Abstract
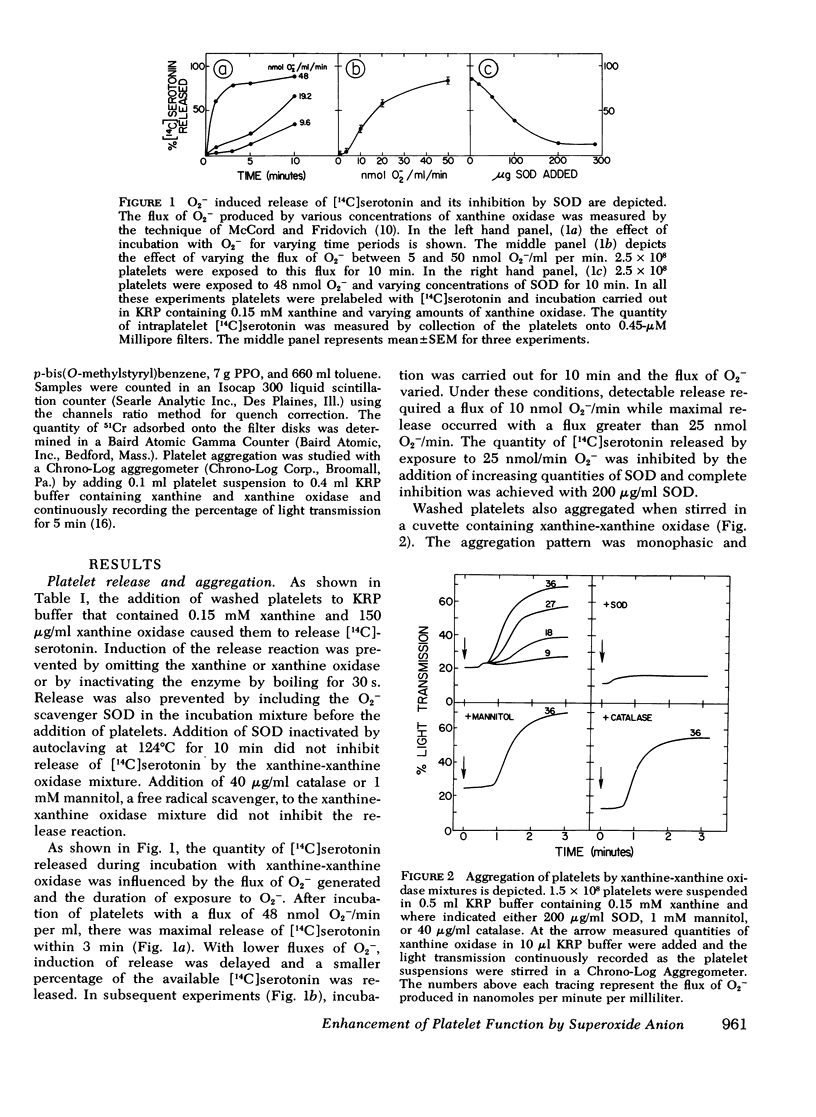
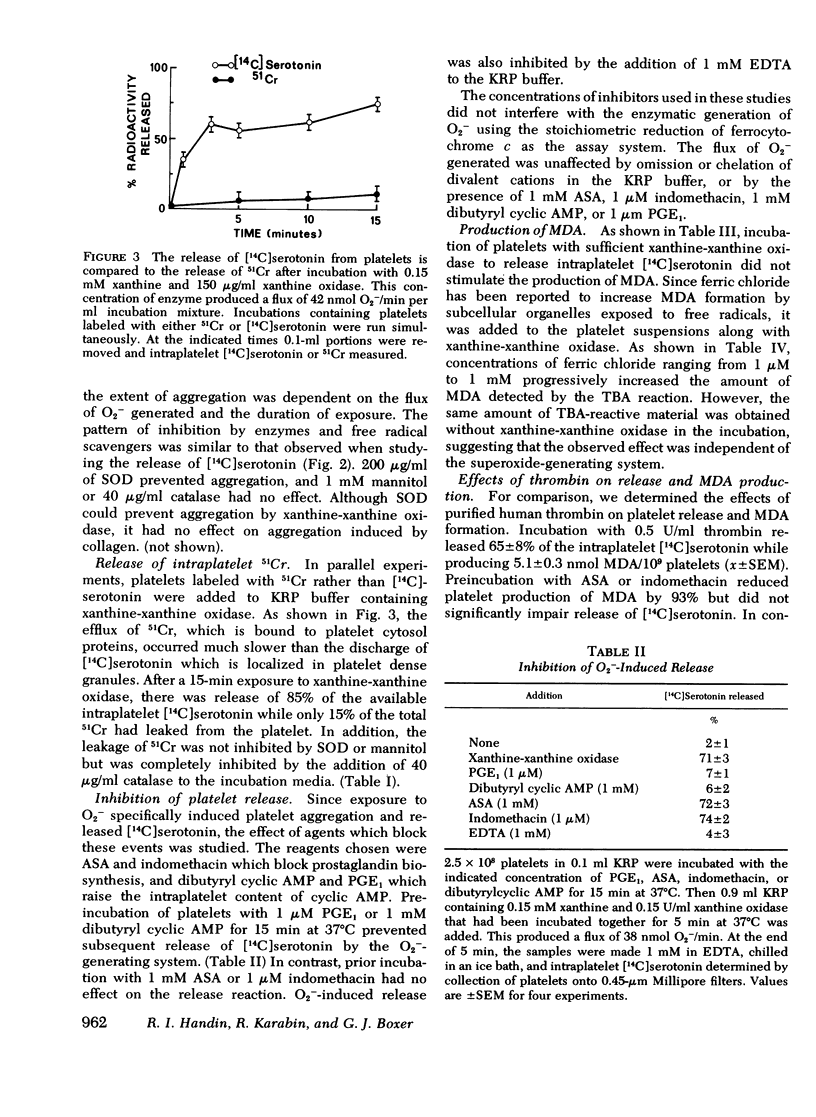
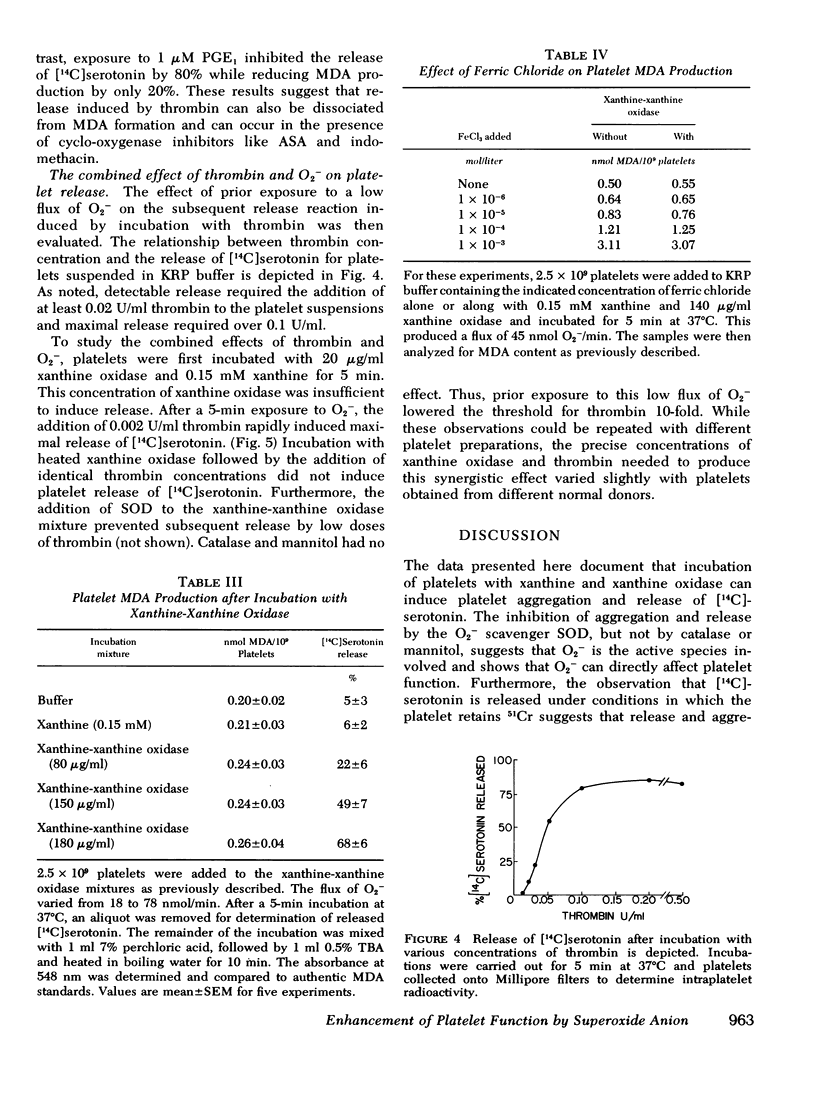
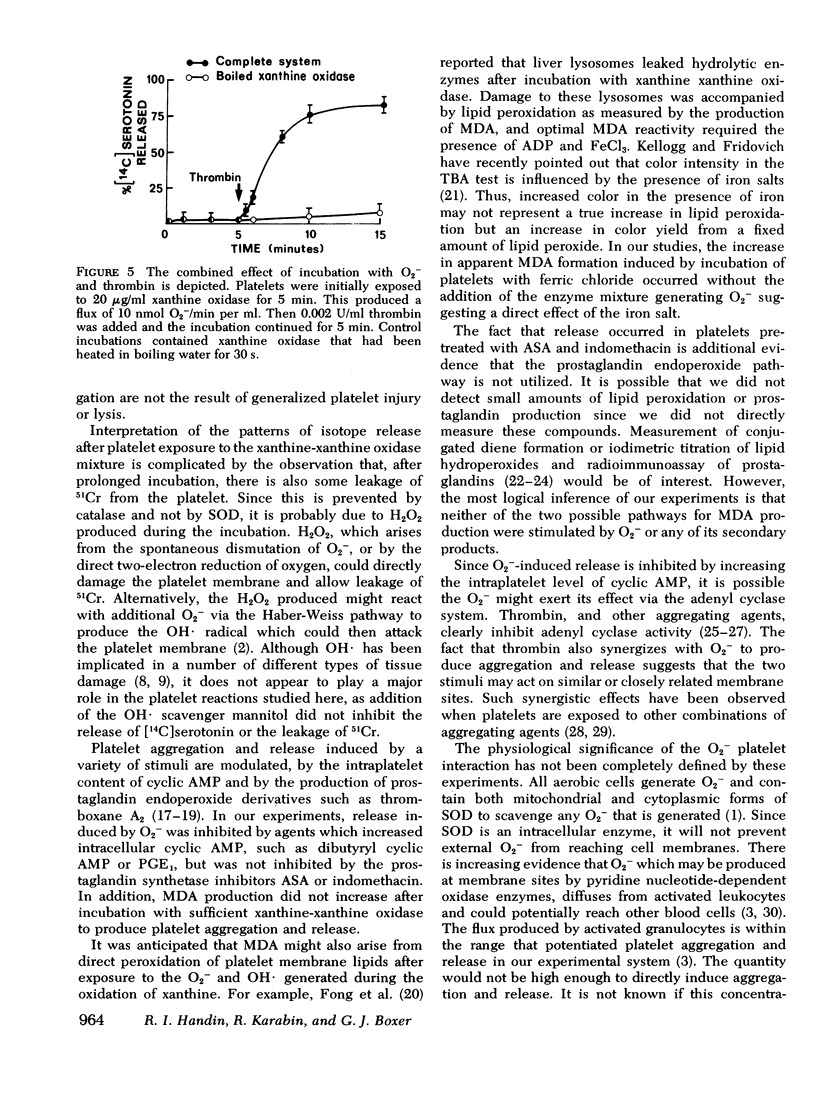
During the aerobic conversion of xanthine to uric acid by xanthine oxidase, superoxide anion and hydrogen peroxide are produced along with the hydroxyl radical. Our studies demonstrate that washed human platelets incubated with xanthine and xanthine oxidase aggregated and released [14C]serotonin. Aggregation and release were dependent on the duration of exposure to xanthine oxidase as well as the concentration of enzyme. Both reactions were inhibited by the superoxide scavenger enzyme superoxide dismutase but not by catalase, or the free radical scavenger mannitol, suggesting that they were induced by superoxide anion. Superoxide-dependent release was inhibited by prior incubation of platelets with 1 mM EDTA, 1 micronM prostaglandin E1, or 1 mM dibutyryl cyclic AMP, but was unaffected by 1 mM acetylsalicylic acid or 1 micronM indomethacin. After prolonged incubation with xanthine and xanthine oxidase there was also efflux of up to 15% of intraplatelet 51Cr, a cytosol marker. This leakage was prevented by the addition of catalase to the media but not by superoxide dismutase. Incubation with xanthine and xanthine oxidase did not produce malonyldialdehyde, the three-carbon fatty acid fragment produced during prostaglandin endoperoxide synthesis and lipid peroxidation. Prior exposure of platelets to low fluxes of superoxide anion lowered the threshold for release by subsequent addition of thrombin, suggesting a synergistic effect. We conclude that superoxide-dependent aggregation and release may be a physiologically important method to modulate hemostatic reactions particularly in areas of inflammation or vessel injury which could have high local concentrations of superoxide anion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes B. S. Pyridine nucleotide-dependent superoxide production by a cell-free system from human granulocytes. J Clin Invest. 1975 Oct;56(4):1035–1042. doi: 10.1172/JCI108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Castle W. B. Oxidant injury of caucasian glucose-6-phosphate dehydrogenase-deficient red blood cells by phagocytosing leukocytes during infection. J Clin Invest. 1971 Dec;50(12):2466–2473. doi: 10.1172/JCI106747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie G. N., Baenziger N. L., Chase L. R., Majerus P. W. The effects of thrombin on adenyl cyclase activity and a membrane protein from human platelets. J Clin Invest. 1972 Jan;51(1):81–88. doi: 10.1172/JCI106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoso R. T., Rodvien R., Scoon K., Levine P. H. Hydrogen peroxide and platelet function. Blood. 1974 May;43(5):645–656. [PubMed] [Google Scholar]
- DAHLE L. K., HILL E. G., HOLMAN R. T. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962 Aug;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- Droller M. J., Wolfe S. M. Thrombin-induced increase in intracellular cyclic 3',5'-adenosine monophosphate in human platelets. J Clin Invest. 1972 Dec;51(12):3094–3103. doi: 10.1172/JCI107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gershman H., Powers E., Levine L., Van Vunakis H. Radioimmunoassay of prostaglandins, angiotensin, digoxin, morphine and adenosine-3',5'-cyclic-monophosphate with nitrocellulose membranes. Prostaglandins. 1972 May;1(5):407–423. doi: 10.1016/0090-6980(72)90055-x. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J. Roles of cyclic nucleotides in platelet function. Ciba Found Symp. 1975;35:121–151. doi: 10.1002/9780470720172.ch7. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Levine P. H., Weinger R. S., Simon J., Scoon K. L., Krinsky N. I. Leukocyte-platelet interaction. Release of hydrogen peroxide by granulocytes as a modulator of platelet reactions. J Clin Invest. 1976 Apr;57(4):955–963. doi: 10.1172/JCI108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- OETTE K., PETERSON M. L., MCAULEY R. L. A HIGHLY SENSITIVE METHOD FOR MEASUREMENT OF LIPID HYDROPEROXIDES BY IODIMETRY AND AMPEROMETRIC ENDPOINT. J Lipid Res. 1963 Apr;4:212–215. [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman E. W. Cyclic AMP and platelet function. N Engl J Med. 1972 Feb 17;286(7):358–363. doi: 10.1056/NEJM197202172860708. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]


