Abstract
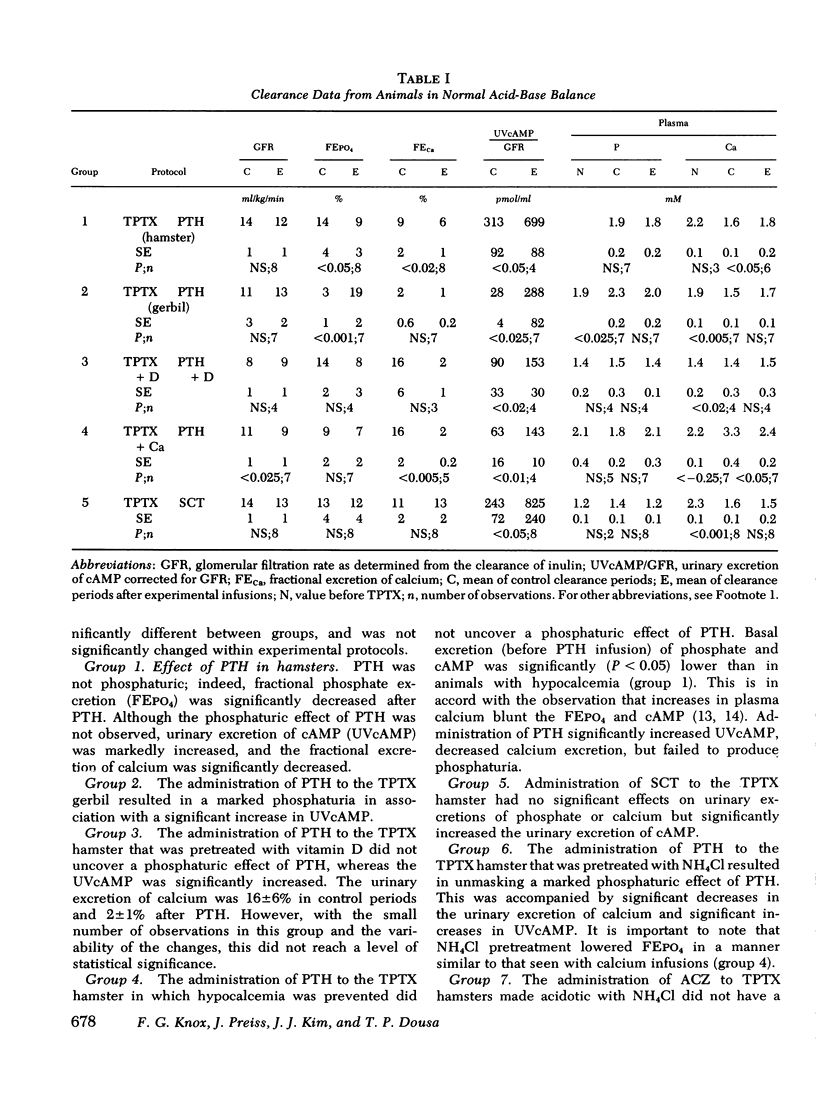
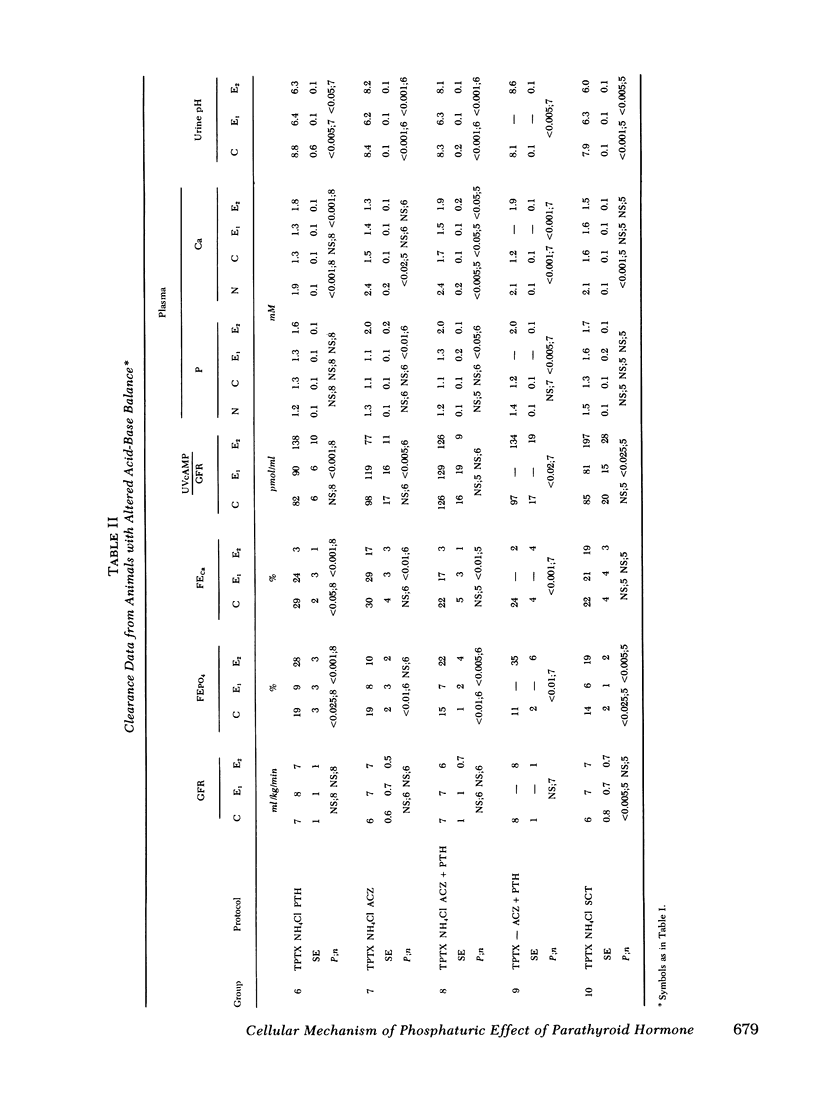
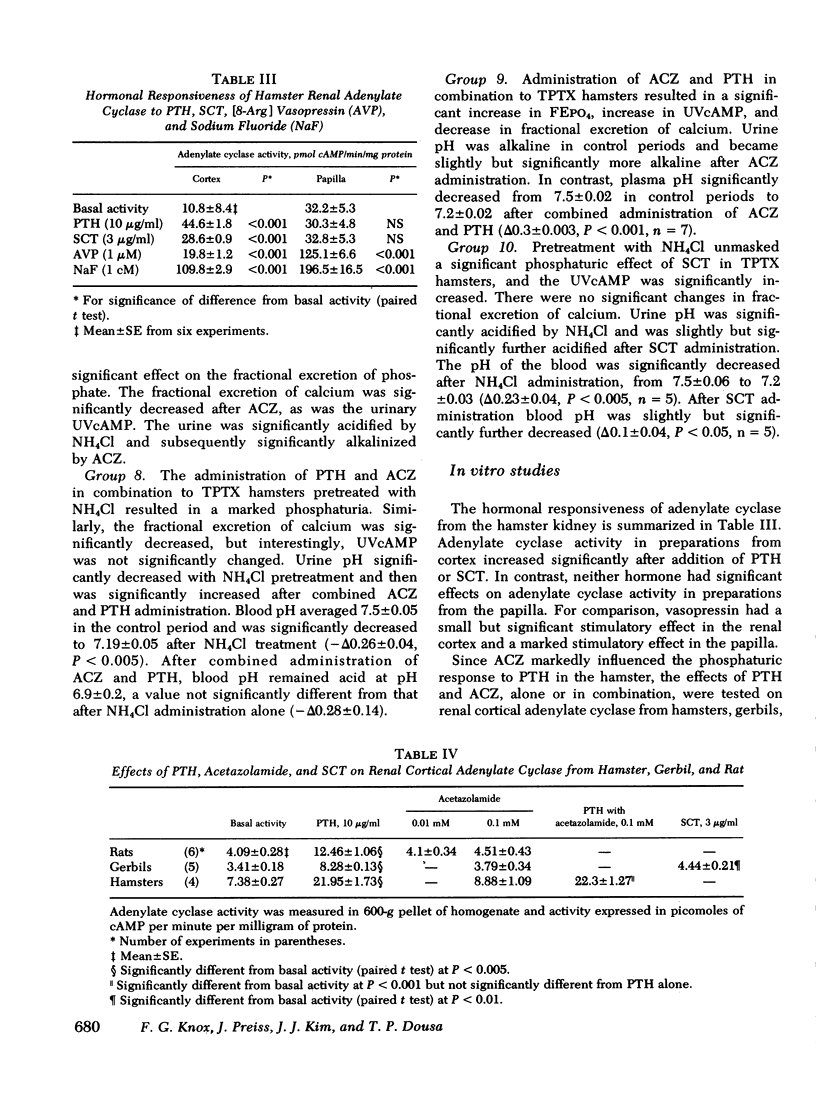
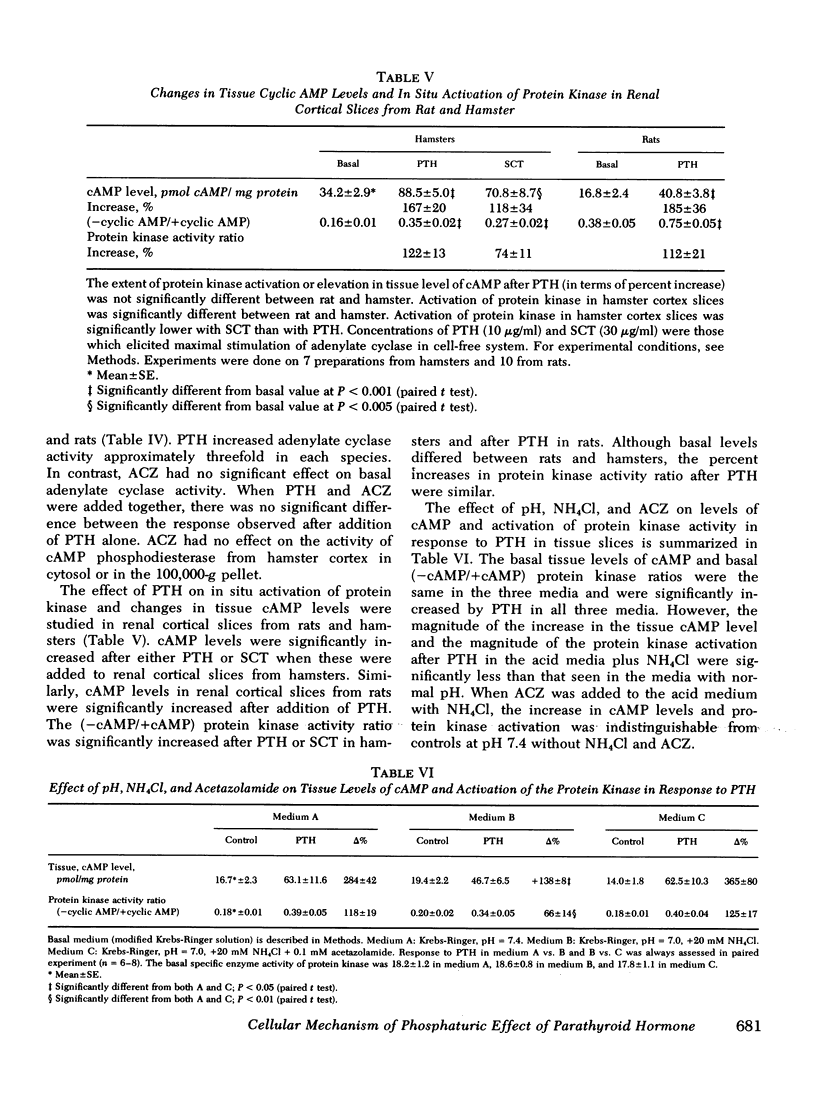
The effect of parathyroid hormone and calcitonin on the renal excretion of phosphate, calcium, and cyclic AMP was evaluated in the thyroparathyroidectomized hamster, a mammal apparently reisstant to the phosphaturic effect of parathyroid hormone. Parathyroid hormone did not increase phosphate excretion, although it decreased excretion of calcium and increased urinary excretion of cyclic AMP. This lack of a phosphaturic response to parathyroid hormone was not reversed by administration of 25-OH vitamin D or infusions of calcium or phosphate. Calcitonin, another potentially phosphaturic hormone, also vailed to increase phosphate excretion but markedly elevated urinary excretion of cyclic AMP. In hamsters pretreated with infusion of urinary ammonium chloride, which decreased plasma and urinary pH, both parathyroid hormone and calcitonin increased excretion of phosphate as well as that of cyclic AMP. Acetazolamide had no phosphaturic effect in ammonium chloride-loaded hamsters, and it decreased cyclic AMP and calcium excretion. Alkalinization of urine by acetazolamide did not prevent the phosphaturic effect of parathyroid hormone in ammonium chloride-loaded hamsters, but it blocked the increase in urinary cyclic AMP excretion. Parathyroid hormone and calcitonin both stimulated adenylate cyclase in a cell-free system (600-g pellet) from hamster renal cortex, elevated tissue cyclic AMP levels, and activated protein kinase in tissue slices from hamster renal cortex. In acid medium, the increase in cyclic AMP and activation of protein kinase in response to parathyroid hormone was diminished, but addition of acetazolamide restored responsiveness of both parameters to control values. Acetazolamide, on the other hand, did not influence adenylate cyclase or its response to parathyroid hormone or cyclic AMP phosphodiesterase activity. We conclude that the lack of a phosphaturic effect of parathyroid hormone and calcitonin in the hamster depends on steps in the cellular action of these hormones, steps that are sensitive to pH subsequent to cyclic AMP generation and protein kinase activation. In addition, acetazolamide may potentiate the phosphaturic effect of parathyroid hormone by promoting accumulation of cyclic AMP in tissue. Thus, the hamster is a particularly useful model for studies of syndromes in which there is renal resistance to phosphaturic hormones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurbach G. D., Heath D. A. Parathyroid hormone and calcitonin regulation of renal function. Kidney Int. 1974 Nov;6(5):331–345. doi: 10.1038/ki.1974.118. [DOI] [PubMed] [Google Scholar]
- Beck N., Kim H. P., Kim K. S. Effect of metabolic acidosis on renal action of parathyroid hormone. Am J Physiol. 1975 May;228(5):1483–1488. doi: 10.1152/ajplegacy.1975.228.5.1483. [DOI] [PubMed] [Google Scholar]
- Beck N., Singh H., Reed S. W., Davis B. B. Direct inhibitory effect of hypercalcemia on renal actions of parathyroid hormone. J Clin Invest. 1974 Mar;53(3):717–725. doi: 10.1172/JCI107610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Cuche J. L., Ott C. E., Marchand G. R., Diaz-Buxo J. A., Knox F. G. Intrarenal calcium in phosphate handling. Am J Physiol. 1976 Mar;230(3):790–796. doi: 10.1152/ajplegacy.1976.230.3.790. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Effects of colchicine and vinblastine on the cellular action of vasopressin in mammalian kidney. A possible role of microtubules. J Clin Invest. 1974 Aug;54(2):252–262. doi: 10.1172/JCI107760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Duarte C. G., Knox F. G. Effect of colchicine on urinary phosphate and regulation by parathyroid hormone. Am J Physiol. 1976 Jul;231(1):61–65. doi: 10.1152/ajplegacy.1976.231.1.61. [DOI] [PubMed] [Google Scholar]
- Drezner M., Neelon F. A., Lebovitz H. E. Pseudohypoparathyroidism type II: a possible defect in the reception of the cyclic AMP signal. N Engl J Med. 1973 Nov 15;289(20):1056–1060. doi: 10.1056/NEJM197311152892003. [DOI] [PubMed] [Google Scholar]
- Hahn T. J., Scharp C. R., Halstead L. R., Haddad J. G., Karl D. M., Avioli L. V. Parathyroid hormone status and renal responsiveness in familial hypophosphatemic rickets. J Clin Endocrinol Metab. 1975 Nov;41(5):926–937. doi: 10.1210/jcem-41-5-926. [DOI] [PubMed] [Google Scholar]
- Harter H. R., Mercado A., Rutherford W. E., Rodriguez H., Slatopolsky E., Klahr S. Effects of phosphate depletion and parathyroid hormone on renal glucose reabsorption. Am J Physiol. 1974 Dec;227(6):1422–1427. doi: 10.1152/ajplegacy.1974.227.6.1422. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Nagata N., Sasaki M., Nakane K. Effects of calcitonin in the concentration of cyclic adenosine 3',5'-monophosphate in rat kidney in vivo and in vitro. Endocrinology. 1974 Jun;94(6):1514–1518. doi: 10.1210/endo-94-6-1514. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. J., Villarreal H., Jr, Klahr S., Slatopolsky E. Pseudohypoparathyroidism type II: restoration of normal renal responsiveness to parathyroid hormone by calcium administration. J Clin Endocrinol Metab. 1974 Oct;39(4):693–701. doi: 10.1210/jcem-39-4-693. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. J., Walls J., Yates J., Klahr S. Effects of acetazolamide on the urinary excretion of cyclic AMP and on the activity of renal adenyl cyclase. J Clin Invest. 1974 Jan;53(1):122–130. doi: 10.1172/JCI107529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Matsushita S., Nakano T., Kimura N., Araki N. Effects of parathyroid hormone in vivo on the protein kinase activity in rat kidney. Endocrinology. 1976 Jun;98(6):1443–1450. doi: 10.1210/endo-98-6-1443. [DOI] [PubMed] [Google Scholar]
- Soderling T. R., Corbin J. D., Park C. R. Techniques for the study of protein kinase activation in intact cells. Methods Enzymol. 1974;38:358–367. doi: 10.1016/0076-6879(74)38052-4. [DOI] [PubMed] [Google Scholar]
- Steiner A. L. Assay of cyclic nucleotides by radioimmunoassay methods. Methods Enzymol. 1974;38:96–105. doi: 10.1016/0076-6879(74)38016-0. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]


