Abstract
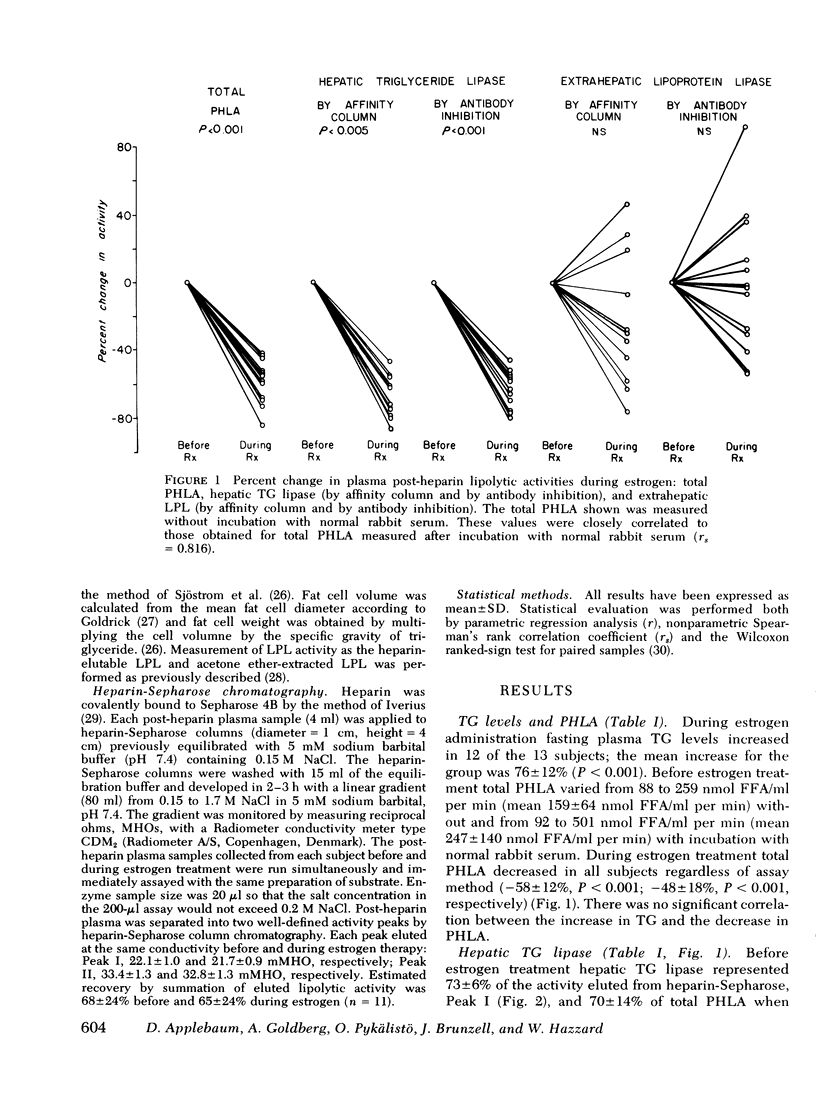
The rise in plasma triglyceride (TG) levels associated with estrogen administration has been thought to arise from impaired clearance because of the uniform suppression of post-heparin lipolytic activity (PHLA). Recently PHLA has been shown to consist of two activities: hepatic TG lipase and extrahepatic lipoprotein lipase (LPL). To determine whether estrogen might induce a selective decline in one of these activities, both hepatic TG lipase and extrahepatic LPL were measured in post-heparin plasma from 13 normal women before and after 2 wk of treatment with ethinyl estradiol (1 mug/kg per day). Hepatic TG lipase and extrahepatic LPL were determined by two techniques: (a) separation by heparin-Sepharose column chromatography, and (b) selective inhibition with specific antibodies to post-heparin hepatic TG lipase and milk LPL. Estrogen uniformly depressed hepatic TG lipase as measured by affinity column (-68 +/- 12%, mean +/- SD, P less than 0.001) or antibody inhibition (-63 +/- 11%, P less than 0.001). Extrahepatic LPL was not significantly changed by affinity column (-22 +/- 40%) or antibody inhibition (-3 +/- 42%). Direct measurement of adipose tissue LPL from buttock fat biopsies also showed no systematic change in the activated form of LPL measured as heparin-elutable LPL (+64 +/- 164%) or in the tissue form of LPL measured in extracts of acetone-ether powders (+21 +/- 77%). The change in hepatic TG lipase correlated with the change in PHLA (r = 0.969, P less than 0.01). However, neither the change in PHLA nor hepatic TG lipase correlated with the increase in TG during estrogen. The decrease in PHLA during estrogen thus results from a selective decline in hepatic TG lipase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Brunzell J. D., Schrott H. G. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. Trans Assoc Am Physicians. 1973;86:245–254. [PubMed] [Google Scholar]
- Davidoff F., Tishler S., Rosoff C. Marked hyperlipidemia and pancreatitis associated with oral contraceptive therapy. N Engl J Med. 1973 Sep 13;289(11):552–555. doi: 10.1056/NEJM197309132891103. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Huttunen J. K., Kinnunen P. J., Miettinen T. A., Nikkilä E. A. Effect of oxandrolone treatment on the activity of lipoprotein lipase, hepatic lipase and phospholipase A1 of human postheparin plasma. N Engl J Med. 1975 Jun 19;292(25):1314–1317. doi: 10.1056/NEJM197506192922503. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K., DAVIS L. L. LIPOLYTIC ACTIVITY OF POST-HEPARIN PLASMA IN HYPERGLYCERIDEMIA. J Lipid Res. 1963 Jan;4:24–33. [PubMed] [Google Scholar]
- Fielding C. J. Human lipoprotein lipase. I. Purification and substrate specificity. Biochim Biophys Acta. 1970 Apr 22;206(1):109–117. doi: 10.1016/0005-2744(70)90087-2. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Fallat R. W., Scheel D. Effects of estrogenic compounds on triglyceride kinetics. Metabolism. 1975 Apr;24(4):537–545. doi: 10.1016/0026-0495(75)90078-5. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Gartside P., Fallat R. W., Mendoza S. Effect of sex hormones on protamine inactivated and resistant postheparin plasma lipases. Metabolism. 1976 Jun;25(6):625–632. doi: 10.1016/0026-0495(76)90059-7. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Scheel D., Fishback J., Steiner P. Estrogen-induced pancreatitis in patients with previously covert familial type V hyperlipoproteinemia. Metabolism. 1972 Jul;21(7):657–666. doi: 10.1016/0026-0495(72)90089-3. [DOI] [PubMed] [Google Scholar]
- Goldrick R. B. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol. 1967 Apr;212(4):777–782. doi: 10.1152/ajplegacy.1967.212.4.777. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., GOLDRICK R. B. SERIAL STUDIES ON THE METABOLISM OF HUMAN ADIPOSE TISSUE. I. LIPOGENESIS AND FREE FATTY ACID UPTAKE AND RELEASE IN SMALL ASPIRATED SAMPLES OF SUBCUTANEOUS FAT. J Clin Invest. 1964 Sep;43:1776–1792. doi: 10.1172/JCI105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh M., Hamosh P. The effect of estrogen on the lipoprotein lipase activity of rat adipose tissue. J Clin Invest. 1975 May;55(5):1132–1135. doi: 10.1172/JCI108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzard W. R., Notter D. T., Spiger M. J., Bierman E. L. Oral contraceptives and triglyceride transport: acquired heparin resistance as the mechanism for impaired post-heparin lipolytic activity. J Clin Endocrinol Metab. 1972 Sep;35(3):425–437. doi: 10.1210/jcem-35-3-425. [DOI] [PubMed] [Google Scholar]
- Hazzard W. R., Spiger M. J., Bagdade J. D., Bierman E. L. Studies on the mechanism of increased plasma triglyceride levels induced by oral contraceptives. N Engl J Med. 1969 Feb 27;280(9):471–474. doi: 10.1056/NEJM196902272800904. [DOI] [PubMed] [Google Scholar]
- Hernell O., Egelrud T., Olivecrona T. Serum-stimulated lipases (lipoprotein lipases). Immunological crossreaction between the bovine and the human enzymes. Biochim Biophys Acta. 1975 Feb 13;381(2):233–241. [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kinnunen P. K., Nikkilä E. A. An immunochemical method for the selective measurement of two triglyceride lipases in human postheparin plasma. Clin Chim Acta. 1975 Sep 16;63(3):335–347. doi: 10.1016/0009-8981(75)90055-8. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekki M., Nikkilä E. A. Plasma triglyceride turnover during use of oral contraceptives. Metabolism. 1971 Sep;20(9):878–889. doi: 10.1016/0026-0495(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kalkhoff R. K. Sex steroid influence on triglyceride metabolism. J Clin Invest. 1975 Oct;56(4):888–896. doi: 10.1172/JCI108168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973 May;5(3):184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- Krauss R. M., Levy R. I., Fredrickson D. S. Selective measurement of two lipase activities in postheparin plasma from normal subjects and patients with hyperlipoproteinemia. J Clin Invest. 1974 Nov;54(5):1107–1124. doi: 10.1172/JCI107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Windmueller H. G., Fredrickson D. S. Comparison of the triglyceride lipase of liver, adipose tissue, and postheparin plasma. J Lipid Res. 1972 May;13(3):356–363. [PubMed] [Google Scholar]
- Persson B. Lipoprotein lipase activity of human adipose tissue in health and in some diseases with hyperlipidemia as a common feature. Acta Med Scand. 1973 May;193(5):457–462. doi: 10.1111/j.0954-6820.1973.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Pukalisto O. J., Smith P. H., Brunzell J. D. Human adipose tissue lipoprotein lipase: comparison of assay methods and expressions of activity. Proc Soc Exp Biol Med. 1975 Jan;148(1):297–300. doi: 10.3181/00379727-148-38526. [DOI] [PubMed] [Google Scholar]
- Pykälistö O., Goldberg A. P., Brunzell J. D. Reversal of decreased human adipose tissue lipoprotein lipase and hypertriglyceridemia after treatment of hypothyroidism. J Clin Endocrinol Metab. 1976 Sep;43(3):591–600. doi: 10.1210/jcem-43-3-591. [DOI] [PubMed] [Google Scholar]
- Rössner S., Larsson-Cohn U., Carlson L. A., Boberg J. Effects of an oral contraceptive agent on plasma lipids, plasma lipoproteins, the intravenous fat tolerance and the post-heparin lipoprotein lipase activity. Acta Med Scand. 1971 Oct;190(4):301–305. doi: 10.1111/j.0954-6820.1971.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Sjöström L., Björntorp P., Vrána J. Microscopic fat cell size measurements on frozen-cut adipose tissue in comparison with automatic determinations of osmium-fixed fat cells. J Lipid Res. 1971 Sep;12(5):521–530. [PubMed] [Google Scholar]
- Stewart J. E., Schotz M. C. Studies on release of lipoprotein lipase activity from fat cells. J Biol Chem. 1971 Sep 25;246(18):5749–5753. [PubMed] [Google Scholar]
- Wynn V., Mills G. L., Doar J. W., Stokes T. Fasting serum triglyceride, cholesterol, and lipoprotein levels during oral-contraceptive therapy. Lancet. 1969 Oct 11;2(7624):756–760. doi: 10.1016/s0140-6736(69)90476-0. [DOI] [PubMed] [Google Scholar]
- Zorrilla E., Hulse M., Hernandez A., Gershberg H. Severe endogenous hypertriglyceridemia during treatment with estrogen and oral contraceptives. J Clin Endocrinol Metab. 1968 Dec;28(12):1793–1796. doi: 10.1210/jcem-28-12-1793. [DOI] [PubMed] [Google Scholar]


