Abstract
Functional imaging studies of healthy participants and previous lesion studies have provided evidence that empathy involves dissociable cognitive functions that rely on at least partially distinct neural networks that can be individually impaired by brain damage. These studies converge in support of the proposal that affective empathy—making inferences about how another person feels—engages at least the following areas: prefrontal cortex, orbitofrontal gyrus, anterior insula, anterior cingulate cortex, temporal pole, amygdala and temporoparietal junction. We hypothesized that right-sided lesions to any one of these structures, except temporoparietal junction, would cause impaired affective empathy (whereas bilateral damage to temporoparietal junction would be required to disrupt empathy). We studied 27 patients with acute right hemisphere ischaemic stroke and 24 neurologically intact inpatients on a test of affective empathy. Acute impairment of affective empathy was associated with infarcts in the hypothesized network, particularly temporal pole and anterior insula. All patients with impaired affective empathy were also impaired in comprehension of affective prosody, but many patients with impairments in prosodic comprehension had spared affective empathy. Patients with impaired affective empathy were older, but showed no difference in performance on tests of hemispatial neglect, volume of infarct or sex distribution compared with patients with intact affective empathy.
Keywords: empathy, stroke, emotion perception, magnetic resonance imaging, prosody
Introduction
Nearly all human interaction requires that we make inferences about what other people think and feel. This ability to take the perspective of another person provides an important foundation for our relationships, communication, negotiations and other social activities. Perspective-taking is an important component of empathy (de Waal, 2012), along with emotional contagion (Stotland and Dunn, 1963; de Waal, 2012), or sharing the other person’s perceived emotional state. Several important neurological and neuropsychiatric diseases are known to disrupt various aspects of empathy, including autism (Dziobek et al., 2008), frontotemporal dementia (Eslinger et al., 2005; Viskontas et al., 2007), traumatic head injury (Eslinger, 1998; McDonald and Flanagan, 2004; Neumann et al., 2012) and schizophrenia (Shamay-Tsoory et al., 2007; Lee et al., 2011). Numerous functional imaging studies have investigated the brain regions engaged in empathy, and have found distinct regions involved in emotional contagion versus cognitive and emotional perspective-taking (the ability to infer what another person thinks or feels). These studies have led to the proposal that there are dissociable neural and cognitive systems of empathy. One is a developmentally and phylogenetically ‘early’ system of emotional contagion that may involve the mirror neuron system and the right inferior frontal gyrus (Bodini et al., 2004; Shamay-Tsoory, 2011), as well as the right temporal pole, superior temporal gyrus, fusiform, insula and amygdala (Carr et al., 2003). Emotional contagion may depend on areas of the brain that are activated in association with recognizing emotions of others through prosody (tone of voice), facial expression and gestures, in right fusiform cortex as well as superior temporal sulcus and amygdala (Gorno-Tempini et al., 2001). The second ‘later’ system, which some think of as the second stage of empathy (Decety and Jackson, 2004) is a higher level perspective-taking system that depends on the medial prefrontal cortex (Eslinger, 1998; Shamay-Tsoory et al., 2003). Perspective-taking can be further subdivided into cognitive and emotional perspective-taking. Perspective-taking may depend on a number of cognitive functions such as cognitive flexibility (Decety and Jackson, 2004; Rankin et al., 2005), attention and working memory, abstract reasoning (Rankin et al., 2006), belief attribution and assignment of agency (Saxe and Kanwisher 2003; Samson et al., 2004; Saxe et al., 2004; Decety and Lamm, 2007). Thus, affective empathy, the ability to recognize and make judgements about how another person feels, includes both emotional contagion and emotional perspective-taking.
The neural networks supporting affective empathy have been studied primarily through task-related and task-free (‘resting-state’) functional MRI. A task-free functional MRI study of neurologically normal adults investigated intrinsic functional dynamics of affective compared with cognitive empathy (Cox et al., 2012). This study showed that a dominance of affective empathy compared to cognitive empathy was associated with functional connectivity between ventral anterior insula, orbitofrontal cortex, amygdala, and anterior cingulate, whereas a dominance of cognitive empathy was associated with functional connectivity between parts of the brainstem, superior temporal sulcus and ventral anterior insula. Task-related functional imaging studies also confirm an important role of various parts of the frontal cortex, anterior insula, anterior cingulate cortex and right amygdala in empathy, as revealed by large coordinate-based activation likelihood estimation meta-analyses. In one activation likelihood estimation meta-analysis of 112 experiments on affective empathy, Bzdok et al. (2012) reported that the following areas showed significant activation in association with empathy tasks across studies: bilateral dorsomedial prefrontal cortex, right greater than left inferior frontal cortex, bilateral anterior insula, anterior and posterior cingulate, bilateral temporoparietal junction, right amygdala, right middle temporal gyrus, right superior temporal sulcus, bilateral thalamus, right hippocampus, midbrain and right pallidum. A few meta-analyses have focused on a particular type of affective empathy: recognizing another person’s negative emotion caused by pain. Gu et al. (2012) reported that anterior insula and anterior cingulate cortex were the areas most commonly activated in association with recognition of another person’s pain, on the basis of an activation likelihood estimation meta-analysis of 28 functional MRI studies on empathetic pain (using keywords ‘empathy’, ‘empathetic’, ‘sympathy’, ‘emotional contagion’, ‘altruism’ or ‘compassion’ combined with ‘pain’ and ‘functional MRI’). Similarly, an image-based meta-analysis of nine independent functional MRI studies of empathy for pain and an activation likelihood estimation meta-analysis of 32 studies of empathy for pain identified activation in bilateral anterior insula, anterior cingulate, precuneus and thalamus, associated with empathy for other people’s pain (Lamm et al., 2011).
Two recent studies have investigated the influence of neuropeptides that affect amygdala function on empathy. The first reported that intranasal oxytocin increased affective, but not cognitive empathy, as measured by a multifaceted empathy task; and improved learning performance on an association task when social, but not non-social, reinforcers were used (Hurlemann et al., 2010). These authors also reported that two females with selective damage to bilateral amygdala due to Urbach-Wiethe disease were impaired in affective, but not cognitive, empathy on their multifaceted empathy task, and showed normal learning on an association task when non-social reinforcers, but not social, reinforcers, were used. Stone et al., (2003) also reported cases of impaired empathy performance associated with bilateral amygdala lesions in two patients. More recently, investigators studied the effects of intranasal vasopressin, which is considered to have opposing effects of oxytocin, on activation in amygdala and other brain regions during empathy in a functional MRI study of affective empathy (Brunnlieb et al., 2013). This study revealed that vasopressin modulated activation of right amygdala associated with affective empathy and increased connectivity between right amygdala and medial prefrontal cortex and inferior parietal cortex in association with the empathy task.
Together, these studies indicate an important role of at least the following areas in recognizing and making inferences about the emotions of another person (affective empathy): prefrontal cortex, orbitofrontal gyrus, anterior insula, anterior cingulate cortex, temporal pole and amygdala. The temporoparietal junction is another area that is nearly always activated in tasks that require perspective-taking (Corbetta et al., 2008), but seems to be important for more general aspects of mentalizing or belief attribution than in affective empathy (Sebastian et al., 2012). For example, it is equally engaged in third person visuospatial judgements as third person judgements of another person’s feelings or emotions (Schnell et al., 2011). Moreover, because it is not specific to emotional processing, we hypothesize that a unilateral lesion would not disrupt its role in assignment of agency or attributing a belief or feeling to another person. Rather, we expect bilateral damage to this region would be required to disrupt its role in empathy.
Lesions localized to some of the regions that are activated in functional imaging studies are reported to cause deficits specific to cognitive versus affective empathy (Hynes et al., 2006; Shamay-Tsoory and Aharon-Peretz, 2007; Gu et al., 2012). Such studies are important to the field, because it is critical to show that regions activated during a task are critical to that task, and not just engaged by it (or actively inhibited during it), as ‘activation’ (blood oxygen level-dependent signal in a region that is correlated with the task) can reflect any of these possibilities (Thompson-Schill et al., 1998; Fellows et al., 2005). However, most of the studies of impaired empathy have been conducted in patients with poorly localized lesions, such as autism, dementia, and traumatic head injury. The few studies of affective empathy in patients with focal lesions, such as stroke, have included just one to two patients with focal lesions (Stuss and Anderson 2004; Samson et al., 2005, 2007; Roldan et al., 2011; Couto et al., 2012) or have involved a heterogeneous population, of which only a few had stroke (and others had meningioma, head injury and other lesions) (Shamay-Tsoory et al., 2003, 2005, 2009; Shamay-Tsoory and Aharon-Peretz, 2007). A voxel-based morphometry study of 123 patients with Alzheimer’s disease, progressive supranuclear palsy, corticobasal degeneration and frontotemporal dementia using caregivers’ ratings on the Interpersonal Reactivity Index (Davis, 1983) to evaluate empathy revealed that impairments in empathy measured by the sum of ‘empathetic concern’ (reflecting emotional contagion) and ‘perspective-taking’ significantly correlated with the volume of grey matter in right temporal pole, fusiform gyrus and medial inferior frontal region (Rankin et al., 2006). Both empathetic concern and perspective-taking subscale scores alone correlated with volume of grey matter volume in right temporal pole. Other studies of behavioural variant frontotemporal dementia, a relatively focal neurodegenerative disease characterized by impaired social conduct and emotional function, including empathy, have also reported a role of right temporal pole and/or orbitofrontal atrophy in empathy deficits (Rankin et al., 2005; Kipps and Hodges, 2006; Viskontas et al., 2007). For example, a detailed study of a patient with hereditary multiple exostoses and frontotemporal dementia revealed severely impaired affective empathy associated with atrophy in right anterior temporal lobe and orbitofrontal gyrus (Narvid et al., 2009). Right insula also showed atrophy. Therefore, we sought to test the hypothesis, derived from functional imaging studies in healthy participants and previous lesion studies, that lesions to components of a network of brain regions involving right prefrontal cortex, orbitofrontal gyrus, temporal pole, anterior insula, anterior cingulate cortex and amygdala cause deficits in affective empathy. We focused on patients with right hemisphere lesions because studies of neurodegenerative disease have consistently shown greater correlation between impairments in empathy and atrophy in the right hemisphere compared to the left hemisphere (Rankin et al., 2005, 2006; Eslinger et al., 2011). Older lesions studies, which frequently compared small numbers of right hemisphere stroke to left hemisphere stroke, without further lesion localization, also found a greater impact of right hemisphere on empathy (Brune and Brune-Cohrs, 2006). Finally, functional imaging studies have also indicated a specialized role of the right hemisphere in emotional aspects of empathy and sympathy (Decety and Chaminade, 2003). We also determined whether deficits in this type of empathy were related to lesion site alone, or also influenced by lesion volume, age, impairments of attention (neglect), or impairments of prosody comprehension. We tested these hypotheses in patients with acute stroke (within 48 h of onset), before the opportunity for reorganization of structure-function relationships, rehabilitation, or recovery. This approach allowed us to determine if a lesion in a particular area had the predicted effect on the task.
Materials and methods
Participants
We enrolled a consecutive series of 27 patients with acute ischaemic right hemisphere stroke who provided informed consent to participate in the study and had none of the following exclusion criteria: (i) neurological disease other than stroke; (ii) reduced level of consciousness or on-going sedation; and (iii) inability to have MRI due to claustrophobia, implanted ferrous metal, or weight >300 lb. We also enrolled 24 patients with transient ischaemic attack who had normal MRI and normal neurological examination at the time of testing to serve as normal controls as they had the same demographic characteristics and the same stressors of hospitalization as the stroke patients. They also had to have none of the exclusion criteria and no stroke.
Imaging
Patients underwent a stroke protocol MRI, including diffusion-weighted imaging, FLAIR and 3D time-of-flight angiography of the intracranial vessels. Diffusion-weighted imaging was acquired using single-shot spin-echo echo-planar imaging, in the transverse plane covering the entire brain with a b-value of 1000 (s/mm2) and with a least diffusion weighting (b0).
Stroke volume measurement
To define boundaries of acute stroke lesion(s) (hereafter termed stroke map) to measure the stroke volume of each participant, a threshold of >30% intensity increase from the unaffected area in the diffusion-weighted image was applied, and then a neurologist (K.O.), who was blinded to the clinical information, manually modified the boundary to avoid false-positive and false-negative areas (Oishi et al., 2009). This procedure was performed on RoiEditor (www.MRIstudio.org).
Image processing
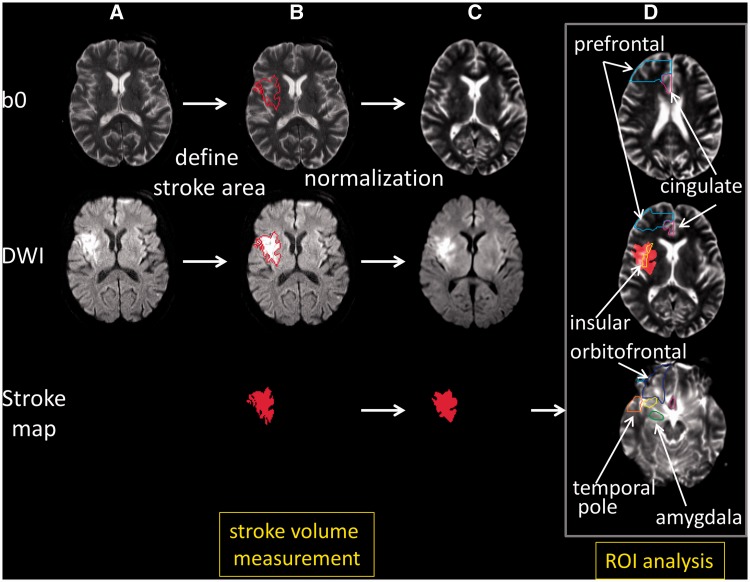
The least diffusion weighted image (b0) with T2-weighted contrast was transformed to the JHU-MNI-b0 atlas using affine transformation followed by the large deformation diffeomorphic metric mapping (Oishi et al., 2009). The resultant matrices were applied to the stroke map for the normalization. Customized version of the JHU-MNI Brain Parcellation Map (cmrm.med.jhmi.edu) was overlaid on the normalized stroke map to investigate % volume of the selected anatomical structures (prefrontal cortex, orbitofrontal gyrus, anterior cingulate cortex, anterior insula, temporal pole and amygdala) affected by acute stroke (Fig. 1). This procedure was performed on DiffeoMap (www.MRIstudio.org).
Figure 1.
Procedures for the image processing. (A) Images in original space. (B) The diffusion weighted image (DWI) was used to 3D define the area with acute infarction (stroke map) shown as a red contour in the least diffusion weighted image (b0) and diffusion weighted imaging, and the red area in the stroke map. (C) The b0 image, with no or minimum signal intensity increase in the infarcted area, was normalized to the atlas space. The resultant transformation matrix was then applied to the stroke map. In this figure, the transformation matrix was also applied to the diffusion weighted imaging to qualitatively demonstrate the accuracy of image normalization. (D) A predefined set of 3D regions of interest (ROI) [right prefrontal (cyan contour), right anterior cingulate (purple contour), right anterior insula (yellow contour), right orbitofrontal (blue contour), right amygdala (green contour), and the right temporal pole (orange contour)] on the atlas space was overlaid on the normalized stroke map to report % volume of each region of interest affected by the infarction. In this figure, the normalized stroke map (red area) was overlaid on the normalized b0 image. Images are all in radiological convention.
Behavioural testing
Patients underwent cognitive testing within 24 h of admission to the hospital. Testing of affective empathy included eight questions requiring inference about emotions of individuals in short videotaped scenarios and two questions requiring inference about emotions of individuals in stories that were read to the patients (Box 1). To control for deficits in general attention and recent memory, patients were also asked factual questions about the stories. To evaluate emotional contagion, the facial expressions, comments, and tone of voice of patients were evaluated when the patients watched the videos and listened to the stories.
Box 1 Example of a story from the Affective Empathy Task.
Participants listen to the following story, and then select a printed word that most accurately depicts the emotion in response to the question.
John was waiting for the bus that would bring his girlfriend, Cathy. He was planning to ask her to marry him. When the bus arrived, Cathy got off the bus, talking to a very handsome man. They were smiling at each other.
How do you thing John feels?
Happy Jealous Scared Bored Excited Relieved
The story continues. Cathy saw John. She smiled and waved. She said, ‘John, I would like you to meet my brother.’
How do you thing John feels now?
Angry Jealous Scared Bored Excited Relieved
Patients were also administered the Interpersonal Reactivity Index. This test is a self-administered test that includes four types of questions designed to evaluate cognitive empathy (perspective-taking and fantasy scales) and affective empathy (empathetic concern and personal distress).
Patients were also administered the prosody comprehension subtest of the Aprosodia Battery (Ross and Monnot, 2008) to evaluate for deficits in comprehension in affective prosody. In this test, participants listen to emotionally-neutral sentences, monosyllables (ba ba ba), or asyllabic tones (ah) produced in different tones of voice, and select (from written choices) the corresponding emotion (e.g. happy, sad, angry, surprised).
Patients were administered a general test of hemispatial neglect, including a test of detecting left versus right-sided gaps in circles scattered across a page (Ota et al., 2001), and copying a scene of a house, two trees and a fence, and bisection of a 10-inch line.
Statistical analysis
We first identified a cut-off score for normal performance on the affective empathy test, by administering the test to 24 neurologically normal control subjects (transient ischaemic attack patients with normal MRI and normal neurological examination at the time of testing). We determined the range and distribution of scores for control subjects with comparable age, education and socioeconomic backgrounds of our stroke patients, which allowed us to identify a cut-off that would be outside of the normal range of scores for this population.
We then identified whether or not a lesion in the proposed network of areas critical for affective empathy (right prefrontal, orbitofrontal gyrus, temporal pole, anterior cingulate, anterior insula, or amygdala) in the right hemisphere is associated with impaired affective empathy, using Fisher’s exact tests, for the entire network as a whole, including only the 27 stroke patients in the analysis. For the two areas most strongly associated with impaired affective empathy, we evaluated the Pearson correlation between severity of impairment (error rate on the affective empathy task) and percentage of damage in each of the two areas.
We then evaluated differences between stroke patients with lesions in this network and patients without lesions in this network (in a case control study), with regard to score on our affective empathy test, tests of affective prosody, tests of hemispatial neglect, as well as age and volume of infarct.
Finally, we evaluated whether or not patients with impaired affective empathy were different from patients with spared affective empathy in terms of scores on prosody comprehension, volume of infarct, age, and scores on neglect.
Results
Affective Empathy Task: range of normal performance
The 24 control participants ranged in age from 35–79 years, mean ± standard deviation (SD) = 52.5 ± 15.5. The stroke patients were similar, ranging in age from 26–75 years, mean ± SD = 54.5 ± 13.6. In both the control group and the stroke group, 33% of patients were female.
The range of scores on the Affective Empathy Task for control patients was 0 to 20% errors. The mean score ± SD was 5.8 ± 7.8. The distribution of scores for controls was as follows: 58.3% made 0% errors; 25% made 10% errors; and 16.7% made 20% errors. Therefore, we used >20% errors as the cut-off for normal performance; ≥30% errors was considered impaired. This score was more than 2 SD above the mean for normal controls.
The effect of acute ischaemic lesions in right hemisphere affective empathy network on affective empathy task performance
We examined the regions of interest in the right hemisphere that most commonly show activation during affective empathy tasks, to determine if a lesion in one or more of the components of the proposed network caused impaired affective empathy (≥30% error). The regions of interest included: right prefrontal cortex, orbitofrontal gyrus, anterior cingulate cortex, anterior insula, temporal pole and amygdala. We considered a region of interest as affected only if at least 1% of the area was infarcted on diffusion weighted imaging trace image. We identified 10 patients with lesions in one or more of the regions of interest. Nine (90%) of the 10 patients had impaired affective empathy. One patient with a lesion involving the anterior insula did not have impaired affective empathy using the 30% error cut-off, although the patient made 20% errors on the task.
Table 1 shows the percentage of infarct in each of the regions of interest for each of the 10 patients with damage to the network. All of these patients who had lesions in the temporal pole also had lesions in the anterior insula. The one patient who had insular damage but no damage to the temporal pole is the patient who made only 20% errors on the affective empathy task (in the normal range).
Table 1.
Error rate and percentage of damage to each region of interest in the ‘empathy network’ for the 10 patients with network lesions
| Case: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Error rate: | 30 | 30 | 30 | 30 | 50 | 40 | 30 | 100 | 100 | 20 |
| Prefrontal cortex | 0 | 0 | 0 | 8.8 | 0 | 0 | 49.8 | 6.7 | 0 | 0 |
| Orbitofrontal gyrus | 0 | 0 | 0 | 0 | 5.0 | 0 | 0 | 3.6 | 0 | 0 |
| STG pole | 14 | 0 | 2.4 | 1.4 | 86.7 | 1.5 | 3.5 | 84.3 | 0 | 0 |
| MTG pole | 0 | 0 | 0 | 0 | 66.0 | 0 | 0 | 57.8 | 0 | 0 |
| Temporal pole | 8.9 | 0 | 1.6 | 0.9 | 79.1 | 0.9 | 2.2 | 74.6 | 0 | 0 |
| Anterior cingulate | 0 | 1.3 | 0 | 0 | 0.6 | 0 | 0 | 2.5 | 0 | 0 |
| Anterior insula | 77.3 | 0 | 57.3 | 47.2 | 95.0 | 39.0 | 23.6 | 96.8 | 0 | 14.4 |
| Amygdala | 0 | 0 | 0 | 0 | 3.1 | 1.9 | 0 | 51.4 | 9.4 | 0 |
MTG = middle temporal gyrus; STG = superior temporal gyrus.
Given the strong association between impaired affective empathy and infarct in right temporal pole, we divided temporal pole into superior temporal gyrus pole and middle temporal gyrus pole, to identify the area most closely associated with impaired affective empathy.
Of the 14 patients with impaired affective empathy, nine (64.3%) had lesions to one or more of the regions of interest we identified on the basis of the functional neuroimaging literature as engaged in affective empathy. The remaining five patients had lesions in the right thalamus (three patients) or right posterior superior frontal gyrus and middle frontal gyrus, posterior segment (two patients). One patient with a posterior superior and middle frontal gyrus lesion also had a lesion in the right precuneus. These are all areas that have shown activation in one or more of the functional imaging studies of affective empathy reviewed above.
There was a very strong association between the presence of a lesion in one or more region of interest in the network hypothesized to underlie affective empathy and impairments on our tasks of affective empathy (Fisher’s exact = 0.004). Figure 2 shows examples of patients with impaired performance on the affective empathy task and lesions in each of the regions of interest. The strongest association (and only significant) associations were between impaired affective empathy and infarct in the temporal pole (Fisher’s exact = 0.04) or in the anterior insula (Fisher’s exact = 0.03). When we further divided the temporal pole into the pole of superior and middle temporal gyri, the strongest association was between impaired affective empathy and infarct of the superior temporal gyrus pole (Fisher’s exact = 0.003). There were seven patients with both superior temporal gyrus pole and anterior insular lesions who had impaired affective empathy. For all 27 stroke patients, there was also a significant correlation between error rate on the affective empathy task and the volume of infarct in (i) temporal pole (r = 0.48; P = 0.014); (ii) superior temporal gyrus pole alone (r = 0.49; P = 0.013); and (iii) anterior insula (r = 0.40; P = 0.047). There were only a few patients with>1% damage to right prefrontal cortex (n = 3), anterior cingulate (n = 2), and amygdala (n = 4). Importantly, however, there were no patients with lesions to >1% of any of these three regions of interest or the temporal pole who had spared affective empathy on our test. Some lesions involved more than one of the regions of interest (Table 1).
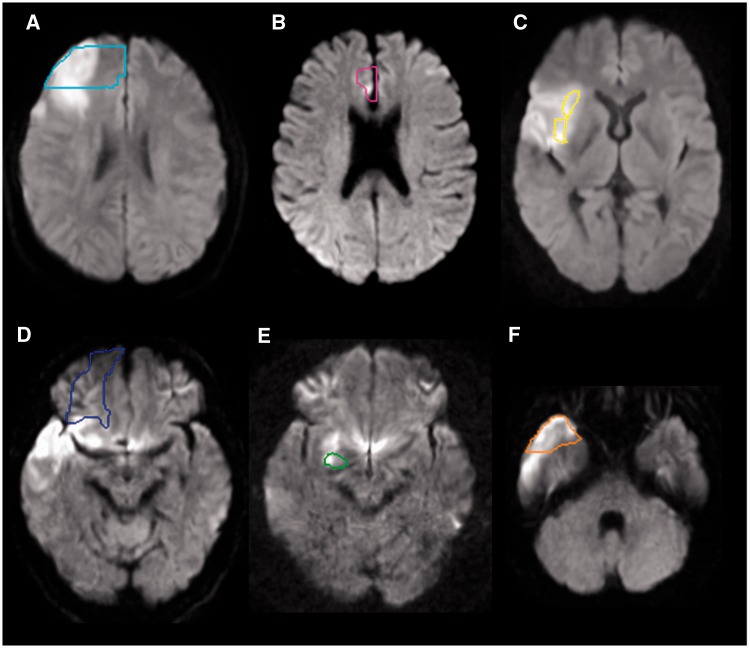
Figure 2.
Representative individuals with acute infarction in the prefrontal cortex (A, cyan contour), anterior cingulate cortex (B, pink contour), anterior insular cortex (C, yellow contour), orbitofrontal cortex (D, blue contour), amygdala (E, green contour) and the temporal pole (F, orange contour). Diffusion weighted images were normalized to the JHU-MNI atlas space and predefined regions of interest were overlaid on the normalized images. Images are all in radiological convention.
As expected, there was no association between impaired affective empathy and a unilateral lesion involving the temporal parietal junction. There were three patients with impaired affective empathy whose lesions included the temporal parietal junction, but also three patients with spared affective empathy whose lesions included that area.
Performance on affective empathy and other cognitive measures in patients with lesions in the hypothesized network supporting affective empathy versus lesions outside the network
Our second goal was to evaluate differences between stroke patients with lesions in the proposed ‘affective empathy network’ and patients with lesions outside this network, with regard to score on our affective empathy test, tests of affective prosody, tests of hemispatial neglect, as well as age and volume of infarct. To accomplish this goal, we carried out a very small case-control study including nine patients with lesions in the regions of interest hypothesized to underlie affective empathy, compared with nine patients with stroke, but not involving any of these six regions of interest, matched relatively closely for lesion volume, age and sex. To identify these patients, we excluded all patients with lesions >100 cm3 and <1.45 cm3. This exclusion yielded patients with the characteristics in Table 2. We compared the two groups using Mann-Whitney tests (because these data were not normally distributed).
Table 2.
Characteristics of patients with and without lesions in the ‘Affective Empathy Network’ (right prefrontal cortex, orbitofrontal gyrus, temporal pole, anterior insula, anterior cingulate, amygdala), excluding those with lesions >100 cm3 and <1.45 cm3
| Patients with empathy network lesions |
Patients without network lesions |
Z | Exact significance | |||
|---|---|---|---|---|---|---|
| Median | Mean ± SD | Median | Mean ± SD | |||
| Empathy error rate | 30% | 34.4 ± 27 | 0% | 8.8 ± 17 | −2.5 | 0.01 |
| Prosody error rate | 60% | 51.3 ± 18 | 36.5% | 37.2 ± 6 | −1.7 | 0.11 |
| Neglect: gap detection error rate | 0% | 0.82 ± 2.3 | 0% | 0.91 ± 2.0 | −0.18 | 0.89 |
| Line bisection deviation (in % of) | 2.2% | 5.5 ± 11.2 | 2.7% | 3.1 ± 1.6 | −1.12 | 0.27 |
| Scene copy error rate | 5.6% | 15.9 ± 28.0 | 2.9% | 8.7 ± 13.4 | −0.98 | 0.33 |
| Volume of infarct in cm3 | 30.6 | 26.6 ± 15.9 | 15.0 | 16.6 ± 16.1 | −1.2 | 0.22 |
| Age | 52 | 51.3 ± 13 | 49 | 50.7 ± 13 | −0.088 | 0.93 |
| % Female | 44% | 33% | 1.00 | |||
Patients who had lesions in the proposed ‘affective empathy network’ (prefrontal cortex, orbitofrontal gyrus, anterior cingulate cortex, anterior insula, temporal pole, and/or amygdala) made significantly more errors on the affective empathy task than patients without lesions to the network (median = 30% versus 0%; Z = −2.5; P = 0.01). There were no significant differences between patients with and without lesions in the ‘affective empathy network’ with regard to lesion volume, age, neglect or prosody scores.
Although patients were administered the Interpersonal Reactivity Index, which has been shown to be a reliably measure of cognitive and affective empathy in neurologically normal individuals and some patient populations (Rankin et al., 2005), we could not use it to detect deficits in empathy in our population. Only one participant had a score that was >1.5 SD below normal on the perspective-taking subscore; and this participant was a transient ischaemic attack (neurologically normal) control subject. Likewise, only one participant (an acute stroke patient) showed performance on the empathetic concern subscore that was >1.5 SD below the mean for normal controls.
We also did not use the scores for emotional contagion based on observations of facial expressions during the videotapes, because nearly all patients were scored as ‘happy’ (given choices of happy, jealous, scared, bored, excited or relieved). The patient testers found it difficult to assess the participants’ emotions while watching the videos. Most patients did not show outward signs of emotion, such as change in facial expression or make spontaneous comments about their feelings. They were scored as happy if they were pleasant and judged to be happy to continue the experiment.
Differences between patients with impaired affective empathy versus patients with spared affective empathy
Our final goal was to identify any significant differences between patients with impaired affective empathy and patients with spared affective empathy, in terms of scores on prosody comprehension, volume of infarct, age and scores on neglect. We compared all stroke patients with impaired performance on the affective empathy task (≥30% errors; n = 14) to those with normal performance on the affective empathy task (≤20% errors; n = 13) using Mann-Whitney tests.
A subset of 25 of the patients completed the prosody comprehension testing. Patients with deficits in affective empathy were significantly more impaired in comprehension of affective prosody compared to patients with normal performance on the affective empathy task (overall median error rate on prosody comprehension: 61% versus 40.3% errors; Z = −2.6; P = .009) (see Table 3 for mean and standard deviations). All patients with impaired affective empathy had impaired affective prosody comprehension; however, there were 13 patients with impaired affective prosody comprehension who had normal performance on the affective empathy task.
Table 3.
Characteristics of patients with and without impairments in affective empathy
| Patients with impaired affective empathy |
Patients with intact affective empathy |
Z | Exact Significance | |||
|---|---|---|---|---|---|---|
| Median | Mean ± SD | Median | Mean ± SD | |||
| Prosody error rate | 65% | 61.4 ± 12 | 37% | 38.0 ± 14 | −2.5 | 0.008 |
| Neglect: gap detection error rate | 0% | 0.4 ± 1.0 | 0% | 1.1 ± 2.4 | −0.02 | 1 |
| Line bisection deviation (in % of line neglected) | 2.6% | 4.8 ± 9.4 | 2.7% | 3.1 ± 1.7 | −0.47 | 0.65 |
| Scene copy error rate | 4.2% | 11.3 ± 23.9 | 5.5% | 10.1 ± 14.5 | −0.06 | 1 |
| Volume of infarct in cm3 | 9.8 | 26.5 ± 45.8 | 16.5 | 33.6 ± 58.1 | −0.82 | 0.43 |
| Age | 61 | 60.8 ± 11.4 | 46 | 48.6 ± 13.8 | −2.3 | 0.02 |
| % Female | 29% | 38% | 1 | |||
In contrast, performance of patients with impaired affective empathy was not significantly different from that of patients with normal affective empathy on tests of hemispatial neglect (Table 3). Those with impaired affective empathy were older (median 60.8 versus 49.1 years; Z = −2.3; P = 0.02); they were no more likely to be male (71% versus 62%; not significant).
Discussion
Previous functional neuroimaging studies have consistently shown that particular areas of the brain are engaged during tasks that involve recognizing and making inferences about how another person feels, sometimes called affective empathy. However, lesion studies have produced more inconsistent results, only some of which indicate that the areas that show activation during affective empathy are also required for this function. One limitation of previous studies is that patients have been heterogeneous in aetiology and time post-onset of lesion. Some lesions, such as meningiomas and other slow growing tumours, often fail to produce deficits, even when they arise in areas of the brain where a stroke or other sudden onset lesion would typically cause a specific deficit. Furthermore, when patients are studied a long time after stroke or other focal lesion, they may have originally had the deficit associated with the lesioned area, but may have recovered due to rehabilitation or spontaneous recovery, as other areas of the brain can assume the functions of the damaged ones. The few previous lesion studies of empathy have included patients with slow growing tumours or patients who are a long time post onset of stroke or other focal injury. We tried to correct for these particular limitations of previous studies by studying patients immediately after onset of acute stroke, before the opportunity for reorganization, recovery or rehabilitation.
We were able to confirm that lesions within the hypothesized network were associated with an error rate on an affective empathy task that is outside the error rate made by neurological normal controls. We also showed, in a small case-control study that patients with lesions involving the network had higher error rates on the affective empathy task than a fairly well-matched group of patients with similar sized lesions that do not involve the network.
The most commonly affected areas in the patients with impaired affective empathy in this study were the anterior insula and the temporal pole (specifically the pole of the superior temporal gyrus). The anterior insula is an area that is commonly affected in acute middle cerebral artery stroke (Finley et al., 2003). Nevertheless, we found that those who had impaired affective empathy were more likely to have lesions in the anterior insula than those who did not have impaired affective empathy. The temporal pole is not an area commonly involved in stroke (Caviness et al., 2002). Yet, 50% of the patients with impaired affective empathy had lesions in temporal pole, and no patients with lesions in temporal pole had normal performance on the affective empathy task. In both areas, percentage of damage correlated with error rate on the affective empathy task. All patients with temporal pole lesions had anterior insular lesions, raising the possibility that damage to only one or the other may be associated with affective empathy. However, there are converging data from other sources for a role of each of these areas in affective empathy. As reviewed above, the anterior insula nearly always shows activation in association with affective empathy tasks in functional imaging studies (Bzdok et al., 2012). The temporal pole is an area where it has been more difficult to reveal activation in functional imaging studies, but recent studies have shown activation in association with affective empathy (Jimura et al., 2010). Furthermore, both right anterior insula and right temporal pole atrophy have been associated with impaired affective empathy in neurodegenerative disease (Rankin et al., 2005, 2006; Kipps and Hodges, 2006; Narvid et al., 2009; Lee et al., 2012). These areas were not the only critical components of the network; other areas included right prefrontal and fronto-orbito cortex, anterior cingulate and amygdala.
All but five patients with significant impairments in affective empathy had lesions in one or more of the regions of interest in the proposed network. Two of these patients had small lesions in part of the right posterior superior frontal gyrus and middle frontal gyrus, close to ventromedial prefrontal cortex (ventral superior frontal gyrus). It is possible that a larger part of the frontal cortex, rather than just ventromedial prefrontal cortex, as proposed by some authors, is critical for affective empathy.
The three remaining patients with impaired affective empathy had right thalamic infarcts. The thalamus has an important role in relaying sensory and multimodal information to the prefrontal cortex for further processing. It is reasonable to assume that it may play an important role in perception of auditory and visual cues about another person’s emotions, and relaying this information to the cortex to enable inferences to be drawn. Four out of nine patients with right thalamic infarcts had impairments in affective empathy. The thalamus consists of several nuclei with quite distinct functions, so whether or not affective empathy was disrupted may have depended on the nuclei involved. However, the resolution of our imaging was not adequate to determine which nuclei were included in the infarct. Of note, consistent with our hypothesis that the thalamus may be important in affective empathy, the activation likelihood estimation meta-analysis of 112 functional MRI studies of affective empathy revealed the right posterior thalamus and left anterior thalamus among the areas commonly activated in association with empathy (Bzdok et al., 2012).
The temporoparietal junction is an area that consistently is engaged in ‘mentalizing’ or cognitive perspective-taking, although it does not appear to be specific to empathy (Decety and Lamm, 2007; Schnell et al., 2011; Sebastian et al., 2012). Although one study has indicated that a right parietal lesion might cause impairment in empathy (Shamay-Tsoory et al., 2003), most studies have raised the possibility that either temporoparietal junction alone might be adequate to handle the role in perspective-taking, which may include attributing a belief to another person (Shamay-Tsoory et al., 2003). In this case, a unilateral temporoparietal junction would not be expected to substantially impair empathy. Consistent with this view, in our study, lesions in this area alone were not associated with impaired affective empathy.
Not surprisingly, patients with impaired affective empathy also had significantly higher error rates in the closely related task of recognizing the emotion in tone of voice. However, we do not believe that impaired recognition of prosody was responsible for the deficit in affective empathy, because recognition of emotion from tone of voice was not required to answer any of the questions correctly. Furthermore, a patient with severely impaired recognition and production of prosody performed this task accurately (and scored normally on the Interpersonal Reactivity Index) (Dara et al., 2012). Furthermore, in this study, 13 patients had impaired prosody comprehension, but normal performance on the empathy task. Rather, we believe that some of the areas of the brain necessary for affective empathy may also be necessary for comprehension of affective prosody.
Finally, we found that impairments in affective empathy appear to be independent of impairments in spatial attention (neglect) after right hemisphere stroke, indicating that it is not likely to simply be a marker of ‘severe’ right cortical stroke.
One limitation of the study is that we were not able to demonstrate a dissociation between perspective-taking and emotional contagion; that is, we could not distinguish between neurologically normal participants and stroke patients in emotional contagion, either using the self-administered Interpersonal Reactivity Index or technicians’ observations of facial expressions, gestures, and tone of voice during videos and stories. Acute right hemisphere stroke patients are known to have other deficits that interfere with expression of emotional concern (e.g. impaired expression of emotion through prosody; Ross et al., 1997) and self-assessment of deficits (anosognosia, perhaps including changes in emotional contagion compared to baseline). Therefore, if they have impaired emotional contagion, we were unable to detect it using the Interpersonal Reactivity Index or observation of facial expression/gestures. Our task of affective empathy may have conflated emotional contagion and affective perspective-taking, because the questions about videos may have been answered in part by recognizing and sharing in the emotions of another person. Future studies may use the caregiver assessments of emotional contagion as well as perspective-taking, as has been done for patients with frontotemporal dementia, who also have limited insight (Rankin et al., 2006; Eslinger et al., 2011). Like our stroke patients, patients with frontotemporal dementia showed no difference from controls on their self-assessment of empathy, although caregivers rated them as having impaired perspective-taking and empathetic concern at the same time (Eslinger et al., 2011). Alternatively, emotional contagion can be assessed with autonomic responses, such as skin conductance response and heart rate changes, when presented with emotional scenes or stories (Balconi and Bortolotti, 2012).
Another limitation is that we did not assess patients with left hemisphere stroke in this study. Previous studies of dementia (Rankin et al., 2006; Eslinger et al., 2011) and focal lesions (Shamay-Tsoory et al., 2003, 2005) have indicated that right hemisphere lesions are more likely than left hemisphere lesions to cause deficits in empathy. However, one study of penetrating traumatic brain injuries found that lesions involving left but not right ventromedial frontal cortex affected performance on affective theory of mind tasks (Leopold et al., 2012). One might argue that penetrating traumatic brain injuries, although they have a focal component, also have a more diffuse and bilateral impact that may have affected performance. Nevertheless, the role of the left medial prefrontal cortex deserves further investigation.
Despite its limitations, our study is a relatively large study of the effect of acute focal ischaemic lesions in carefully selected regions of interest on affective empathy. This study provides converging evidence that areas identified as engaged in the task are indeed necessary for the task. Furthermore, the study indicates that one or more nucleus of the right thalamus also likely plays an important role in this network.
Acknowledgements
We are grateful to the patients who participated in this study.
Funding
This work was supported by: National Institute of Neurological Disorders and Stroke, RO1NS47691 (to A.E.H.), and The Yousem Family Research Fund and NICHD R01 HD065955 (to K.O.).
References
- Balconi M, Bortolotti A. Empathy in cooperative versus non-cooperative situations: the contribution of self-report measures and autonomic responses. Appl Psychophysiol Biofeedback. 2012;37:161–9. doi: 10.1007/s10484-012-9188-z. [DOI] [PubMed] [Google Scholar]
- Bodini B, Iacoboni M, Lenzi GL. Acute stroke effects on emotions: an interpretation through the mirror system. Curr Opin Neurol. 2004;17:55–60. doi: 10.1097/00019052-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Brune M, Brune-Cohrs U. Theory of mind—evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev. 2006;30:437–55. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Munte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;1499:29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–96. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V, Makris N, Montinaro E, Sahin N, Bates J, Schwamm L, et al. Anatomy of stroke, Part I: An MRI-based topographic and volumetric system of analysis. Stroke. 2002;33:2549–56. doi: 10.1161/01.str.0000036083.90045.08. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto B, Sedeno L, Sposato LA, Sigman M, Riccio PM, Salles A, et al. Insular networks for emotional processing and social cognition: comparison of two case reports with either cortical or subcortical involvement. Cortex. 2013;49:1420–34. doi: 10.1016/j.cortex.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di MA, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2012;7:727–37. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dara C, Kirsch-Darrow L, Ochfeld E, Slenz J, Agranovich A, Vasconcellos-Faria A, et al. Impaired emotion processing from vocal and facial cues in frontotemporal dementia compared to right hemisphere stroke. Neurocase. 2012 doi: 10.1080/13554794.2012.701641. Advance Access published on July 25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–26. [Google Scholar]
- de Waal FB. The antiquity of empathy. Science. 2012;336:874–6. doi: 10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: a new cognitive neuroscience view on psychological identification. Conscious Cogn. 2003;12:577–96. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–93. [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the multifaceted empathy test (MET) J Autism Dev Disord. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Neurological and neuropsychological bases of empathy. Eur Neurol. 1998;39:193–9. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2005;76:1630–5. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsych Clin Neurosci. 2011;23:74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17:850–8. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Finley A, Saver J, Alger J, Pregenzer M, Leary M, Ovbiagele B. Diffusion weighted imaging assessment of insular vulnerability in acute middle cerebral artery infarctions [abstract] Stroke. 2003;34:259. discussion. [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–35. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Asari T, Miyashita Y. Temporal pole activity during understanding other persons' mental states correlates with neuroticism trait. Brain Res. 2010;1328:104–12. doi: 10.1016/j.brainres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR. Theory of mind in frontotemporal dementia. Soc Neurosci. 2006;1:235–44. doi: 10.1080/17470910600989847. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lee SE, Seeley WW, Poorzand P, Rademakers R, Karydas A, Stanley CM, et al. Clinical characterization of bvFTD due to FUS neuropathology. Neurocase. 2012;18:305–17. doi: 10.1080/13554794.2011.604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41:2297–304. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A, Krueger F, Dal Monte O, Pardini M, Pulaski SJ, Solomon J, et al. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Soc Cogn Affect Neurosci. 2012;7:871–80. doi: 10.1093/scan/nsr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Flanagan S. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability and social communication. Neuropsychology. 2004;18:572–9. doi: 10.1037/0894-4105.18.3.572. [DOI] [PubMed] [Google Scholar]
- Narvid J, Gorno-Tempini ML, Slavotinek A, Dearmond SJ, Cha YH, Miller BL. Of brain and bone: the unusual case of Dr A. Neurocase. 2009;15:190–205. doi: 10.1080/13554790802632967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Zupan B, Babbage DR, Radnovich AJ, Tomita M, Hammond F, et al. Affect recognition, empathy and dysosmia after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1414–20. doi: 10.1016/j.apmr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage. 2009;46:486–99. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Fujii T, Suzuki K, Fukatsu R, Yamadori A. Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology. 2001;57:2064–9. doi: 10.1212/wnl.57.11.2064. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(Pt 11):2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Roldan GE, Cerquetti D, Tenca E, Leiguarda R. The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase. 2011;17:270–5. doi: 10.1080/13554791003730618. [DOI] [PubMed] [Google Scholar]
- Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104:51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ross ED, Thompson RD, Yenkosky J. Lateralization of affective prosody in brain and the callosal integration of hemispheric language functions. Brain Lang. 1997;56:27–54. doi: 10.1006/brln.1997.1731. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else's belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Humphreys GW. Error analyses reveal contrasting deficits in ‘theory of mind’: neuropsychological evidence from a 3-option false belief task. Neuropsychologia. 2007;45:2561–9. doi: 10.1016/j.neuropsychologia.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–11. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in ‘theory of mind’. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54:1743–54. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Fontaine NM, Bird G, Blakemore SJ, Brito SA, McCrory EJ, et al. Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Soc Cogn Affect Neurosci. 2012;7:53–63. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054–67. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90:274–83. doi: 10.1016/j.schres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired ‘affective theory of mind’ is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41:209–20. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Stotland E, Dunn RE. Empathy, self-esteem and birth order. J Abnorm Soc Psychol. 1963;66:532–40. doi: 10.1037/h0042891. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Anderson V. The frontal lobes and theory of mind: developmental concepts from adult focal lesion research. Brain Cogn. 2004;55:69–83. doi: 10.1016/S0278-2626(03)00271-9. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–45. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]