Abstract
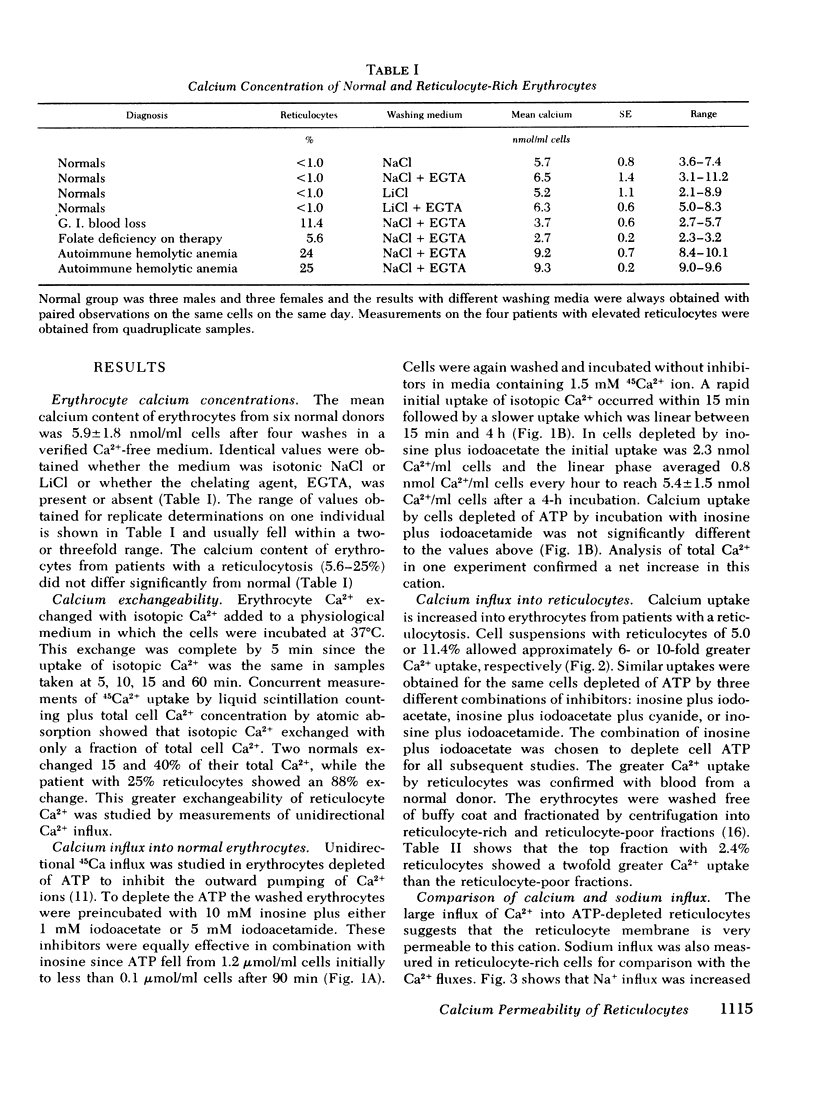
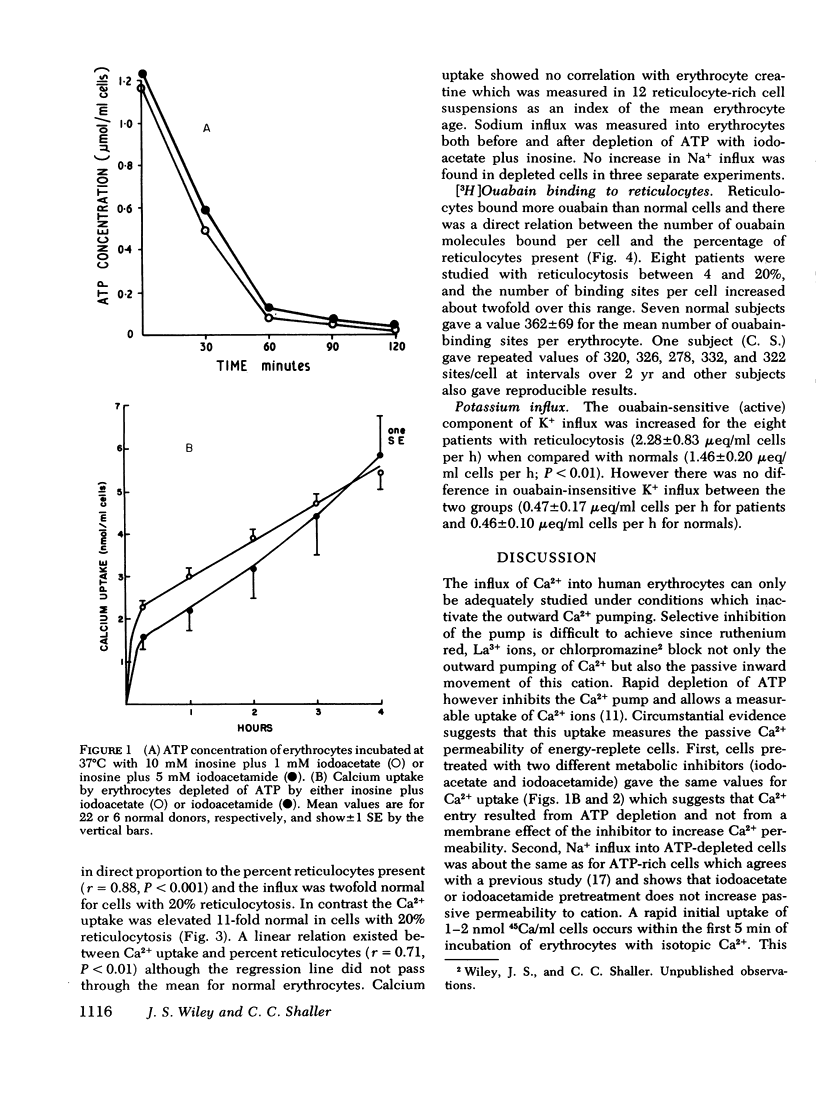
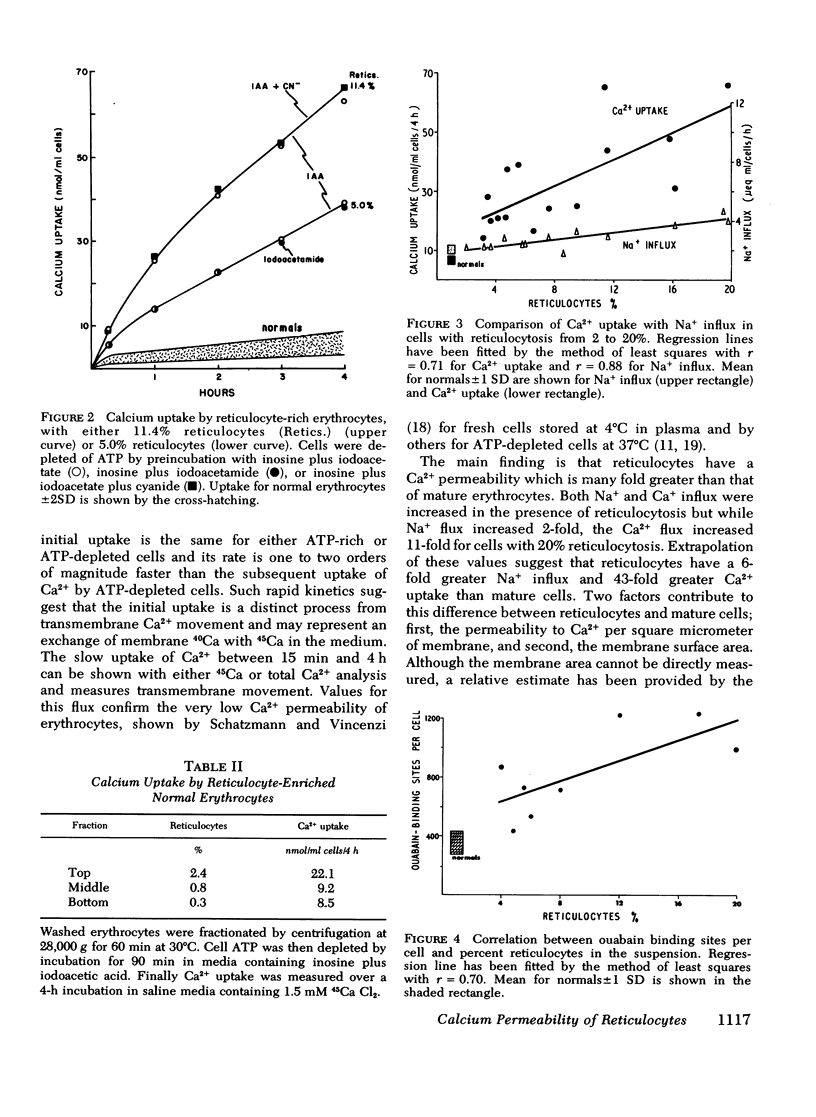
Calcium and sodium permeability of human reticulocytes have been studied and compared to mature erythrocytes. Mature erythrocytes had extremely low Ca2+ permeability which was less than 0.1% of values published for squid axon or HeLa cells. Calcium entry was markedly increased in reticulocyte-rich suspensions and the uptake was linearly related to the percentage of reticulocytes present. The data suggest that reticulocytes are 43-fold more permeable to Ca2+ than mature cells although their Ca2+ concentration is not increased. Sodium influx into reticulocyte-rich suspensions was also increased in direct proportion to the percent of reticulocytes present. Reticulocytes are sixfold more permeable to Na+ than mature cells so the ratio of Ca2+:Na+ permeability falls by sevenfold as the reticulocyte changes to an erythrocyte. [3H]Ouabain binding was increased in reticulocyte-rich cell suspensions and the correlation suggested a value of about 4,000 sites per reticulocyte compared with 362+/-69 per mature cell. Maturation of the human reticulocyte produces disproportionate changes in cation permeability and in particular a selective loss of Ca2+ permeability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. I. Calcium influx. J Gen Physiol. 1969 Jan;53(1):43–56. doi: 10.1085/jgp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Cittadini A., Scarpa A., Chance B. Calcium transport in intact Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Jan 2;291(1):246–259. doi: 10.1016/0005-2736(73)90416-1. [DOI] [PubMed] [Google Scholar]
- Come S. E., Shohet S. B., Robinson S. H. Surface remodeling vs. whole-cell hemolysis of reticulocytes produced with erythroid stimulation or iron deficiency anemia. Blood. 1974 Dec;44(6):817–830. [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. Bile salts and cholesterol in the pathogenesis of target cells in obstructive jaundice. J Clin Invest. 1968 Apr;47(4):809–822. doi: 10.1172/JCI105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Keynes R. D. Binding of tritiated digoxin to human red cell ghosts. Nature. 1969 Feb 22;221(5182):776–776. doi: 10.1038/221776a0. [DOI] [PubMed] [Google Scholar]
- Erdmann E., Hasse W. Quantitative aspects of ouabain binding to human erythrocyte and cardiac membranes. J Physiol. 1975 Oct;251(3):671–682. doi: 10.1113/jphysiol.1975.sp011115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Ganzoni A., Hillman R. S., Finch C. A. Maturation of the macroreticulocyte. Br J Haematol. 1969 Jan-Feb;16(1):119–135. doi: 10.1111/j.1365-2141.1969.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. J., Fitzpatrick M. The effect of age on the creatine in red cells. Br J Haematol. 1967 Mar;13(2):175–180. doi: 10.1111/j.1365-2141.1967.tb08728.x. [DOI] [PubMed] [Google Scholar]
- Griffiths W. J., Lothian E. J. Erythropoiesis, red-cell creatine and plasma aldolase activity in anaemia in the rabbit and man. Br J Haematol. 1969 Nov;17(5):477–484. doi: 10.1111/j.1365-2141.1969.tb01396.x. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Koch P. A., Gardner F. H., Carter J. R., Jr Red cell maturation: loss of a reticulocyte-specific membrane protein. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1296–1299. doi: 10.1016/0006-291x(73)91128-5. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M., Hoffman J. F. Some kinetic and metabolic characteristics of calcium-induced potassium transport in human red cells. J Gen Physiol. 1972 Oct;60(4):406–429. doi: 10.1085/jgp.60.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Joiner C. H. Increased potassium transport and ouabain binding in human Rhnull red blood cells. Blood. 1976 Sep;48(3):457–468. [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Divalent cation content of normal and ATP-depleted erythrocytes and erythrocyte membranes. Nouv Rev Fr Hematol. 1972 Nov-Dec;12(6):799–813. [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Cooper R. A. Maturation of macroreticulocyte membranes in vivo. J Lab Clin Med. 1972 Feb;79(2):215–227. [PubMed] [Google Scholar]
- Stryckmans P. A., Cronkite E. P., Giacomelli G., Schiffer L. M., Schnappauf H. The maturation and fate of reticulocytes after in vitro labeling with tritiated amino acids. Blood. 1968 Jan;31(1):33–43. [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Ellory J. C., Shuman M. A., Shaller C. C., Cooper R. A. Characteristics of the membrane defect in the hereditary stomatocytosis syndrome. Blood. 1975 Sep;46(3):337–356. [PubMed] [Google Scholar]
- Wiley J. S., Gill F. M. Red cell calcium leak in congenital hemolytic anemia with extreme microcytosis. Blood. 1976 Feb;47(2):197–210. [PubMed] [Google Scholar]
- Wise W. C. Maturation of membrane function: transport of amino acid by rat erythroid cells. J Cell Physiol. 1975 Dec;87(2):199–201. doi: 10.1002/jcp.1040870208. [DOI] [PubMed] [Google Scholar]


