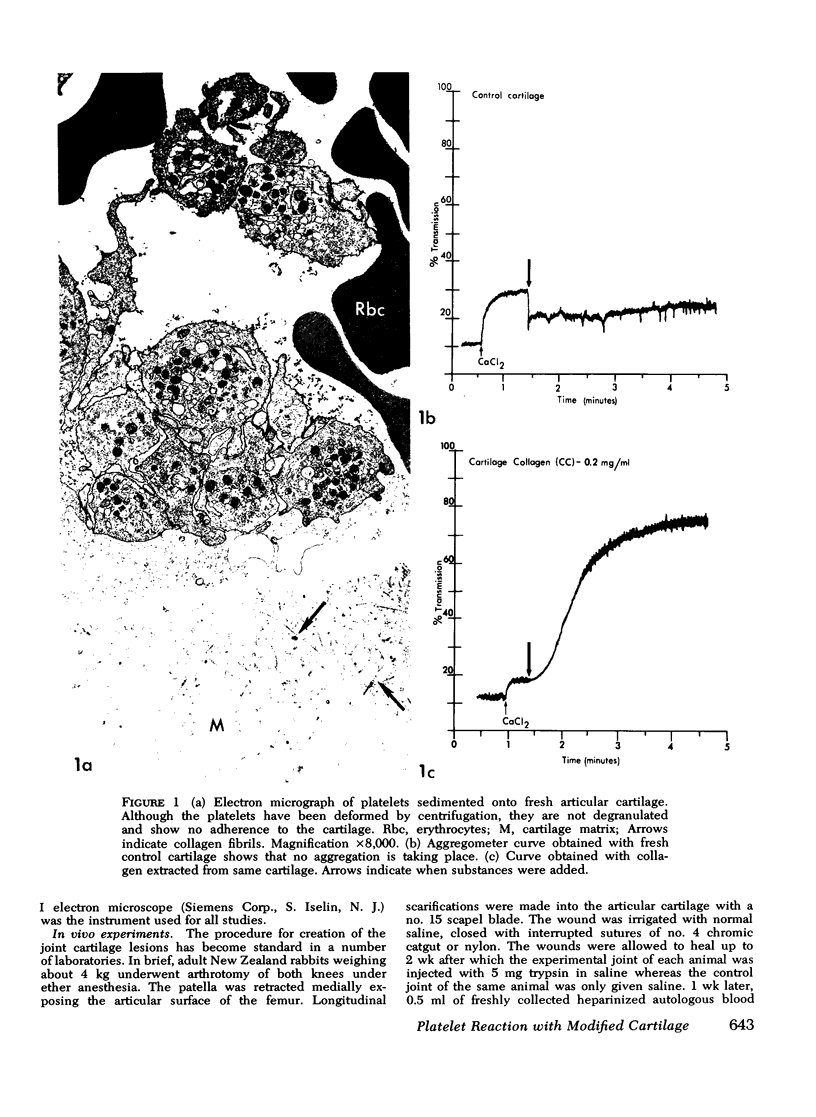
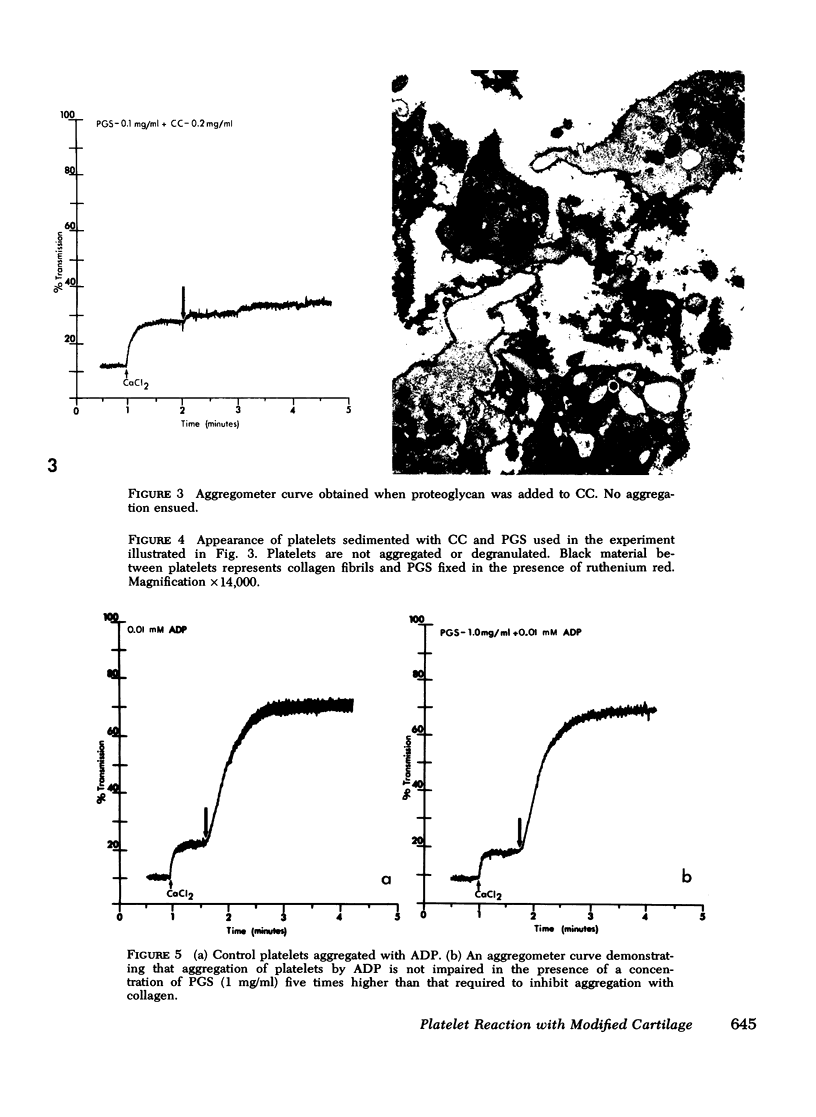
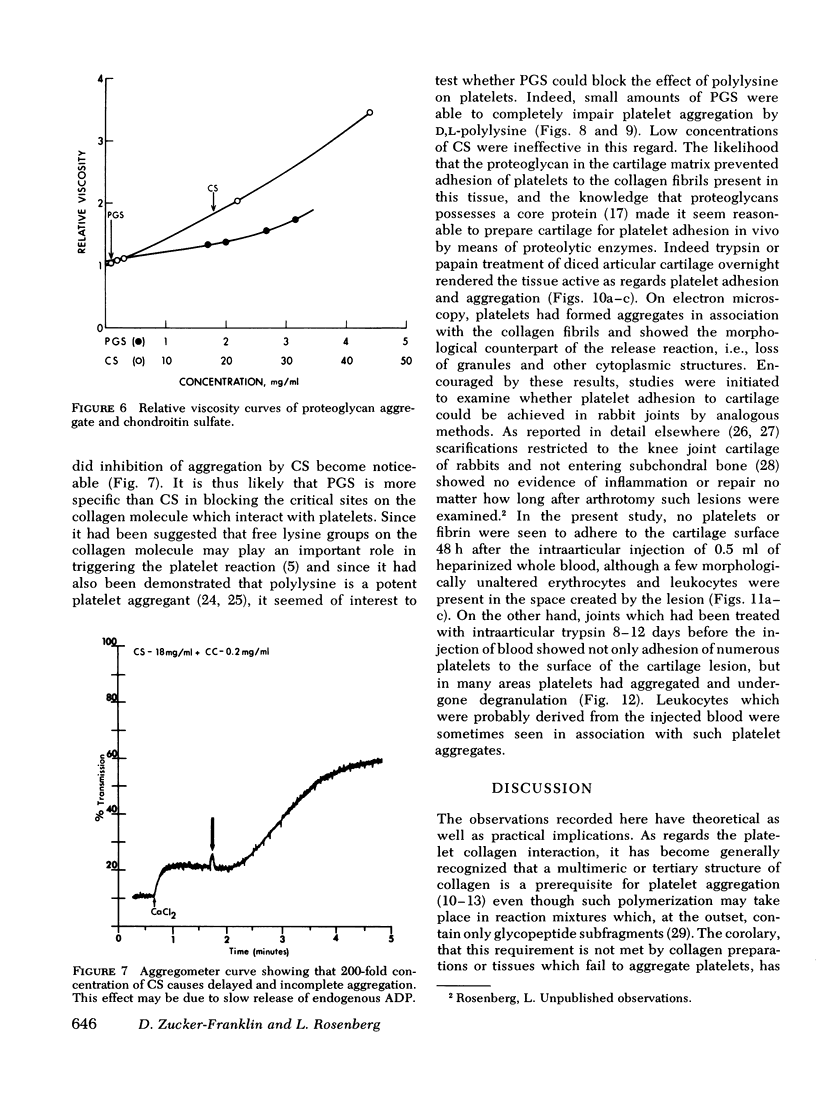
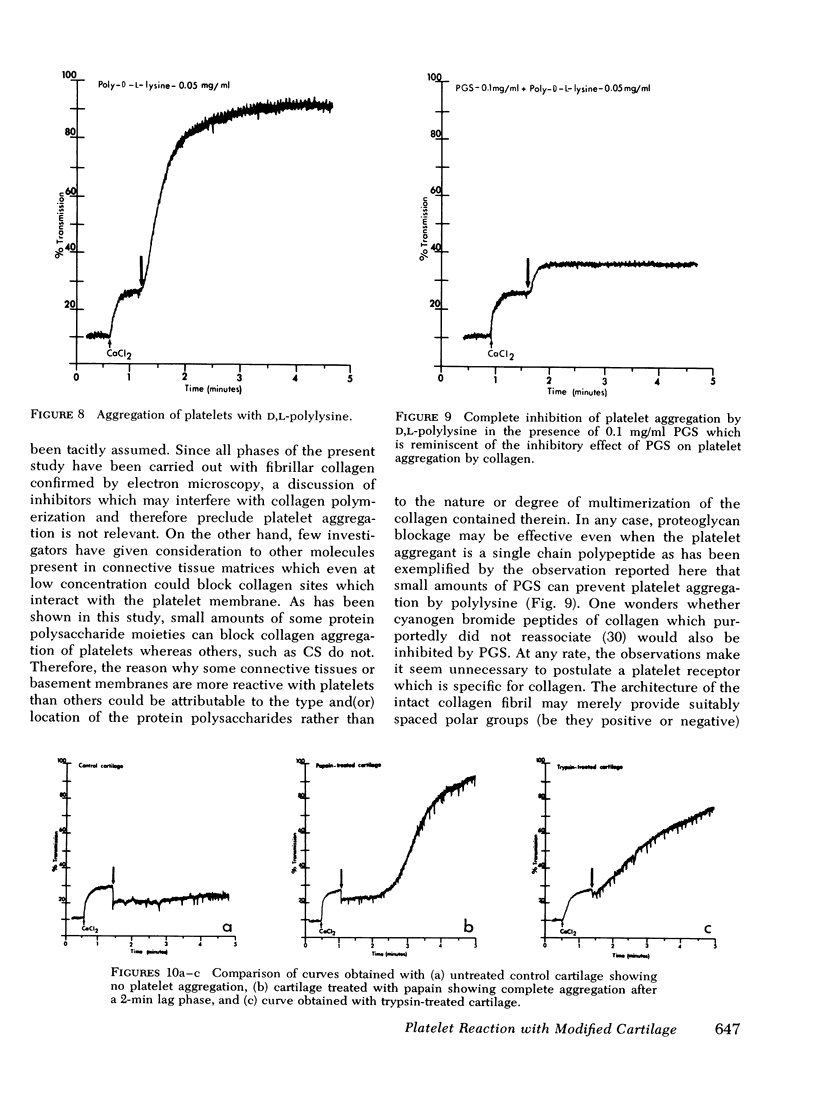
Abstract
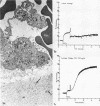
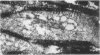
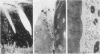
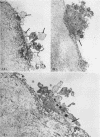
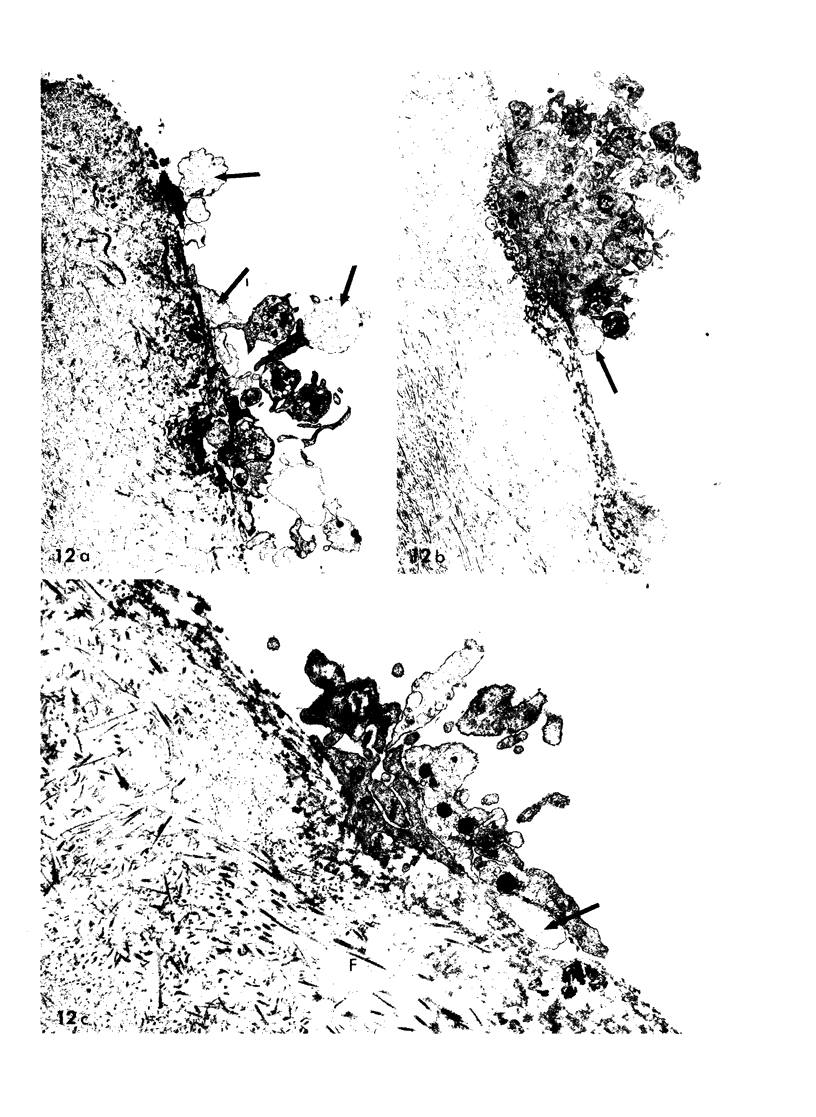
During studies concerned with the platelet-collagen interaction, it was observed that platelets did not adhere to bovine or human articular cartilage and that cartilage did not induce platelet aggregation in vivo or in vitro. To study the mechanism responsible for this observation, the role of proteoglycans was examined. Purified cartilage collagen proved to be fully active as a platelet aggregant. Addition of small amounts of proteoglycan subunit (PGS) blocked platelet aggregation, whereas chondroitin sulfate, a major glycosaminoglycan component of cartilage matrix, impaired platelet aggregation only at concentrations which resulted in a marked increase in viscosity. Moreover, PGS abolished aggregation of platelets by polylysine but did not prevent aggregation by ADP, suggesting that PGS may block strategically placed lysine sites on the collagen molecule. Treatment of fresh articular cartilage with proteolytic enzymes rendered the tissue active as a platelet aggregant. In vivo experiments demonstrated that surgical scarification of rabbit articular cartilage does not result in adhesion of autologous platelets. Treatment of rabbit knee joints with intraarticular trypsin 1 wk before the injection of blood resulted in adhesion and aggregation of platelets on the surface of the lesions. Since there is evidence from other studies that some degree of cartilage healing may take place after initiation of an inflammatory response, it is postulated that induction of platelet-cartilage interaction may eventuate in cartilage repair.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Platelet collagen adhesion characterization of collagen glucosyltransferase of plasma membranes of human blood platelets. Biochim Biophys Acta. 1971 Dec 21;252(3):533–545. doi: 10.1016/0304-4165(71)90156-5. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Bensusan H. B. The role of collagen quaternary structure in the platelet: collagen interaction. J Clin Invest. 1974 Dec;54(6):1480–1487. doi: 10.1172/JCI107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Critical role of the carbohydrate side chains of collagen in platelet aggregation. J Clin Invest. 1972 Oct;51(10):2693–2701. doi: 10.1172/JCI107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Beachey E. H., Kang A. H. Interaction of a chick skin collagen fragment (alpha1-CB5) with human platelets. Biochemical studies during the aggregation and release reaction. J Biol Chem. 1975 Sep 10;250(17):6916–6922. [PubMed] [Google Scholar]
- Fuller J. A., Ghadially F. N. Ultrastructural observations on surgically produced partial-thickness defects in articular cartilage. Clin Orthop Relat Res. 1972 Jul-Aug;86:193–205. doi: 10.1097/00003086-197207000-00031. [DOI] [PubMed] [Google Scholar]
- HUGUES J. [Binding of platelets to collagen]. C R Seances Soc Biol Fil. 1960;154:866–868. [PubMed] [Google Scholar]
- Hodge A. J., Schmitt F. O. THE CHARGE PROFILE OF THE TROPOCOLLAGEN MACROMOLECULE AND THE PACKING ARRANGEMENT IN NATIVE-TYPE COLLAGEN FIBRILS. Proc Natl Acad Sci U S A. 1960 Feb;46(2):186–197. doi: 10.1073/pnas.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe R., Deykin D. Evidence for a structural requirement for the aggregation of platelets by collagen. J Clin Invest. 1974 Mar;53(3):875–883. doi: 10.1172/JCI107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. H., Beachey E. H., Katzman R. L. Interaction of an active glycopeptide from chick skin collagen (alpha 1-CB5) with human platelets. J Biol Chem. 1974 Feb 25;249(4):1054–1059. [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Mankin H. J. The reaction of articular cartilage to injury and osteoarthritis (first of two parts). N Engl J Med. 1974 Dec 12;291(24):1285–1292. doi: 10.1056/NEJM197412122912406. [DOI] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. On the mechanism by which cell contact induces the release reaction of blood platelets; the effect of cationic polymers. Thromb Diath Haemorrh. 1972 Feb 29;27(1):121–133. [PubMed] [Google Scholar]
- Mitchell N., Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976 Mar;58(2):230–233. [PubMed] [Google Scholar]
- Mustard J. F. Hemostasis and thrombosis. Semin Hematol. 1968 Apr;5(2):91–106. [PubMed] [Google Scholar]
- Puett D., Wasserman B. K., Ford J. D., Cunningham L. W. Collagen-mediated platelet aggregation. Effects of collagen modification involving the protein and carbohydrate moieties. J Clin Invest. 1973 Oct;52(10):2495–2506. doi: 10.1172/JCI107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Pal S., Beale R. J. Proteoglycans from bovine proximal humeral articular cartilage. J Biol Chem. 1973 May 25;248(10):3681–3690. [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Schubert M. The proteinpolysaccharides of bovine nucleus pulposus. J Biol Chem. 1967 Oct 25;242(20):4691–4701. [PubMed] [Google Scholar]
- Ross R. Connective tissue cells, cell proliferation and synthesis of extracellular matrix-a review. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):247–259. doi: 10.1098/rstb.1975.0049. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Kübler W., Gross R. Induction of blood platelet aggregation by cationic polypeptides. Thromb Diath Haemorrh. 1968 Mar 31;19(1):307–307. [PubMed] [Google Scholar]
- Shepard N., Mitchell N. The localization of proteoglycan by light and electron microscopy using safranin O. A study of epiphyseal cartilage. J Ultrastruct Res. 1976 Mar;54(3):451–460. doi: 10.1016/s0022-5320(76)80029-9. [DOI] [PubMed] [Google Scholar]
- Simons E. R., Chesney C. M., Colamn R. W., Harper E., Samberg E. The effect of the conformation of collagen on its ability to aggregate platelets. Thromb Res. 1975 Jul;7(1):123–139. doi: 10.1016/0049-3848(75)90130-9. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., Procupez T. L. Aggregation of platelets by collagen: polar active sites of insoluble human collagen. Am J Physiol. 1971 Apr;220(4):1074–1079. doi: 10.1152/ajplegacy.1971.220.4.1074. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G. The actin and myosin filaments of human and bovine blood platelets. J Clin Invest. 1972 Feb;51(2):419–430. doi: 10.1172/JCI106828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. Microfibrils of blood platelets: their relationship TO MICROTUBULES AND THE CONTRACTILE PROTEIN. J Clin Invest. 1969 Jan;48(1):165–175. doi: 10.1172/JCI105965. [DOI] [PMC free article] [PubMed] [Google Scholar]