Abstract
After antireflux surgery for gastroesophageal reflux disease, 10% to 15% of patients may have unsuccessful results as a result of abnormal restoration of the esophagogastric junction. The purpose of this study was to evaluate the postoperative endoscopic and radiologic characteristics of the antireflux barrier and their correlation with the postoperative results. After surgery, endoscopic and radiologic features of the antireflux wrap were evaluated in 120 consecutive patients. Jobe's classification of the postoperative valve was used for the definition of a “normal” or “defective” wrap. Patients were evaluated 3 to 5 years later in order to determine the clinical and objective failed fundoplication. A “normal” antireflux wrap was associated with successful results in 81.7% of the patients. On the contrary, defective radiologic or endoscopic antireflux wrap was observed in 19% of cases. Among these patients, hypotensive lower esophageal sphincter was observed in 50% to 65% of patients, abnormal 24-hour pH monitoring in 91%, and recurrent postoperative erosive esophagitis in 50% of patients, respectively (P < 0.001). “Defective” antireflux fundoplication is associated with recurrent reflux symptoms, presence of endoscopic esophagitis, hypotensive lower esophageal sphincter, and abnormal acid reflux.
Keywords: Antireflux surgery, Postoperative failures, Defective fundoplication
Patients with gastroesophageal reflux disease (GERD) have anatomic defects of the esophagogastric junction (EGJ) as dilatation of the cardia and/or presence of hiatal hernia.1–4 These defects have been associated with incompetence of the lower esophageal sphincter (LES) and pathologic acid reflux. The aim of antireflux surgery is to create an antireflux barrier by increasing the competence of the LES and to avoid this abnormal acid reflux. However, failure of antireflux surgery has been reported in several publications and pathologic acid reflux test can be observed in 5% to 15% of GERD patients.5,6 Zaninotto et al6 reported 13.2% of reflux recurrence in patients with GERD (6.2% in patients with grade 0 to I esophagitis and 7% in patients with grade-II esophagitis, respectively). Presence of abnormal acid reflux has been reported after Nissen fundoplication as well as after Toupet or cardial calibration in a very similar percentage of cases.7–9 The restoration of the cardial anatomic integrity is followed by a successful outcome after surgery. On the contrary, some authors have published endoscopic or radiologic failures after fundoplication, with defective antireflux wrap associated with a high rate of symptomatic and objective reflux recurrence reaching up to 25% of cases.2,10
The purpose of this prospective study was to correlate the presence of postoperative radiologic and endoscopic defects of the new antireflux barrier after surgery and its association with persistence of symptoms, endoscopic esophagitis, hypotensive LES, and abnormal 24-hour pH monitoring.
Patients and Methods
Patients studied
In this prospective study, we included 120 consecutive patients with chronic gastroesophageal reflux disease. They comprised 49 men and 71 women, with a mean age of 41.8 years (range, 27–73 years), submitted to calibrated fundoplication and posterior gastropexy. They corresponded to 53 patients with esophagitis type A or B of the Los Angeles classification, 35 patients with esophagitis and short-segment Barrett's esophagus, and 20 patients with type-I axial hiatal hernia. Exclusion criteria included patients with complicated Barrett's esophagus with ulcer or stricture, scleroderma, previous antireflux operations, and paraesophageal or large hiatal hernias. All patients gave their written informed consent to be included in the present study.
Preoperative and postoperative evaluation
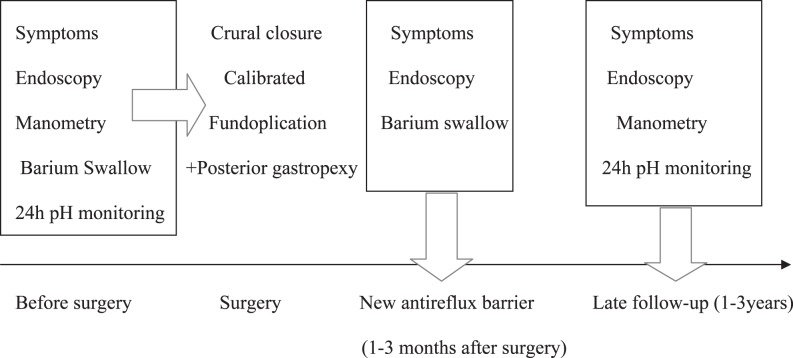
All patients were strictly submitted to a complete preoperative and postoperative evaluation, which included a clinical questionnaire, upper gastrointestinal (GI) endoscopy, manometry, 24-hour intraesophageal pH monitoring, and a radiologic esoph-agogastroduodenal study with barium sulphate, before and during the follow-up after the operation. Figure 1 shows the design of the entire study including the follow-up.
Fig. 1.
Study design: preoperative study, early postoperative evaluation, and late evaluation.
Clinical questionnaire
A careful clinical assessment was performed in each patient before and 1 to 3 years after surgery, asking about the presence of heartburn, regurgitation, dysphagia, chest pain, or other extra-esophageal symptoms; graded as absent, occasional (less than once a week), and frequent (more than once a week). The presence of frequent symptoms was considered an indicator of recurrent or persistent reflux after surgery.
Radiologic evaluation
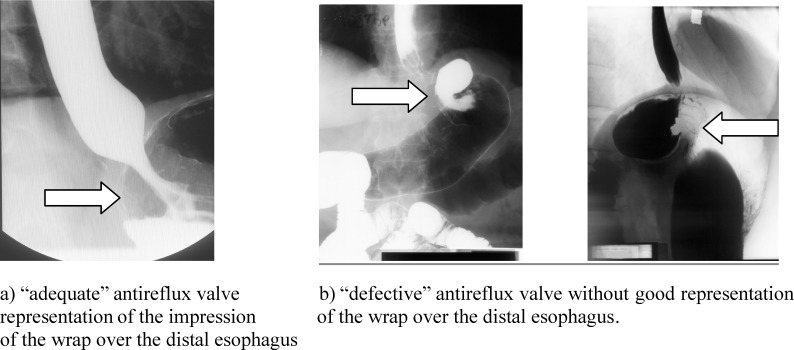
Before surgery, patients were studied with barium sulphate swallow. This test was performed after an overnight fast, using a low-density barium sulfate suspension (45% weight in volume). Patients were instructed to drink the amount of barium that they could tolerate without regurgitation or aspiration (usually between 150 and 200 mL). With the patient in an upright position, 4 X-rays (35 × 35 cm) were taken between 1 and 4 minutes after the last swallow of barium. Then, the patient was placed in a supine position, and the other 4 films were taken. The radiologic characteristics of the EGJ were evaluated according to the method previously reported.11 One month after surgery, the study was repeated in all patients, considering the symmetric or asymmetric configuration of the wrap, impression of the wrap over the distal esophagus, dilated cardia, in order to exclude postoperative hiatal hernia, slipping, displacement, strictures, or the presence of other deformities after surgery. According to these findings, fundoplication was defined as follows:
normal: adequate His angle, diameter of the EGJ less than 25 mm, without hiatal hernia, and having good representation of the antireflux wrap (Fig. 2a);
defective: without normal His′ angle, dilated EGJ more than 26 mm, presence of recurrent hiatal hernia, no clear representation of the antireflux barrier without normal impression of the wrap over the distal esophagus, or having saccular deformities (Fig. 2b).
Fig. 2.
Radiologic appearance of (a) a normal and (b) a defective fundoplication.
Endoscopic and histologic evaluation
In all patients, before and 3 months after the operation, endoscopic examination was performed using an Olympus video-endoscope. The anatomic characteristics of the cardia and the presence of a hiatal hernia observed by “U-turn” procedure were recorded. Preoperative anatomic features of the cardia were described employing Hill's classification.12 Special care was taken to measure the exact location of the squamo-columnar junction at the beginning and at the end of the procedure, avoiding the “push” and “pull” effects of the endoscope. After surgery, the anatomic aspects of the antireflux valve were classified according to Seltman and Jobe's classification for definition of a “normal” fundoplication,13,14 based on the following findings:
tight adherence to scope
circumferences of the cardia less than 35 mm (considered as normal in the Seltman's report)13
no cardia dilatation
valve length (body) 3 to 4 cm
nipple or coil type (Jobe classification)
intra-abdominal location and proper repair position.
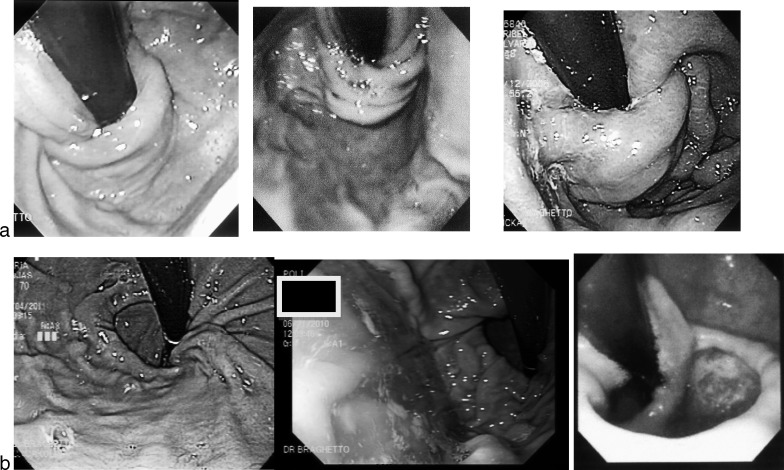
On the contrary, if a persistent dilated cardia, asymmetric deformities, or presence of hiatal hernia were demonstrated, it was defined as “defective” fundoplication. Figure 3a shows the normal endoscopic images during the endoscopic postoperative evaluation compared with abnormal images (Fig. 3b)
Fig. 3.
(a) Endoscopic representation of “normal” fundoplication with restoration of the normal diameter of cardia and acute His angle. Images demonstrate 3- to 4-cm-long valve, tight adherence to scope nipple or coil type, and good intra-abdominal position. These images accomplish the requirements suggested by Jobe and Kahrilas for a good antireflux valve.13,15 Patients after surgery did not present severe dysphagia. (b) Endoscopic “defective” fundoplication: short valve or dilated cardia in poor position. These images did not meet the required characteristics for a good antireflux valve.
Manometric studies
Manometric testing was carried out after a 12-hour fast with the patient in the supine position. The complete details of this procedure have been fully explained in previous reports.6,7 The resting pressure of the lower esophageal sphincter was measured. The location of the distal and proximal end of the lower esophageal sphincter was also measured in centimeters from the incisors in order to measure the total and abdominal length of the sphincter. According to the normal values of our laboratory (18 ± 3 mm), a resting lower esophageal sphincter pressure less than 12 mmHg was considered a hypotensive sphincter.
24-Hour intraesophageal pH study
A 24-hour intraesophageal pH study was performed after a 12-hour fast, introducing the catheter through the nose until the stomach was reached (Digitrapper; Synectics, Sweden). The catheter was then placed 5 cm above the manometric upper border of the lower esophageal sphincter (manometry is always done before this procedure). Positive acid reflux was considered according to the DeMeester parameters.8 This test was carried out in all patients before and 1 to 3 years after surgery when the last evaluation was performed. The complete details of these procedures have been published very completely elsewhere.6,8
Follow-up
All patients were submitted to late follow-up 3 to 5 years after the operation in order to precise presence of reflux symptoms (considered as positive reflux symptoms), heartburn, regurgitation, erosive esophagitis, and incompetence of LES or acid reflux.
Surgical technique
After pneumoperitoneum with 5 working ports and complete removal of the fatty tissue around the angle of His, we divided the first ascending branch of the left gastric artery at the lesser curvature and continued the dissection around the cardia in order to completely clear the esophagogastric junction, also dividing 1 or 2 short gastric vessels via the posterior approach. In this way, the anterior, posterior, right and left portions of the abdominal esophagus were completely exposed. The hiatus was closed with 2 to 3 nonabsorbable stitches. We proceeded to perform seromuscular 2/0 nonabsorbable sutures in the anterior wall of the stomach on the lesser curvature, 1 to 2 cm distal and perpendicular to the anatomic border of the cardia, without including the esophagus, including the sling fibers and the inferior clasp fibers. The stomach was then rotated to expose the posterior wall of the esophagus and gastric fundus in order to place stitches in the corresponding points of the anterior wall and posterior wall of stomach in a symmetric fashion, and we performed a calibrated fundoplication, wrapping 3 cm of the distal esophagus.15 To avoid extreme narrowing of the esophagus and cardia, an intraluminal 40 French bougie was used. The more proximal stitch was also fixed to the diaphragmatic pillar.
Statistical analysis
Data were managed in Microsoft Excel database; for statistical analysis contingence, 2 × 2 tables were made: Fisher exact test and χ2 test for proportions. Tests were applied to establish the significant difference between postoperative exams, using SPSS 6.0 (Statistical Program for Social Sciences, Chicago University, 1975); P < 0.05 was considered significant.
Results
Before operation, radiologic cardial dilatation or hiatal hernia with hypotensive LES and an abnormal acid reflux were present in all patients included in this study. Table 1 shows the manometric and 24-hour pH monitoring characteristics in patients with radiologic or endoscopic “normal” fundoplication compared with patients with postoperative defective wrap.
Table 1.
Postoperative lower esophageal sphincter pressure (LESP) and 24-hour pH monitoring in patients with “normal” or “defective” fundoplication
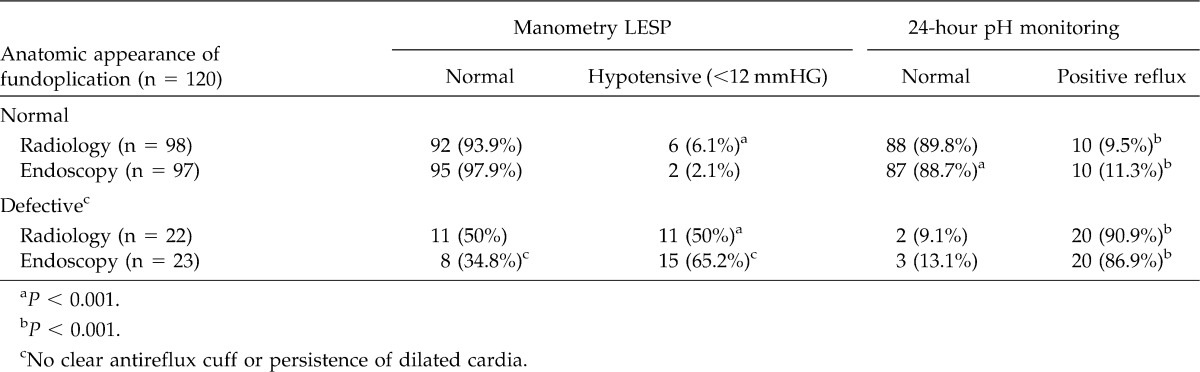
After surgery, the radiologic aspects of the cardia were defined as “normal” in 98 patients. Among them, normal manometry was observed in 92 (93.6%) (P < 0.001). Defective fundoplication was observed in 22 patients, and 11 (50%) of them had hypotensive LES late after surgery (P < 0.001). Almost the same results were observed during the evaluation of 24-hour pH monitoring. Among patients presenting a “normal” radiologic fundoplication late after surgery, abnormal acid reflux was observed in 10 patients (9.5%). On the contrary, among 22 patients who presented “defective” radiologic fundoplication, 20 (90.9%) showed positive reflux (P < 0.001).
Endoscopic evaluation after surgery, was “normal” in 97 (80.8%) patients (very similar to radiologic evaluation), associated with normal lower esophageal sphincter pressure (LESP) in 95 of them (97.9%). In 23 patients, “defective” wrap was associated with hypotensive LES in 15 of them (65.2%) (P < 0.001). Positive acid reflux was present in 10 of 97 (11.3%) patients with “normal” endoscopic fundoplication. Surgery failed to create a good fundoplication in 23 patients, 20 (86.9%) of them demonstrating abnormal acid reflux late after surgery (P < 0.001). Therefore, when dividing patients as “refluxers” or “non-refluxers,” the majority of patients (near 90%) with defective fundoplication confirmed with endoscopic or radiologic assessment were refluxers, while only 10% of patients with normal fundoplication were refluxers (P < 0.001).
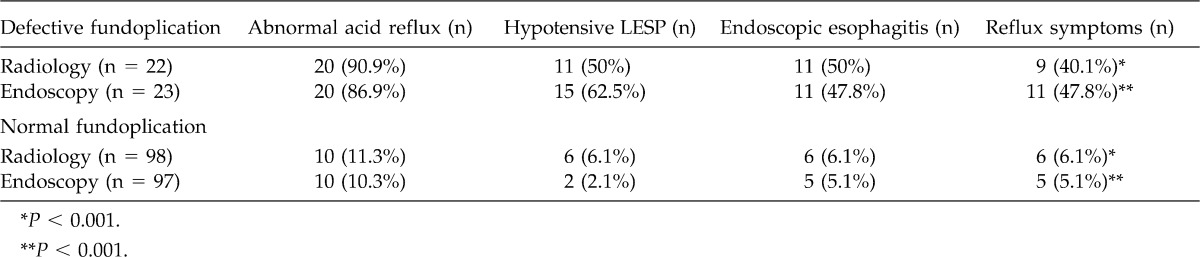
Table 2 shows the results of patients with radiologic or endoscopic defective fundoplication compared with patients with normal fundoplication and its correlation with manometry, acid reflux, endoscopic esophagitis, and symptoms 1 to 3 years after surgery. In patients with radiologic defective fundoplication, almost 90% of them presented with abnormal acid reflux, 50% presented with hypotensive LES, 50% with erosive esophagitis, and only 9 (40%) presented with reflux symptoms. In patients with defective endoscopic fundoplication, 20 patients (86.9%) had positive reflux, 15 patients (62.5%) had hypotensive LES, and 11 (47.8%) presented with erosive esophagitis as well as reflux symptoms. Among patients with either radiologic or endoscopic “normal” fundoplication, hypotensive LES, endoscopic esophagitis, and reflux symptoms were significantly less frequent (P < 0.001).
Table 2.
Postoperative radiologic and endoscopic evaluation of anatomic characteristics of cardia and defective antireflux barrier correlated to postoperative manometry, endoscopic esophagitis, and postoperative reflux symptoms
Therefore, after this objective evaluation after surgery, abnormal acid reflux was observed in 30 out of 120 operated patients (25%), almost 90% of them as a result of defective antireflux surgery. We observed a good correlation comparing adequate radiologic and endoscopic fundoplication with normal manometry and 24-hour pH monitoring. In patients with defective fundoplication, endoscopic evaluation seems to have better sensitivity compared with radiologic assessment.
Discussion
Several studies have demonstrated that increased cardiac circumference or cardiac dilatation correlates closely with the severity of GERD. Hill12 proposed a classification based on the diameter of the cardia visualization during endoscopic U-turn view, demonstrating that patients with GERD, Barrett's esophagus or hiatal hernia presented a dilated cardia type III or IV of his classification. Korn et al3 and Csendes et al4 also correlated the anatomic dilatation of the cardia and competence of the LES. The conclusion of these studies also demonstrated that dilatation of the cardia is accompanied by incompetent LES and GERD. Seltman and Kahrilas,14 measuring the circumference of the cardia, confirmed a close relationship between this parameter and more advanced disease. Both studies propose that endoscopic assessment could be superior to manometry for separating GERD patients from normal subjects. They suggest that the presence of GERD would be unlikely with a cardiac circumference less than 34 mm. In addition, other studies performed by the same authors, using endoscopic appraisal of the gastroesophageal valve, suggest that these criteria can be employed for evaluating the results after antireflux surgery. Jobe et al described 10 endoscopic criteria in order to establish a lexicon determining the integrity or normality of antireflux valve.15 The purpose of each antireflux surgery is to create a new barrier for reflux, which includes reduction of hiatal hernia (if it exists), crural closure, creation of an intra-abdominal segment of distal esophagus, and creation of a “competent” antireflux valve. Our technique includes all of these considerations. We have employed a combination of Hill's, Jobe's, and Kahrilas's classifications of the cardia characteristics after our antireflux surgery procedure, taking into account the cardia diameter and valve configuration in order to correlate them with late failures. Fundoplication attempts to restore the physiology of LES by wrapping the distal 3 cm of the esophagus with gastric fundus, submerging the abdominal esophagus within the wrap, and producing an acute His angle, which is clearly observed endoscopically. If we compare these criteria and the valve configuration after Nissen fundoplication described by Jobe, the endoscopic images observed after our procedure are quite similar, confirming that our technique is a combination of calibration of the cardia plus a fundoplication.
If a very careful technique is performed with construction of an effective antireflux procedure, excellent results after surgery have been observed. There are many reports that confirm the safety of laparoscopic antireflux surgery for patients undergoing primary repair in hands of experienced surgeons.16–22 However, there are several citations in the literature reporting failure rates of laparoscopic fundoplication from 2% to 17%.22–28 These lower published rates probably reflect shorter follow-up rather than an intrinsically better operation. Besides, the majority of these reports do not include objective evaluation of the success or failure of antireflux surgery, such as endoscopic appearance, manometric measurements, or 24-hour pH studies. After laparoscopic fundoplication, anatomic alterations of the EGJ are observed in 2% in non–Barrett's esophagus patients versus 16% in patients with Barrett's esophagus.19–28 Martínez de Haro et al,29 in 51 patients with reflux esophagitis followed for more than 6 years, obtained good results in 89% of patients, but endoscopic recurrence was higher (14%) and pathologic acid reflux confirmed by 24-hour pH monitoring was 20% after surgery. In Barrett's esophagus patients, the results are even worse.30
Symptoms and antireflux medication after surgery are poor indicators of GERD after fundoplication, because a high proportion of these patients who improve after proton pump inhibitors still have pathologic levels of acid reflux assessed by 24-hour pH monitoring.31–34
Bonatti et al have reported as high a proportion as 43% to 62% taking acid suppression medications after fundoplication.32–36 Lord and others,36 described that 20 of 86 patients (23%) studied had a positive pH study, 33% of patients with any grade of esophagitis and 46% of these patients presented abnormal fundoplication. Among patients in whom fundoplication was not intact or was in an abnormal position, 75% presented with positive postoperative acid reflux. Therefore, our results agree with this report. Asymptomatic patients with normal endoscopy but abnormal acid exposure also have been observed during the postoperative follow-up.36
The majority of authors agree that the main reasons for failures are technical errors in the performance of surgery such as asymmetric wrap, early disruption, wrap migration, and slipped fundoplication producing abnormal deformities, clearly demonstrated by radiologic and endoscopic assessment.37–42 The recurrence rate of erosive esophagitis resulting from failure of antireflux operation ranges from 3% to 16%, most of them caused by a misperformed surgical procedure with a high proportion of positive acid reflux tests after the operation.23
Mickevicius et al43 suggests that a wrap length is important in fundoplication in order to avoid postsurgical failures. These findings in some way agree with our results in that a good valve assessed early after surgery could ensure successful late results in agreement with Jobe's study. The purpose of our study was to correlate the radiologic endoscopic images obtained during the early assessment and compared them with the late result after surgery. The images obtained in the majority of our patients are very similar to those published by Jobe. However, in some patients, these typical images were not obtained, and bad results were observed during the follow-up. Horgan et al38 describes 25% of misperformed fundoplication and almost 70% of hiatal hernias in patients with failed antireflux procedures, and other reports present even worst results in patients with Barrett's esophagus.44–50
In the present article, we have concluded that abnormalities of the new antireflux valve may be followed by persistence of reflux symptoms in a small group of patients, which is in agreement with the data reported by Ferguson.51
Acknowledgments
All contributing authors declare that they have no conflicts of interest.
References
- 1.Watson D. I., Foreman D., Devitt P. G., Jamieson G. C. Preoperative endoscopic grading of esophagitis vs. outcome after laparoscopic Nissen fundoplication. Am J Gastroenterol. 1997;92(2):222–225. [PubMed] [Google Scholar]
- 2.Desai K. M., Frisella M. M., Soper N. J. Clinical outcome after laparoscopic antireflux surgery in patients with and without preoperative endoscopic esophagitis. J Gastrointest Surg. 2003;7(1):44–52. doi: 10.1016/S1091-255X(02)00135-X. [DOI] [PubMed] [Google Scholar]
- 3.Korn O., Csendes A., Burdiles P., Braghetto I., Stein H. Anatomic dilatation of the cardia and competence of the lower esophageal sphincter: a clinical and experimental study. J Gastrointest Surg. 2000;4(1):398–401. doi: 10.1016/s1091-255x(00)80019-0. [DOI] [PubMed] [Google Scholar]
- 4.Csendes A., Miranda M., Espinoza M., Velasco N., Henríquez A. Perimeter and location of the muscular gastroesophageal junction or cardia in control subjects and in patients with reflux esophagitis or achalasia. Scand J Gastroenterol. 1981;16(7):951–956. doi: 10.3109/00365528109181829. [DOI] [PubMed] [Google Scholar]
- 5.Yau P. I., Watson D., Devitt P. Laparoscopic antireflux surgery in the treatment of gastroesophageal reflux in patients with Barrett's esophagus. Arch Surg. 2000;135(7):801–805. doi: 10.1001/archsurg.135.7.801. [DOI] [PubMed] [Google Scholar]
- 6.Zaninotto G., Portale G., Costantini M., Rizzetto Ch, Guirroli E., Ceolin M., et al. Long term results (6–10 years) of laparoscopic fundoplication. J Gastrointest Surg. 2007;11(9):1138–1145. doi: 10.1007/s11605-007-0195-y. [DOI] [PubMed] [Google Scholar]
- 7.Hafez J., Brba F., Lengingler J., Miholic J. Fundoplication for gastroesophageal reflux and factor associated with the outcome 6–10 years after the operation: multivariate analysis of prognostic factors using the propensity score. Surg Endosc. 2008;22(10):1763–1768. doi: 10.1007/s00464-008-9872-5. [DOI] [PubMed] [Google Scholar]
- 8.Hagedorn C., Jönson C., Lönroth H., Ruth M., Thune A., Lundell L. Efficacy of an anterior as compared with a posterior laparoscopic partial fundoplication: results of a randomized, controlled clinical trial. Ann Surg. 2003;238(2):189–196. doi: 10.1097/01.sla.0000080821.08262.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J. Y., Woloshin S., Laycock W. S., Schwartz L. M. Late outcome after laparoscopic surgery for gastroesophageal reflux. Arch Surg. 2002;137(4):437–401. doi: 10.1001/archsurg.137.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Luostarinen M. Nissen fundoplication for reflux esophagitis: long term clinical and endoscopic results in 109 of 127 consecutive patients. Ann Surg. 1993;217(4):329–337. doi: 10.1097/00000658-199304000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graziani L., De Nigis E., Pesaresi A. Reflux esophagitis: radiological-endoscopical correlation in 39 symptomatic patients. Gastrointest Radiol. 1983;8(1):1–6. doi: 10.1007/BF01948078. [DOI] [PubMed] [Google Scholar]
- 12.Hill L. D., Korazek R. A., Kraemer S. J., Aye R. W., Mercer C. D., Low D. E., et al. The gastroesophageal flap valve: in vitro and in vivo observation. Gastrointest Endosc. 1996;44(5):541–547. doi: 10.1016/s0016-5107(96)70006-8. [DOI] [PubMed] [Google Scholar]
- 13.Seltman A. K., Kahrilas P., Chang E. Y., Mori M., Hunter J. G., Jobe B. A. Endoscopic measurement of cardia circumference as an indicator of GERD. Gastrointest Endosc. 2006;63(1):22–31. doi: 10.1016/j.gie.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Jobe B. A., Kahrilas P., Vernon A. H., Sandone C., Deepak M. A., Goyal D. V., et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol. 2004;99(2):233–243. doi: 10.1111/j.1572-0241.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 15.Braghetto I., Korn O., Debandi A., Burdiles P., Valladares H., Csendes A. Laparoscopic cardial calibration and gastropexy for treatment of patients with reflux esophagitis: pathophysiological basis and results. World J Surg. 2005;29(5):636–644. doi: 10.1007/s00268-005-7416-x. [DOI] [PubMed] [Google Scholar]
- 16.Horgan S., Pellegrini C. A. Surgical treatment of gastroesophageal reflux. Surg Clin North Am. 1997;77(5):1063–1082. doi: 10.1016/s0039-6109(05)70605-8. [DOI] [PubMed] [Google Scholar]
- 17.Christen D. J., Buyske J. Current status of antireflux surgery. Surg Clin North Am. 2005;85:931–947. doi: 10.1016/j.suc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Eubanks T. R., Omelanczuk K. P., Richards C., Pohl D., Pellegrini C. A. Outcomes of laparoscopic antireflux procedures. Am J Surg. 2000;179(5):391–395. doi: 10.1016/s0002-9610(00)00363-9. [DOI] [PubMed] [Google Scholar]
- 19.Bowers S., Mattar S., Smith C. Clinical and histological follow up after antireflux surgery for Barrett's esophagus. J Gastrointest Surg. 2002;6(4):532–533. doi: 10.1016/s1091-255x(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter W., Peters J., De Meester T. R. Long term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg. 2001;234(4):532–539. doi: 10.1097/00000658-200110000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oelschlager B., Barreca M., Chang L., Oleynikov D., Pellegrini C. Clinical and pathological response of Barrett's esophagus to laparoscopic antireflux surgery. Ann Surg. 2003;238(4):458–466. doi: 10.1097/01.sla.0000090443.97693.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith C. D. Antireflux surgery. Surg Clin North Am. 2008;88(5):943–958. doi: 10.1016/j.suc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Hatch K. F., Daily M. F., Christensen B. J., Glasgow R. E. Failed fundoplications. Am J Surg. 2004;188(6):786–791. doi: 10.1016/j.amjsurg.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal A., Awad Z., Simkins J., Shah R., Haider M., Salinas V., et al. Repair of 104 failed antireflux operations. Ann Surg. 2006;244(1):42–51. doi: 10.1097/01.sla.0000217627.59289.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soper N. J., Dunnegan D. Anatomic fundoplication failure after laparoscopic antireflux surgery. Ann Surg. 1999;229(5):669–676. doi: 10.1097/00000658-199905000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb P. J., Myers J. C., Jamieson G. G., Thompson S. K., Devitt P. G., Watson D. L. Long-term outcomes of revisional surgery following laparoscopic fundoplication. Br J Surg. 2009;96(4):391–397. doi: 10.1002/bjs.6486. [DOI] [PubMed] [Google Scholar]
- 27.Khajanchee Y. S., O'Rourke R., Cassera M. A., Gatta P., Hansen P. D., Swanstrom L. L. Laparoscopic reintervention for failed antireflux surgery. Arch Surg. 2007;142(8):785–792. doi: 10.1001/archsurg.142.8.785. [DOI] [PubMed] [Google Scholar]
- 28.Stein H. J., Feussner H., Siewert R. Failure of antireflux surgery: causes and management strategies. Am J Surg. 1996;171(1):36–40. doi: 10.1016/S0002-9610(99)80070-1. [DOI] [PubMed] [Google Scholar]
- 29.Martinez de Haro L. F., Ortiz A., Parrilla P., García Mancilla J. A., Aguayo J. L., Morales G. Long term results of Nissen fundoplication in reflux esophagitis without strictures: clinical, endoscopic and pH-metric evaluation. Dig Dis Sci. 1992;37(4):523–527. doi: 10.1007/BF01307574. [DOI] [PubMed] [Google Scholar]
- 30.Csendes A., Braghetto I., Burdiles P., Puente G., Korn O., Díaz J. C., et al. Long term results of classic antireflux surgery in 152 patients with Barrett's esophagus: clinical, radiologic, endoscopic, manometric and acid reflux test analysis before and late after operation. Surgery. 1998;123(6):645–657. [PubMed] [Google Scholar]
- 31.Galvani C., Fisichella P., Gorodner M. V., Perrotta S., Patti M. Symptoms are poor indicator of reflux status after fundoplication for gastroesophageal reflux disease. Arch Surg. 2003;138(5):514–519. doi: 10.1001/archsurg.138.5.514. [DOI] [PubMed] [Google Scholar]
- 32.Bonatti H., Bammer T., Achem S. R., Lukens F., De Vault K. R., Klaus A., et al. Use of acid suppressive medications after laparoscopic antireflux surgery: prevalence and clinical indications. Dig Dis Sci. 2007;52(1):267–272. doi: 10.1007/s10620-006-9379-7. [DOI] [PubMed] [Google Scholar]
- 33.Spechler S. J., Lee E., Ahmen D., Goyal R. K., Hirano I., Ramirez F., et al. Long term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285(18):2331–3238. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson A. D., Kadirkamanathan S. S., Scott S. M., Yazaki E., Evans D. F. Relationship between response and oesophageal acid exposure after medical and surgical treatment for gastroesophageal reflux disease. Brit J Surg. 2004;91(11):1460–1465. doi: 10.1002/bjs.4614. [DOI] [PubMed] [Google Scholar]
- 35.Wijnhoven B. P. L., Lally C. J., Kelly J. J., Myers J. C., Watson D. I. Use of antireflux medication after antireflux surgery. J Gastrointest Surg. 2008;12(3):510–517. doi: 10.1007/s11605-007-0443-1. [DOI] [PubMed] [Google Scholar]
- 36.Lord R. N., Kaminsky A., Oberg S., Bowrey D. J., Hagen J. A., DeMeester T. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg. 2002;6(1):3–10. doi: 10.1016/s1091-255x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 37.Sandbu R., Khamis H., Gustavsson S., Haglund U. Long term results of antireflux surgery indicate the need for a randomized clinical trial. Brit J Surg. 2002;89(2):225–230. doi: 10.1046/j.0007-1323.2001.01990.x. [DOI] [PubMed] [Google Scholar]
- 38.Horgan S., Pohl D., Bogetti D., Eubanks T., Pellegrini C. Failed antireflux surgery: what have we learned from reoperations? Arch Surg. 1999;134(2):809–817. doi: 10.1001/archsurg.134.8.809. [DOI] [PubMed] [Google Scholar]
- 39.Watson D. I., Jamieson G. G., Game P. A., Williams R. S., Devitt P. G. Laparoscopic reoperation following failed antireflux surgery. Brit J Surg. 1999;86(1):98–101. doi: 10.1046/j.1365-2168.1999.00976.x. [DOI] [PubMed] [Google Scholar]
- 40.Granderath F. A., Kamolz T., Schweiger U. M., Pasiut M., Hass C. F., Wykypiel H., et al. Long-term results of laparoscopic antireflux surgery. Surg Endosc. 2002;16(5):753–757. doi: 10.1007/s00464-001-9103-9. [DOI] [PubMed] [Google Scholar]
- 41.Graziano K., Teitelbaum D. H., McLean, Hirschl R. B., Coran A. G., Geiger J. D. Recurrence after laparoscopic and open Nissen fundoplication. Surg Endosc. 2003;17(5):704–707. doi: 10.1007/s00464-002-8515-5. [DOI] [PubMed] [Google Scholar]
- 42.Braghetto I., Csendes A., Korn O., Burdiles P., Valladares H., Cortes C., et al. Anatomical deformities after laparoscopic antireflux surgery. Int Surg. 2004;89(4):227–235. [PubMed] [Google Scholar]
- 43.Mickevicius A., Endzinas Z., Kiudelis M., Jonaitis L., Kupcinskas L., Maleckas A., et al. Influence of wrap length on the effectiveness of Nissen and Toupet fundoplication: a prospective randomized study. Surg Endosc. 2008;22(10):2269–2276. doi: 10.1007/s00464-008-9852-9. [DOI] [PubMed] [Google Scholar]
- 44.Jackson C. C., DeMeester S. R. Surgical therapy for Barrett's esophagus. Thorac Surg Clin. 2005;15(3):429–436. doi: 10.1016/j.thorsurg.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Little A. G. Failed antireflux operations: pathophysiology and treatment. Chest Surg Clin N Am. 1994;4(4):697–704. [PubMed] [Google Scholar]
- 46.Oeschlager B. K., Pellegrini C. A. Minimally invasive surgery for gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech. 2001;11(6):341–349. doi: 10.1089/10926420152761851. [DOI] [PubMed] [Google Scholar]
- 47.Farrell T. M., Smith C. D., Metreveli R. E. Fundoplication provides effective and durable symptoms relief in patients with Barrett's esophagus. Am J Surg. 1999;178(1):18–21. doi: 10.1016/s0002-9610(99)00111-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen L. Q., Ferraro P., Martini J., Duranceau A. C. Antireflux surgery or Barrett's esophagus: comparative results of the Nissen and Collins-Nissen. Dis Esophagus. 2005;18(5):320–328. doi: 10.1111/j.1442-2050.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 49.Jamieson G. G., France M., Watson D. I. Results of laparoscopic antireflux operation in patients who have Barrett's esophagus. Chest Surg Clin N Am. 2002;12(1):149–155. doi: 10.1016/s1052-3359(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 50.Furnée E. J., Draaisma W. A., Broeders I. A., Smout A. J., Goozen H. G. Surgical reintervention after antireflux surgery for gastroesophageal reflux disease: a prospective cohort study in 130 patients. Arch Surg. 2008;143(3):267–274. doi: 10.1001/archsurg.2007.50. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson M. K. Pitfalls and complications of antireflux surgery: Nissen and Collis Nissen techniques. Chest Surg Clin N Am. 1997;7(3):489–509. [PubMed] [Google Scholar]