Abstract
Estrogen is known to play a pivotal role in granulosa cell responses to follicle-stimulating hormone (FSH) that is critical for the establishment of dominant follicles and subsequent ovulation in mammals. Thus, elucidating the cellular and molecular mechanisms that regulate FSH activity is important to understand female fertility. We previously discovered that the oocyte is required for estrogen to exert its positive effects on FSH activity in rat granulosa cells. This finding supports the new concept that estrogen action in granulosa cells is mediated by the oocyte. In the current study, we explored the underlying mechanism. In the presence of oocytes, estrogens enhanced FSH-induced increases in aromatase, steroidogenic acute regulatory protein and FSH receptor mRNA expression as well as cAMP production. However, as forskolin did not mimic FSH activity this indicated that coexistence of estrogen/oocytes increases FSH activity at a site upstream of adenylate cyclase in granulosa cells. We therefore sought a possible involvement of the autoregulatory molecules for FSH receptor, G protein-coupled receptor kinases (GRKs) and β-arrestins in enhancing FSH activity in response to the estrogen/oocyte co-treatment in granulosa cells. Among the seven known GRK and two β-arrestin molecules, we found that estrogens with oocytes suppressed FSH-induced GRK-6 mRNA expression. Consistent with this finding, transfecting granulosa cells with small interfering RNA of GRK-6 significantly increased FSH induction of aromatase mRNA, suggesting that endogenous GRK-6 plays an inhibitory role in FSH-induced aromatase mRNA expression. Consequently, these findings strongly suggest that GRK-6 is involved in the mechanism by which estrogen and oocytes synergistically augment FSH activity in granulosa cells.
Keywords: Estrogen, Follicle-stimulating hormone (FSH), Granulosa cells, Oocyte, G protein-coupled receptor kinase (GRK) and Steroidogenesis
INTRODUCTION
A fundamental concept in female reproductive biology is that FSH is essential for dominant follicle formation and no other ligand by itself can serve in this regulatory capacity. Therefore, to understand follicle dominance one must understand the mechanisms governing FSH action and sensitivity in target granulosa cells. In any given ovarian cycle, the cohort of follicles that respond to FSH and develop into dominant follicles destined for ovulation is tightly controlled, such that the number of ovulated oocytes in a cycle is appropriate for a particular species. Given that FSH is the predominant factor that promotes follicle dominance and that the entire ovary is theoretically exposed to the same levels of FSH, the local governance of the sensitivity of a particular follicle to FSH is an important element in the determination of dominant preovulatory follicles.
In this context, it has been demonstrated previously that estrogen acts as a physiological regulator of FSH action in the rat based on in vivo studies using the immature hypophysectomized diethylstilbestrol (DES)-primed rats [1,2] and on in vitro studies using primary cultures of rat granulosa cells from these animals [3,4]. Based on these classic observations, it is now widely accepted that the activation of estrogen signaling pathways in the granulosa cells enhances FSH action. A particularly important action of estrogen is the enhancement of FSH-induced P450 aromatase (P450arom) activity, resulting in the production of more estrogens. This feed-forward attribute of estrogen activity may perpetuate continuous growth of the selected dominant follicles in the face of declining circulating FSH levels.
We previously discovered that the oocyte is required for estrogen to enhance FSH action in rat granulosa cells [5] and proposed the new concept that estrogen action in granulosa cells is mediated by the oocyte. While it has been known that oocytes play essential roles in the regulation of the function of granulosa cells throughout the course of folliculogenesis, our finding has extended the role of the oocyte to include the role of estrogen mediating in the enhancement of FSH action in granulosa cells. The question is how oocytes and estrogen act synergistically to enhance FSH action in granulosa cells.
It is well known that G-protein-coupled receptor (GPCR) signaling is subject to regulation by the GPCR-kinase (GRK)/β-arrestin system [6–9]. The general mechanism by which the GRK/β-arrestin system causes receptor desensitization involves the following steps i) upon binding of a ligand to its GPCR, the cytoplasmic domain of the GPCR is phosphorylated by members of the GRK family, desensitizing the receptor by causing the uncoupling of the receptor from G proteins; ii) after phosphorylation of the GPCR, β-arrestins can then interact with and internalize GPCRs by a clathrin-mediated mechanism [10,11]. The role of the GRK/β-arrestin system has been extended to the FSH receptor (FSHR) by studies that have used various strategies in rat primary sertoli cells, a sertoli cell line (MSC-1) and cell lines engineered to overexpress FSHR. However, the role of GRK/β-arrestin system in the ovary remains to be investigated.
In the current study, we have tested our hypothesis that the GRK system is involved in the cellular and molecular mechanisms by which oocytes and estrogen act to enhance FSH action in granulosa cells. We provide the first analysis of the ovarian GRK, which is implicated in the mediation of the FSHR activity regulated by estrogen and oocytes.
MATERIALS AND METHODS
Primary culture of granulosa cells and co-culture with oocytes
Silastic capsules containing 10 mg of DES were implanted in 22-day-old female SD rats to increase the number of granulosa cells. After 4 days of DES exposure, ovarian follicles were punctured with a 28-gauge needle, and the isolated mixture of granulosa cells and oocytes was cultured in serum-free McCoy’s 5A medium supplemented with penicillin-streptomycin at 37°C. For indicated experiments, granulosa cells were separated from oocytes by filtering the oocyte/granulosa cell suspension through an additional 40-μm nylon mesh (BD Falcon) that allowed granulosa cells but not oocytes to pass through [12,13]. The animal protocols were approved by Okayama University Institutional Animal Care and Use Committee.
Measurements of cAMP
To assess cellular cAMP synthesis, granulosa cells (1 × 105) were cultured in 96-well plates with or without 20 oocytes with 200 μl of serum-free McCoy’s 5A medium containing 0.1 mM of IBMX (specific inhibitor of phosphodiesterase activity). The ratio of the oocyte and granulosa cell numbers in this co-culture system was determined based on our previous finding [12]. FSH (30 ng/ml) was added to the culture medium either alone or in combination with 5 × 10−8 M of DES and estradiol. After 48-h culture with indicated treatments, the conditioned medium was collected and stored at −80°C until assay. The extracellular contents of cAMP were determined by an enzyme immunoassay (Assay Designs, Ann Arbor, MI) after acetylation of each sample with assay sensitivity of 0.039 nM.
Cellular RNA extraction, RT and quantitative real-time PCR
Granulosa cells (5 × 105) with or without oocytes (102) were cultured in 12-well plates with 1 ml of serum-free McCoy’s 5A medium. FSH (30 ng/ml) was added to the culture medium either alone or in combination with 5 × 10−8 M of DES and estradiol. After 48-h culture, total cellular RNA was extracted using TRIzol (Invitrogen Corp.). The extracted RNA (1 μg) was subjected to an RT-PCR reaction. PCR primer pairs are listed in Supplemental Table 1. The primer set for GRK-6 was designed to detect both splicing variants of GRK-6a and -6b mRNAs; the latter of which is only 2-bp longer than the former [14], thus their PCR products are indistinguishable by the agarose gel elctrophoresis. For the quantification of P450arom, FSHR and GRK-6 mRNA expression, real-time PCR was performed using a LightCycler-FastStart DNA master SYBR Green I system (Roche Diagnostic Co., Tokyo, Japan). PCR primer pairs for real-time PCR are listed in Supplemental Table 2. Accumulated levels of fluorescence for each product were analyzed by the second derivative method (Roche Diagnostic Co.). The expression levels of target gene mRNA were normalized by RPL19 mRNA level in each sample.
Transient transfection
Granulosa cells (5 × 105) with or without oocytes (102) were cultured in 12-well plates in 1 ml of serum-free medium. After 1-h preculture, cells were transiently transfected with 10 μM GRK-6 siRNA or control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) using Fugene 6 for 3 h. Cells were then treated with FSH (30 ng/ml) either alone or in combination with 5 × 10−8 M of DES or estradiol for 48 h. After 48-h culture, total cellular RNA was isolated and stored at −80°C until assay.
Statistical analysis
All results are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The data were subjected to ANOVA to determine differences (StatView 5.0 software, Abacus Concepts, Inc., Berkeley, CA). If differences were detected by ANOVA, Fisher’s PLSD test was used to determine which means differed (StatView 5.0 software). P values < 0.05 were accepted as statistically significant.
RESULTS
We reported previously that oocytes must be present in the cell cultures for DES to augment FSH activity in primary rat granulosa cells [13]. Fig. 1A shows not only the confirmation of our previous finding but also new data demonstrating that a physiological estrogen, estradiol, plays a role in evoking positive estrogen response in P450arom mRNA expression in rat granulosa cells in the presence, but not absence, of oocytes. Likewise, StAR and FSHR mRNA expression in granulosa cells was also increased by estrogen treatments only when the cells were co-cultured with oocytes (Fig. 1B and 1C). These results suggested that estrogen augments FSH action in granulosa cells in cooperation with oocytes.
Fig. 1. Effects of estrogens on P450arom, StAR and FSHR mRNA expression in granulosa cells co-cultured with or without oocytes.
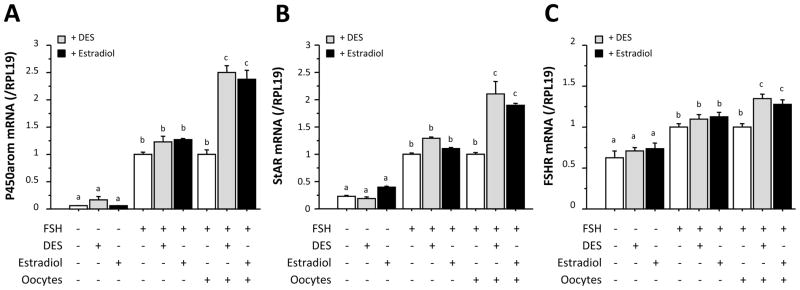
Granulosa cells (5 × 105) with or without oocytes (102) were treated with FSH (30 ng/ml) either alone or in combination with 5 × 10−8 M of DES or estradiol. After 48-h culture, total cellular RNA was extracted, and P450arom (A), StAR (B) and FSHR (C) mRNA levels were determined by real-time PCR. The expression levels of target mRNA were standardized by RPL19 mRNA level in each sample, and levels of each target mRNA were expressed as fold changes. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. For each result within a panel, values with different superscript letters are significantly different at P < 0.05.
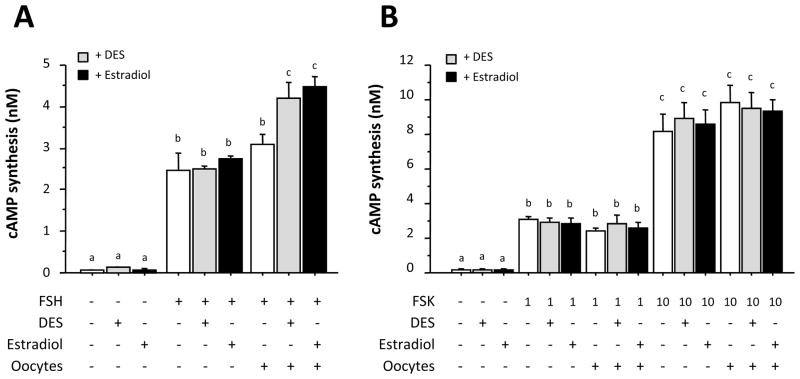
Next we examined the effects of estrogens on FSH-induced cAMP synthesis by granulosa cells. DES and estradiol increased FSH-induced cAMP production in the presence of oocytes, although neither of the estrogens had a significant effect on basal or FSH-induced cAMP synthesis by granulosa cells in the absence of oocytes (Fig. 2A). In contrast, DES and estradiol failed to increase forskolin-induced cAMP synthesis by granulosa cells regardless of co-culture with oocytes (Fig. 2B). These data suggested that the stimulatory effect of estrogens on FSH-induced cAMP synthesis is manifested at a site upstream of adenylate cyclase.
Fig. 2. Effects of estrogen on cAMP synthesis by granulosa cells co-cultured with or without oocytes.
FSH (30 ng/ml) (A) or forskolin (1 or 10 μM) (B) was added to the culture medium either alone or in combination with 5 × 10−8 M of DES or estradiol. After 48-h culture with indicated treatments, the conditioned medium was collected and stored at −80°C until assay. The extracellular contents of cAMP were determined by EIA after acetylation of each sample. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. For each result within a panel, values with different superscript letters are significantly different at P < 0.05.
In order to elucidate the mechanism by which estrogens upregulate FSH activity in granulosa cells in an oocyte-dependent manner, we focused on the activity of autoregulatory molecules for FSHR, G protein-coupled receptor kinases (GRKs) and β-arrestins. There are seven GRKs (GRK-1, -2, -3, -4, -5, -6 and -7) and two β-arrestins known to date. Among these, expression of GRK-1 and -7 are confined to retinal photoreceptors, while the expression of GRK-4 is restricted to testicular germ cells [10]. Therefore, we attempted a preliminary study to examine whether DES and oocytes have an effect on the expression of GRK-2, -3, -5, -6, β-arrestin-1 and β-arrestin-2 in granulosa cells treated with FSH. Neither DES or oocytes alone, nor taken together affected the mRNA levels of GRK-2, -3, -5, β-arrestin-1 and β-arrestin-2; however, DES in the presence of oocytes did cause a reduction in the expression levels of GRK-6 mRNA (Supplemental Fig. 1). These findings suggested that a selective inhibition of GRK-6 mRNA expression, and thus prevention of FSHR desensitization, caused by a combination of DES and oocytes may be involved in the mechanism of their enhancement of the FSH activity.
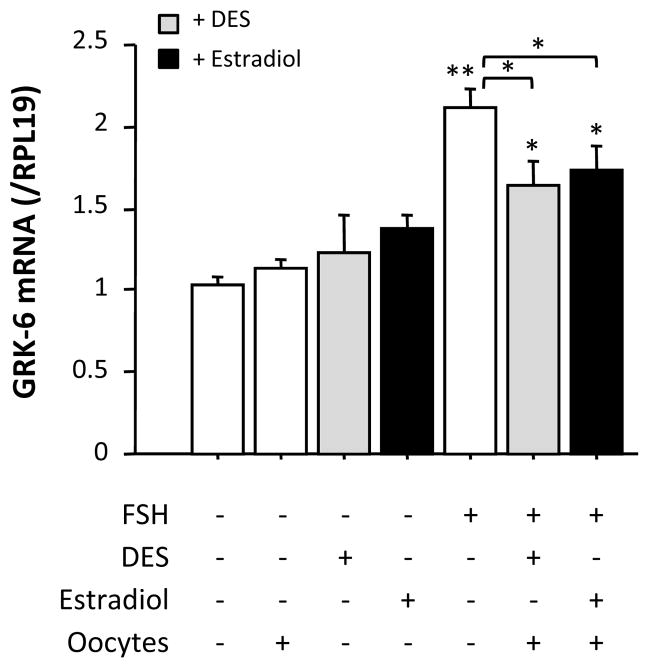
We confirmed the preliminary data presented in Supplemental Fig. 1 by real-time PCR in more detailed settings (Fig. 3). Interestingly, FSH increased GRK-6 mRNA level while the stimulatory effect of FSH was suppressed by a combination of either DES/oocyte or estradiol/oocyte. These results suggested that downregulation of GRK-6 by estrogens in combination with oocytes was functionally involved in the amplification of FSHR signaling activity.
Fig. 3. Effects of estrogens on GRK-6 mRNA expression in granulosa cells.
Granulosa cells (5 × 105) with or without oocytes (102) were cultured in 12-well plates with 1 ml of serum-free medium in the presence or absence of FSH (30 ng/ml) either alone or in combination with 5 × 10−8 M of DES or estradiol. After 48-h culture, total cellular RNA was extracted, and then GRK-6 mRNA expression levels were determined by real-time PCR. The expression levels of target mRNA were standardized by RPL19 mRNA level in each sample, and levels of each target mRNA were expressed as fold changes. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA and, when a significant effect due to treatment was observed (P < 0.05), subsequent comparisons of group means were conducted. *, P < 0.05 and **, P < 0.01 vs. control or between the indicated groups.
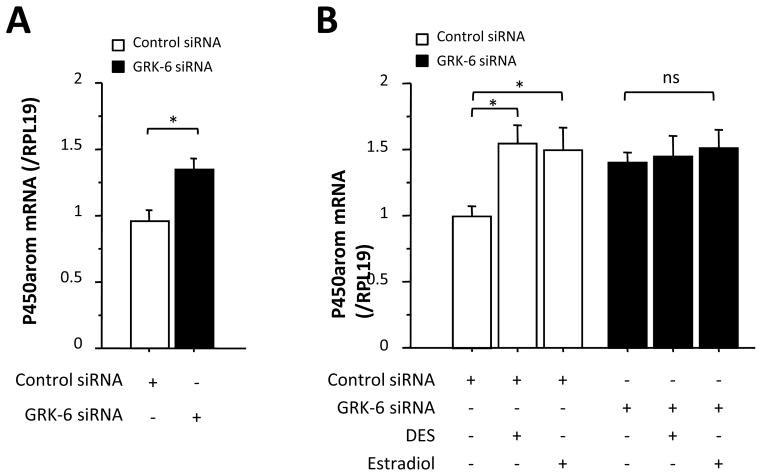
To directly determine whether GRK-6 is involved in FSH-induced P450arom mRNA expression, we attempted to silence endogenous GRK-6 mRNA level in granulosa cells using siRNA specific for GRK-6. As a preliminary experiment, we first examined the time-course effect of GRK-6 siRNA. As shown in Supplemental Fig. 2, transfection with GRK-6 siRNA for 48 h reduced the GRK-6 mRNA level. Using this experimental paradigm, it was found that P450arom mRNA level was significantly higher in granulosa cells transfected with GRK-6 siRNA as compared with that treated with the control siRNA (Fig. 4A), suggesting that the endogenous GRK-6 plays an inhibitory role in FSH-induced P450arom mRNA expression. In contrast, in the presence of both estrogens and oocytes, GRK-6 siRNA failed to further increase the levels of FSH-induced P450arom mRNA expression (Fig. 4B). This failure is most likely because the levels of GRK-6 mRNA were already reduced below functional levels due to the action of the estrogen/oocyte co-treatment. Consequently, these findings strongly suggest that GRK-6 is involved in the mechanism by which estrogen/oocytes augments FSH action in granulosa cells.
Fig. 4. Effects of GRK-6 siRNA on P450arom mRNA expression in granulosa cells co-cultured with oocytes in the presence of FSH.
Granulosa cells (5 × 105) and oocytes (102) were plated in 12-well plates were transiently transfected with 10 μM control siRNA or GRK-6 siRNA for 3 h. FSH (30 ng/ml) was then added to the culture either alone (A) or in combination with 5 × 10−8 M of DES or estradiol (B). After 48-h culture, total cellular RNA was extracted, and P450arom mRNA expression levels determined by real-time PCR. The expression levels of target mRNA were standardized by RPL19 mRNA level in each sample, and levels of each target mRNA were expressed as fold changes. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA and, when a significant effect due to treatment was observed (P < 0.05), subsequent comparisons of group means were conducted. *, P < 0.05 vs. control or between the indicated groups. ns, not significant.
DISCUSSION
The responsiveness of individual follicles to FSH is a predominant mechanism for the determination of the establishment of dominant follicles or for their demise by atresia. The physiological regulation of FSH signaling through the FSHR is an essential means by which granulosa cell function is dynamically yet precisely governed in particular follicles throughout ovarian cycles. In this regard, there are numerous physiological mechanisms that have been identified by which the sensitivity and/or responsiveness of granulosa cells to FSH can be regulated, such as the regulation of FSHR mRNA expression [15] or the modulation of adenylate cyclase activity [16]. However, there have been no reports that demonstrate the presence of a functional GRK/β-arrestin system in granulosa cells of any species. In the current study, we have shown that GRK-2, -5, -6 and β-arrestin-1 and -2 mRNAs are expressed in rat granulosa cells. Further, we have demonstrated that co-treatment of granulosa cells with estrogen and oocytes causes the selective reduction in GRK-6 mRNA, suggesting that the GRK/β-arrestin system in granulosa cells may be regulated over the course of folliculogenesis. We have also provided the first demonstration that the GRK/β-arrestin system in primary granulosa cells can affect FSH sensitivity by demonstrating that gene silencing of GRK-6 with siRNA augments FSH-induced P450arom mRNA expression by granulosa cells.
Our data indicate that oocytes must be present in the cell cultures for estrogen to augment FSH activity. However, it is unknown whether estrogen acts through estrogen receptors (ERs) in granulosa cells or oocytes. Studies using rodent ovaries have demonstrated that the ERβ is strongly expressed in the granulosa cells of all growing follicles, and faintly expressed in the oocyte, whereas ERα is moderately expressed in the theca and secondary interstitial cells, and weakly expressed in subpopulations of granulosa cells and some growing oocytes [17–22]. The view emerging from these experiments is that ERβ most likely acts as a transducer of the estrogen responses in the granulosa cells. However, one cannot rule out the possibility that some of the estrogen responses might involve the activation of ERα signaling pathways in oocytes and subtypes of granulosa cells. Future studies would include the identification of the direct cellular target of estrogen action (i.e. the oocyte, granulosa cell or both) and exploration of the mechanism as to how oocytes and granulosa cells communicate in order for estrogen to stimulate FSH action.
The central question of the aforementioned experiments in rodents is to what extent the estrogen concept can be extrapolated to humans. This is an important issue when evaluating the clinical relevance of the positive regulatory role of estrogen in FSH signaling. Although the answer to this question is still far from being clear, there are some supporting data. First, it has been reported [23] that human granulosa cells of all developing follicles strongly express ERβ, which is similar to the expression in rats and mice. It is also important to mention that the ERα expression has been reported in human oocytes [24]. Therefore, it is not unreasonable to hypothesize that estrogen/ER signaling might play a physiological role in human granulosa cells and/or oocytes during folliculogenesis, as it does in rats and mice. However, it raises an intriguing question: why there is no direct evidence as of yet for a biological function of estrogen in human granulosa cells in in vitro culture? In searching for reasons, an important clue is that the human granulosa cell experiments do not contain oocytes in striking contrast to those of the rat in which the mixture of granulosa cells and oocytes were traditionally used for in vitro culture. Given our finding that oocytes play a crucial role in mediating the estrogen effect, we propose a compelling hypothesis that the absence of oocytes in the culture medium is responsible for the negative data in human granulosa cells. If the lack of oocytes in the culture medium is the reason that previous studies have failed to identify an estrogen effect in human granulosa cells, then this would open up a new line of clinically relevant research in women’s health.
GRK-6 mRNA is expressed in many brain regions, including primary dopaminergic areas, such as substantia nigra and dorsal and ventral striatum. GRK-6 deficient mice are supersensitive to the locomotor-stimulating effect of psychostimulants, including cocaine and amphetamine [25]. Heterozygote and homozygote GRK-6 KO mice are viable but nothing has been reported about their reproductive phenotype. As seen in many other genes, it is possible that the GRK system is functionally redundant or that GRK molecules compensate one another to regulate GPCR activity. Nevertheless, we hypothesize that GRK-6 expression is down-regulated in granulosa cells of large dominant follicles in vivo due to elevated intrafollicular estrogen as they gain dominance during the final stages of folliculogenesis. This hypothesis could be tested by analyzing the expression levels of GRK-6 mRNA and protein in granulosa cells of developing dominant follicles in vivo. We predict that specific alterations of the GRK/β-arrestin system will be correlated with the component phases of folliculogenesis. The correlation of the GRK/β-arrestin system with the progress of folliculogenesis will most likely identify new approaches for the future study of the regulation of FSH sensitivity throughout the ovarian cycle.
In summary, the current study demonstrates that the oocyte plays an obligatory role in mediating the mechanisms of estrogen-stimulated FSH action and that GRK-6 is involved in the mechanism by which estrogen/oocytes augments FSH action in granulosa cells. It is our belief that this new insight into the mechanisms by which estrogen/oocyte interactions lead to increased FSH action will help us understand the physiological and pathological processes leading to reproductive dysfunctions in women, in particular during aging.
Supplementary Material
Supplemental Fig. 1. Expression of GRK and β-arrestin mRNAs in granulosa cells and the effects of DES and oocytes. Granulosa cells (5 × 105) with or without oocytes (102) and/or 5 × 10−8M of DES were cultured in 12-well plates with 1 ml of serum-free medium containing FSH (30 ng/ml). After 48-h culture, total cellular RNA was extracted and subjected to RT-PCR.
Supplemental Fig. 2. Effects of GRK-6 siRNA on the expression of endogenous GRK-6 mRNA in granulosa cells. Granulosa cells (2 × 105) were transfected with 10 μM GRK-6 siRNA for the indicated periods of time followed by the analysis of GRK-6 mRNA expression by semi-quantitative RT-PCR.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research, Daiichi-Sankyo Foundation of Life Science and Yamaguchi Endocrine Research Foundation, Kurozumi Medical Foundation, Foundation for Growth Science (to F.O.) and the National Institutes of Health (NIH) Grants R01 HD41494 and R21 HD060032, and by the National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to S.S.).
Abbreviations
- DES
diethylstilbestrol
- FSHR
follicle-stimulating hormone receptor
- GRK
G protein-coupled receptor kinase
- IBMX
3-isobutyl-1-methylxanthine
- P450arom
P450 aromatase
- siRNA
small interfering RNA
- StAR
steroidogenic acute regulatory protein
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Richards JS. Estradiol receptor content in rat granulosa cells during follicular development: modification by estradiol and gonadotropins. Endocrinology. 1975;97:1174–1184. doi: 10.1210/endo-97-5-1174. [DOI] [PubMed] [Google Scholar]
- 2.Richards JS, Hedin L. Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization. Annu Rev Physiol. 1988;50:441–463. doi: 10.1146/annurev.ph.50.030188.002301. [DOI] [PubMed] [Google Scholar]
- 3.Adashi EY, Hsueh AJ. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982;257:6077–6083. [PubMed] [Google Scholar]
- 4.Hsueh AJ, Adashi EY, Jones PB, et al. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5:76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka F, Miyoshi T, Murakami K, et al. An extra-adrenal abdominal pheochromocytoma causing ectopic ACTH syndrome. Am J Hypertens. 2005;18:1364–1368. doi: 10.1016/j.amjhyper.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Marion S, Robert F, Crepieux P, et al. G protein-coupled receptor kinases and beta arrestins are relocalized and attenuate cyclic 3′,5′-adenosine monophosphate response to follicle-stimulating hormone in rat primary Sertoli cells. Biol Reprod. 2002;66:70–76. doi: 10.1095/biolreprod66.1.70. [DOI] [PubMed] [Google Scholar]
- 7.Reiter E, Marion S, Robert F, et al. Kinase-inactive G-protein-coupled receptor kinases are able to attenuate follicle-stimulating hormone-induced signaling. Biochem Biophys Res Commun. 2001;282:71–78. doi: 10.1006/bbrc.2001.4534. [DOI] [PubMed] [Google Scholar]
- 8.Lazari MF, Liu X, Nakamura K, et al. Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor. Mol Endocrinol. 1999;13:866–878. doi: 10.1210/mend.13.6.0289. [DOI] [PubMed] [Google Scholar]
- 9.Troispoux C, Guillou F, Elalouf JM, et al. Involvement of G protein-coupled receptor kinases and arrestins in desensitization to follicle-stimulating hormone action. Mol Endocrinol. 1999;13:1599–1614. doi: 10.1210/mend.13.9.0342. [DOI] [PubMed] [Google Scholar]
- 10.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 11.Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. discussion 352–313. [PubMed] [Google Scholar]
- 12.Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuka F, Moore RK, Wang X, et al. Essential role of the oocyte in estrogen amplification of follicle-stimulating hormone signaling in granulosa cells. Endocrinology. 2005;146:3362–3367. doi: 10.1210/en.2005-0349. [DOI] [PubMed] [Google Scholar]
- 14.Firsov D, Elalouf JM. Molecular cloning of two rat GRK6 splice variants. Am J Physiol. 1997;273:C953–961. doi: 10.1152/ajpcell.1997.273.3.C953. [DOI] [PubMed] [Google Scholar]
- 15.Otsuka F, Yamamoto S, Erickson GF, et al. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka F, Moore RK, Shimasaki S. Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem. 2001;276:32889–32895. doi: 10.1074/jbc.M103212200. [DOI] [PubMed] [Google Scholar]
- 17.Mowa CN, Iwanaga T. Differential distribution of oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J Endocrinol. 2000;165:59–66. doi: 10.1677/joe.0.1650059. [DOI] [PubMed] [Google Scholar]
- 18.Sar M, Welsch F. Differential expression of estrogen receptor-beta and estrogen receptor-alpha in the rat ovary. Endocrinology. 1999;140:963–971. doi: 10.1210/endo.140.2.6533. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SC, Clemens JW, Pisarska MD, et al. Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology. 1999;140:4320–4334. doi: 10.1210/endo.140.9.6965. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165:359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- 22.Hiroi H, Momoeda M, Inoue S, et al. Stage-specific expression of estrogen receptor subtypes and estrogen responsive finger protein in preimplantational mouse embryos. Endocr J. 1999;46:153–158. doi: 10.1507/endocrj.46.153. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 24.Wu TC, Wang L, Wan YJ. Detection of estrogen receptor messenger ribonucleic acid in human oocytes and cumulus-oocyte complexes using reverse transcriptase-polymerase chain reaction. Fertil Steril. 1993;59:54–59. [PubMed] [Google Scholar]
- 25.Gainetdinov RR, Bohn LM, Sotnikova TD, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Expression of GRK and β-arrestin mRNAs in granulosa cells and the effects of DES and oocytes. Granulosa cells (5 × 105) with or without oocytes (102) and/or 5 × 10−8M of DES were cultured in 12-well plates with 1 ml of serum-free medium containing FSH (30 ng/ml). After 48-h culture, total cellular RNA was extracted and subjected to RT-PCR.
Supplemental Fig. 2. Effects of GRK-6 siRNA on the expression of endogenous GRK-6 mRNA in granulosa cells. Granulosa cells (2 × 105) were transfected with 10 μM GRK-6 siRNA for the indicated periods of time followed by the analysis of GRK-6 mRNA expression by semi-quantitative RT-PCR.