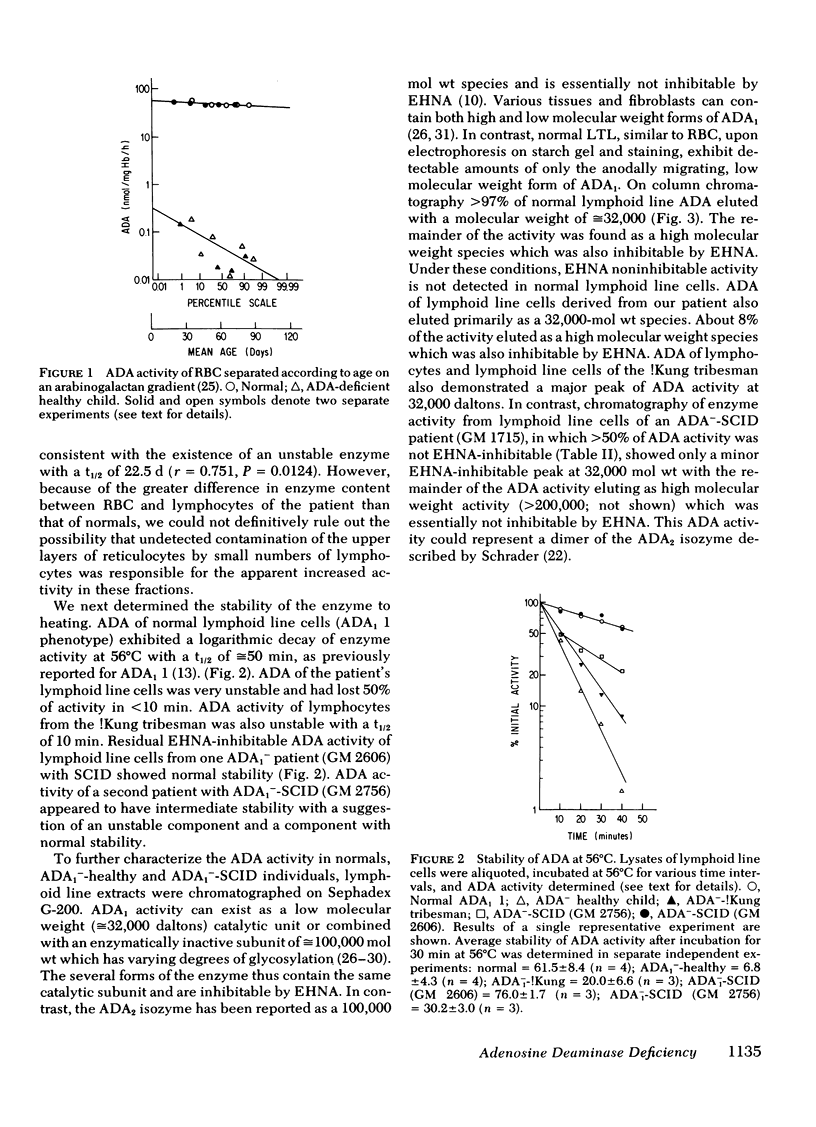
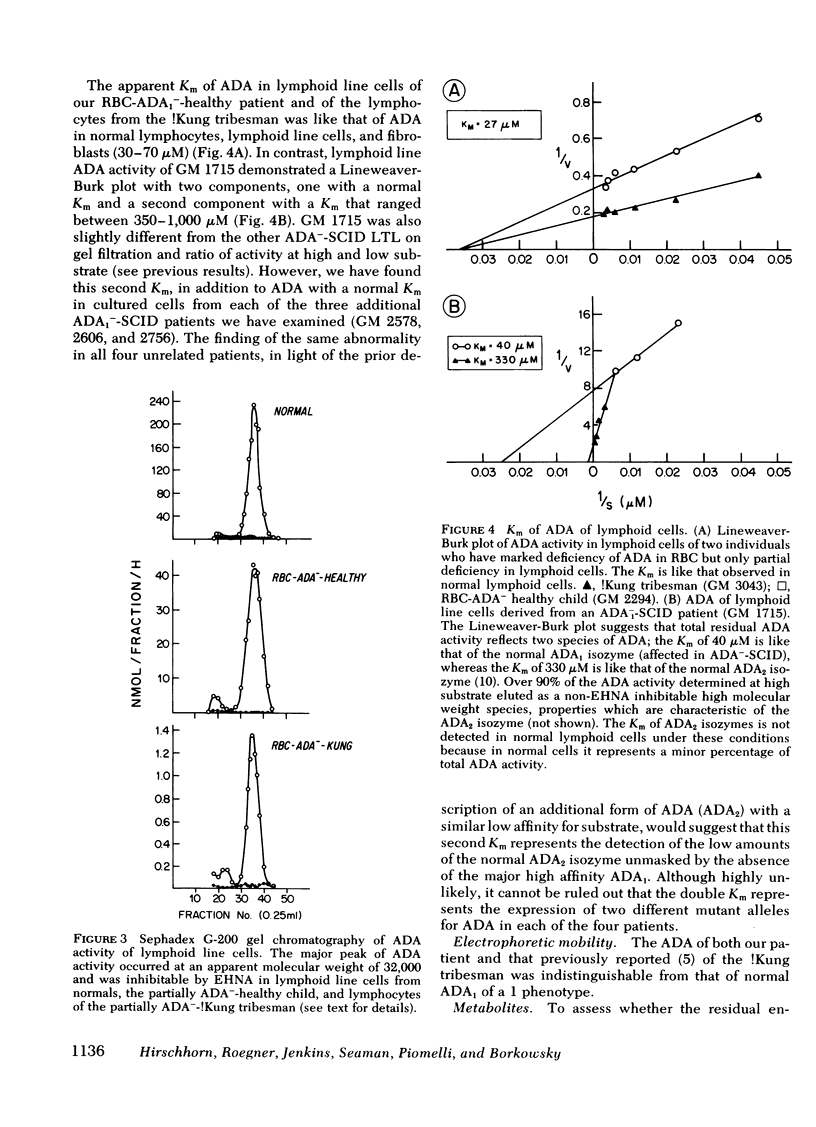
Inherited deficiency of the purine salvage enzyme adenosine deaminase (ADA) gives rise to a syndrome of severe combined immunodeficiency (SCID). We have studied a 2.5-yr-old immunologically normal child who had been found to lack ADA in his erythrocytes during New York State screening of normal newborns. His erythrocytes were not detectably less deficient in ADA than erythrocytes of ADA−-SCID patients. In contrast, his lymphocytes and cultured long-term lymphoid cells contained appreciably greater ADA activity than those from patients with ADA−-SCID. This residual ADA activity had a normal molecular weight and Km but was markedly unstable at 56°C. His residual erythrocytes-ADA activity also appeared to have diminished stability in vivo. ADA activity in lymphoid line cells of a previously reported erythrocyte-ADA-deficient!Kung tribesman was found to contain 50% of normal activity and to exhibit diminished stability at 56°C. ATP content of erythrocytes from both partially ADA-deficient individuals was detectably greater than normal (12.3 and 6.1 vs. normal of 2.6 nmol/ml packed erythrocytes). However, the dATP content was insignificant compared to that found in erythrocytes of ADA−-SCID patients (400-1,000 nmol/ml packed erythrocytes). The New York patient, in contrast to normals, excreted detectable amounts of deoxyadenosine, but this was <2% of deoxyadenosine excreted by ADA−-SCID patients. Thus, the residual enzyme in cells other than erythrocytes appears to be sufficient to almost totally prevent accumulation of toxic metabolites.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Carson D. A., Goldblum R., Seegmiller J. E. Quantitative immunoassay of adenosine deaminase in combined immunodeficiency disease. J Immunol. 1977 Jan;118(1):270–273. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Scott C. R., Swedberg D. R. Heterogeneity for adenosine deaminase deficiency: Expression of the enzyme in cultured skin fibroblasts and amniotic fluid cells. Am J Hum Genet. 1975 Jan;27(1):46–52. [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Human adenosine deaminase binding protein. Assay, purification, and properties. J Biol Chem. 1978 Jul 10;253(13):4617–4623. [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Beratis N. G., Martiniuk F. Adenosine deaminase. Alterations in activity and isozymes during growth of normal and genetically deficient fibroblasts. Exp Cell Res. 1978 Nov;117(1):103–109. doi: 10.1016/0014-4827(78)90432-9. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Beratis N., Rosen F. S. Characterization of residual enzyme activity in fibroblasts from patients with adenosine deaminase deficiency and combined immunodeficiency: evidence for a mutant enzyme. Proc Natl Acad Sci U S A. 1976 Jan;73(1):213–217. doi: 10.1073/pnas.73.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Beratis N., Rosen F. S., Parkman R., Stern R., Polmar S. Adenosine-deaminase deficiency in a child diagnosed prenatally. Lancet. 1975 Jan 11;1(7898):73–75. doi: 10.1016/s0140-6736(75)91075-2. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Conversion of human erythrocyte-adenosine deaminase activity to different tissue-specific isozymes. Evidence for a common catalytic unit. J Clin Invest. 1975 Mar;55(3):661–667. doi: 10.1172/JCI107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson D. A., Cook P. J., Harris H. Further data on the adenosine deaminase (ADA) polymprphism and a report of a new phenotype. Ann Hum Genet. 1969 May;32(4):361–367. doi: 10.1111/j.1469-1809.1969.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Lane A. B., Nurse G. T., Hopkinson D. A. Red cell adenosine deaminase (ADA) polymorphism in Southern Africa, with special reference to ADA deficiency among the !Kung. Ann Hum Genet. 1979 May;42(4):425–433. doi: 10.1111/j.1469-1809.1979.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Rabson A. R., Nurse G. T., Lane A. B. Deficiency of adenosine deaminase not associated with severe combined immunodeficiency. J Pediatr. 1976 Nov;89(5):732–736. doi: 10.1016/s0022-3476(76)80792-5. [DOI] [PubMed] [Google Scholar]
- Jenkins T. Red-blood-cell adenosine deaminase deficiency in a "healthy" Kung individual. Lancet. 1973 Sep 29;2(7831):736–736. doi: 10.1016/s0140-6736(73)92568-3. [DOI] [PubMed] [Google Scholar]
- Krstulovic A. M., Brown P. R., Rosie D. M. Identification of nucleosides and bases in serum and plasma samples by reverse-phase high performance liquid chromatography. Anal Chem. 1977 Dec;49(14):2237–2241. doi: 10.1021/ac50022a032. [DOI] [PubMed] [Google Scholar]
- McKusick V. A. Phenotypic diversity of human diseases resulting from allelic series. Am J Hum Genet. 1973 Jul;25(4):446–456. [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Chen S. H., Scott C. R. Use of the integrated steady state rate equation to investigate product inhibition of human red cell adenosine deaminase and its relevance to immune dysfunction. J Biol Chem. 1978 Jan 25;253(2):323–325. [PubMed] [Google Scholar]
- Osborne W. R., Spencer N. Partial purification and properties of the common inherited forms of adenosine deaminase from human erythrocytes. Biochem J. 1973 May;133(1):117–123. doi: 10.1042/bj1330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polmar S. H., Stern R. C., Schwartz A. L., Wetzler E. M., Chase P. A., Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med. 1976 Dec 9;295(24):1337–1343. doi: 10.1056/NEJM197612092952402. [DOI] [PubMed] [Google Scholar]
- Rubinstein A., Hirschhorn R., Sicklick M., Murphy R. A. In vivo and in vitro effects of thymosin and adenosine deaminase on adenosine-deaminase-deficient lymphocytes. N Engl J Med. 1979 Feb 22;300(8):387–392. doi: 10.1056/NEJM197902223000802. [DOI] [PubMed] [Google Scholar]
- Schrader W. P., Pollara B., Meuwissen H. J. Characterization of the residual adenosine deaminating activity in the spleen of a patient with combined immunodeficiency disease and adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):446–450. doi: 10.1073/pnas.75.1.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. P., Stacy A. R. Purification and subunit structure of adenosine deaminase from human kidney. J Biol Chem. 1977 Sep 25;252(18):6409–6415. [PubMed] [Google Scholar]
- Solter A. W., Handschumacher R. E. A rapid quantitative determination of deoxyribonucleoside triphosphates based on the enzymatic synthesis of DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):585–590. doi: 10.1016/0005-2787(69)90288-3. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Evans L., Hopkinson D. A. Several of the adenosine deaminase isozymes are glycoproteins. Nature. 1977 Sep 15;269(5625):261–262. doi: 10.1038/269261a0. [DOI] [PubMed] [Google Scholar]
- Uberti J., Lightbody J. J., Wolf J. W., Anderson J. A., Reid R. H., Johnson R. M. The effect of adenosine on mitogenesis of ADA-deficient lymphocytes. Clin Immunol Immunopathol. 1978 Aug;10(4):446–458. doi: 10.1016/0090-1229(78)90157-5. [DOI] [PubMed] [Google Scholar]


