Abstract
Oxidized low-density lipoprotein (OxLDL) contributes to the atherosclerotic plaque formation and progression by several mechanisms, including the induction of endothelial cell activation and dysfunction, macrophage foam cell formation, and smooth muscle cell migration and proliferation. Vascular wall cells express on their surface several scavenger receptors that mediate the cellular effects of OxLDL. The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is the main OxLDL receptor of endothelial cells, and it is expressed also in macrophages and smooth muscle cells. LOX-1 is almost undetectable under physiological conditions, but it is upregulated following the exposure to several proinflammatory and proatherogenic stimuli and can be detected in animal and human atherosclerotic lesions. The key contribution of LOX-1 to the atherogenic process has been confirmed in animal models; LOX-1 knockout mice exhibit reduced intima thickness and inflammation and increased expression of protective factors; on the contrary, LOX-1 overexpressing mice present an accelerated atherosclerotic lesion formation which is associated with increased inflammation. In humans, LOX-1 gene polymorphisms were associated with increased susceptibility to myocardial infarction. Inhibition of the LOX-1 receptor with chemicals or antisense nucleotides is currently being investigated and represents an emerging approach for controlling OxLDL-LOX-1 mediated proatherogenic effects.
1. Introduction
Atherosclerosis is a chronic inflammatory vascular disease, having as ultimate outcome the atheromatous plaque, a focal lesion located within the intima of large and medium sized arteries [1, 2]. Subendothelial retention of low density lipoprotein (LDL) and its oxidative modification represent the initial event in atherogenesis, which is followed by infiltration and activation of blood inflammatory cells. Oxidized LDL (OxLDL) in fact activates endothelial cells (ECs) by inducing the expression of several cell surface adhesion molecules which mediate the rolling and adhesion of blood leukocytes (monocytes and T cells); after adhesion to the endothelium, leukocytes migrate into the intima in response to chemokines. Monocytes then differentiate into macrophages that upregulate both toll-like receptors (TLRs), involved in macrophage activation, and scavenger receptors (SRs), that internalize apoptotic cell fragments, bacterial endotoxins, and OxLDL, leading to lipid accumulation and foam cell formation [2]. Macrophage activation leads to the release of proinflammatory cytokines, reactive oxygen species (ROS), proteolytic enzymes involved in matrix degradation and thus in atherosclerotic plaque destabilization. T cells respond to local peptide antigens present on the surface of antigen-presenting cells, become activated, and release proinflammatory cytokines [2].
OxLDL acts via binding to several SRs, including SR-A, SR-BI, CD36, and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) [3]. LOX-1 is a type II integral membrane glycoprotein consisting of a short N-terminal cytoplasmic domain, a transmembrane domain, a neck region, which regulates receptor oligomerization, and an extracellular C-type lectin-like extracellular domain, involved in ligand binding (Figure 1) [4]. LOX-1 has been identified first in ECs as the major OxLDL receptor [5]; however also macrophages and smooth muscle cells express LOX-1 together with other SRs [6]. Basal cellular LOX-1 expression is very low, but it can be induced by several proinflammatory and proatherogenic stimuli [7, 8]. In vitro, LOX-1 expression is induced by many stimuli related to atherosclerosis, including proinflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin-1 (IL-1), and interferon-gamma (IFNγ); angiotensin II; endothelin-1; OxLDL and other modified lipoproteins; free radicals; and fluid shear stress [8–10] (Table 1). In vivo, the expression of LOX-1 is upregulated in the presence of pathological conditions including atherosclerosis, hypertension, and diabetes [8] (Figure 2). In human atherosclerotic lesions, LOX-1 is overexpressed in ECs especially in the early stage of atherogenesis; in advanced atherosclerotic plaques, LOX-1 is overexpressed in ECs of neovascular formations [11]. Furthermore, LOX-1 is highly expressed by intimal smooth muscle cells (SMCs) and macrophages in human carotid atherosclerotic plaques [11], suggesting the roles for LOX-1 in endothelial activation and in foam cell formation. Finally, the results obtained in LOX-1 knockout or LOX-1 overexpressing mice have suggested a key contribution of LOX-1 in the inflammatory response and lipid deposition in heart vessels [12, 13].
Figure 1.
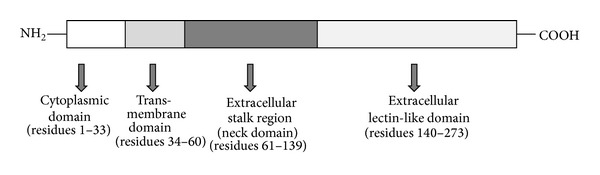
Schematic representation of LOX-1 structure. LOX-1 consists of four domains: a cytoplasmic N-terminal domain, a single transmembrane domain, an extracellular neck domain, and an extracellular lectin-like domain.
Table 1.
LOX-1 inducers.
| Proinflammatory cytokines | |
| Tumor necrosis factor α (TNFα) | |
| Interleukin-1 (IL-1) | |
| Interferon γ (IFNγ) | |
| Lipopolysaccharide (LPS) | |
| C-reactive protein (CRP) | |
| Modified lipoproteins | |
| OxLDL (copper-oxidized LDL) | |
| 15-Lipoxygenase-modified LDL | |
| 15-Lipoxygenase-modified HDL3 | |
| Glycoxidized-LDL | |
| Lysophosphatidylcholine (LPC) | |
| Palmitic acid | |
| Hypertension-related stimuli | |
| Angiotensin II | |
| Endothelin-1 | |
| Fluid shear stress | |
| Hyperglycemic stimuli | |
| High glucose | |
| Advanced glycation end-products (AGEs) | |
| Other stimuli | |
| Homocysteine | |
| Free radicals |
Figure 2.
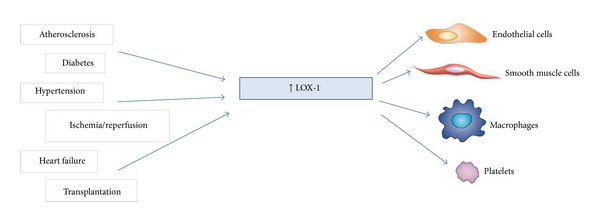
In vivo stimuli of LOX-1. Several pathological conditions can upregulate LOX-1 expression resulting in vascular wall cell activation.
Besides OxLDL, LOX-1 binds multiple ligands, including other forms of modified lipoproteins, advanced glycation end-products, activated platelets, and apoptotic cells [8–10] (Table 2). LOX-1 also binds delipidated OxLDL, suggesting that LOX-1 recognizes the modified apolipoprotein B; furthermore, LOX-1 binds with higher affinity to mildly oxidized LDL rather than extensively oxidized LDL, suggesting the ability to recognize also oxidized lipids (i.e., lipids that are covalently bound to the protein and are not removed by the delipidation process) [3] (Table 2). OxLDL is rapidly internalized into cells following the interaction with LOX-1; this internalization process is inhibited by LOX-1-blocking antibody which acts by preventing the binding of OxLDL with the receptor [14]. After the endocytosis of the complex OxLDL-LOX-1, the receptor is uncoupled from OxLDL, and both are located in separate compartments within the cytosol [14].
Table 2.
LOX-1 ligands.
| Modified lipoproteins | Other ligands |
|---|---|
| OxLDL (copper-oxidized LDL) | Apoptotic cells |
| 15-Lipoxygenase-oxidized LDL | Activated platelets |
| 15-Lipoxygenase-oxidized HDL3 | Advanced glycation end-products (AGEs) |
| Glycoxidized LDL | |
| Delipidated OxLDL | |
| HOCl-modified HDL |
LOX-1 is mainly localized in caveolae/lipid rafts (cholesterol-enriched membrane microdomains) in the plasma membrane; its function is modulated by the cholesterol content of membrane: cholesterol depletion induces the mislocalization of LOX-1, which results in a more diffuse distribution of the receptor in the plasma membrane (without a reduction of the amount of receptor exposed on the cell surface) and in a marked reduction of LOX-1-mediated OxLDL binding and uptake [15]. This finding suggests that the clustered distribution of LOX-1 in specific membrane microdomains is essential for an efficient interaction with OxLDL and for the internalization of OxLDL-LOX-1 complexes.
2. LOX-1-Mediated Endothelial Dysfunction
2.1. LOX-1 Upregulation by OxLDL
Endothelial dysfunction represents a very early stage in the atherogenic process and is a pathological condition characterized by alterations in anti-inflammatory and anticoagulant properties and by impaired ability to regulate vascular tone. LOX-1 is the main receptor for OxLDL in ECs [5], and OxLDL, through LOX-1, contributes to the induction of endothelial dysfunction by several mechanisms (Figure 3). Basal LOX-1 expression is very low, but it can be induced by proinflammatory cytokines generated in a local environment within the arterial wall or by other atherogenic stimuli including OxLDL [8].
Figure 3.
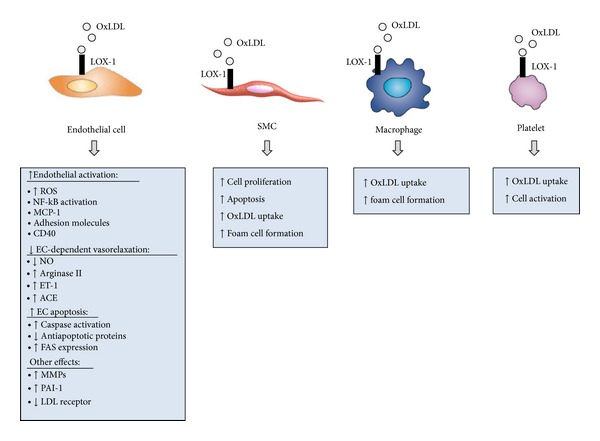
Role of LOX-1 in atherosclerosis. OxLDL binding to LOX-1 induces endothelial activation and dysfunction, supports the recruitment of circulating leukocytes, triggers foam cell formation, and sustains migration and proliferation of smooth muscle cells, thus contributing to the development of the atherosclerotic plaque. Furthermore, OxLDL-LOX-1 interaction may also contribute to plaque destabilization by inducing smooth muscle cell apoptosis and the release of matrix degrading enzymes (MMPs).
OxLDL upregulates LOX-1 mRNA and protein expression in a dose-dependent manner [16]; OxLDL-mediated upregulation of LOX-1 is suppressed by pretreatment of cells with antisense to LOX-1 mRNA [17], suggesting that OxLDL modulates its own receptor through interaction with LOX-1. In vitro, LOX-1 expression can be upregulated in ECs also by 15-lipoxygenase-modified LDL, and LOX-1 overexpression increases the association of 15-lipoxygenase-modified LDL to ECs [9], supporting the hypothesis that also minimally oxidized LDL may contribute to LOX-1 induction and to EC activation. LOX-1 upregulation occurs also in ECs exposed to 15-lipoxygenase-modified HDL3; this modified lipoprotein is also a ligand for LOX-1 [10].
2.2. LOX-1 Mediates OxLDL-Induced EC Activation
After adhesion to the endothelium, monocytes migrate into the intima where they differentiate into macrophages, accumulate lipids, and become foam cells. Recruitment of monocytes involves both chemokines and adhesion molecules. Monocyte chemoattractant protein-1 (MCP-1) is a chemotactic protein for monocytes; incubation of ECs with OxLDL significantly increases MCP-1 expression and monocyte adhesion to ECs; these effects are both suppressed in the presence of an antisense to LOX-1 mRNA [18]. Activation of mitogen-activated protein kinase (MAPK) is required for OxLDL-mediated induction of MCP-1, and antisense to LOX-1 mRNA completely inhibits the OxLDL-induced MAPK activation [18]. Additional chemokines are upregulated by LOX-1 activation in response to OxLDL, including IL-8, chemokine (C-X-C motif) ligands 2 and 3 (CXCL2 and CXCL3) [19].
Upregulation of endothelial adhesion molecules contributes to the leukocyte adhesion; OxLDL significantly increases the expression of E-selectin, P-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) in ECs [20]. These effects are mediated by LOX-1, since antisense to LOX-1 mRNA decreases both LOX-1 expression and adhesion molecule upregulation in response to OxLDL [20]. In addition, pretreatment of ECs with a statin or with polyinosinic acid or carrageenan (two known ligands of LOX-1) lowers OxLDL-induced expression of LOX-1 as well as adhesion molecules, confirming that LOX-1 activation plays an important role in OxLDL-induced expression of adhesion molecules [20, 21]. ICAM-1 expression is upregulated in LOX-1-overexpressing ECs exposed to 15-lipoxygenase-modified LDL [9], providing a role for minimally modified LDL (mmLDL) as proinflammatory particle.
Nuclear factor kappa B (NF-κB), which is activated by several proinflammatory cytokines, modulates the expression of proinflammatory genes, including adhesion molecules, cytokines, and chemokines, and is also involved in LOX-1 upregulation [8, 22]. OxLDL, following the binding with LOX-1, activates NF-κB; preincubation of ECs with anti-LOX-1 antibody markedly attenuates the transcription factor activation [23].
The stimulation of CD40/CD40L signaling results in the induction of proatherogenic pathways (including the expression of adhesion molecules and proinflammatory cytokines) and in EC activation [24]. Incubation of human coronary artery endothelial cells (HCAECs) with OxLDL increases the expression of CD40 and CD40L, while the inhibition of LOX-1 with a blocking antibody reduces the OxLDL-mediated increase of CD40 [25].
2.3. OxLDL-LOX-1 Interaction Impairs Endothelium-Dependent Relaxation
ECs, due to their location at the interface between blood and the vessel wall, play a crucial role in the control of vascular tone and homeostasis, through the production of several vasoactive mediators. Among them, nitric oxide (NO) is produced by endothelial NO synthase (eNOS) in response to several stimuli; functions mainly as a vasodilator; inhibits leukocyte-endothelial cell adhesion, platelet adhesion and aggregation, and smooth muscle cell proliferation; and stimulates angiogenesis [26]. In disease conditions, ECs exhibit a reduced NO bioavailability, due to an increased degradation of NO in response to an enhanced oxidative stress [26]. The release of NO by ECs can be reduced by OxLDL: OxLDL displaces eNOS from its caveolae membrane localization by depleting caveolae of cholesterol, thus inhibiting NO generation [27]. Furthermore, OxLDL inactivates NO through an oxidative mechanism by increasing cellular production of reactive oxygen species (ROS), and in particular superoxide [28]. Anti-LOX-1 monoclonal antibody reduces superoxide formation and increases intracellular NO level, suggesting the involvement of LOX-1 in these OxLDL-induced effects [28]. Additionally, OxLDL activates endothelial arginase II (which regulates NO production by competing with eNOS for the common substrate L-arginine) through the dissociation of arginase from the microtubule cytoskeleton, resulting in a decreased NO production and a raise of ROS [29, 30]. The increased arginase activity is inhibited by monoclonal antibody to LOX-1; furthermore, ECs from LOX-1 knockout (KO) mice do not increase arginase activity and exhibit reduced changes in NO and superoxide production in response to OxLDL [30], thus confirming the involvement of LOX-1 in OxLDL-mediated arginase activity. Further, neutralizing antibodies to LOX-1 restore NO-mediated coronary arteriolar dilation in ApoE KO mice [31].
Dysfunctional ECs can also generate vasoconstrictor factors, including endothelin-1 (ET-1) and angiotensin II (Ang II). ET-1 is a potent vasoconstrictor, proinflammatory, and mitogenic peptide produced by injured vascular ECs in response to several stimuli; a tight interaction exists between ET-1 and OxLDL: OxLDL stimulates the generation of ET-1, and ET-1 enhances the uptake of OxLDL in ECs by promoting the expression of LOX-1 [32]. Incubation of ECs with OxLDL increases ET-1 mRNA and protein expression, an effect inhibited by anti-LOX-1 antibody [33].
Renin-angiotensin system may contribute to the endothelial dysfunction; angiotensin-converting enzyme (ACE), which converts Ang I to Ang II, is mainly expressed in ECs [34]. Ang II induces LOX-1 expression and facilitates OxLDL uptake by ECs [35]; in turn, OxLDL increases the expression of ACE in a concentration- and time-dependent manner. The upregulation of ACE expression in response to OxLDL is mediated by LOX-1, as pretreatment of ECs with LOX-1-blocking antibody significantly reduces the OxLDL-induced expression of ACE [36].
2.4. LOX-1 Mediates OxLDL-Induced Apoptosis of ECs
Incubation with high concentrations of OxLDL induces cellular changes that may result in cell death. OxLDL may induce both necrosis and apoptosis; the last is a highly regulated process and involves multiple pathways, including ROS generation, caspase and protein kinase activation, alteration of calcium homeostasis, and alteration of proapoptotic/antiapoptotic gene expression [37]. EC apoptosis results in increased vascular permeability to cells and lipids, smooth muscle cell proliferation, and increased coagulation, thus contributing to the development of atherosclerotic lesions.
OxLDL induces EC apoptosis through LOX-1; in fact, inhibition with antisense to LOX-1 mRNA or with chemical inhibitors of LOX-1 significantly reduces the number of apoptotic cells in response to OxLDL [17]. NF-κB activation following EC exposure to OxLDL acts as a signal transduction mechanism in LOX-1-mediated apoptosis, and antisense to LOX-1 significantly inhibits OxLDL-induced NF-κB activation [17].
OxLDL also activates caspase-9 and caspase-3 in ECs and induces the release of mitochondrial activators of caspases, while reducing the expression of the antiapoptotic proteins B-cell lymphoma 2 (Bcl-2) and cellular inhibitor of apoptosis protein 1 (c-IAP-1) [38]. These effects are mediated by LOX-1, as pretreatment of cells with antisense to LOX-1 mRNA significantly decreases OxLDL-induced activation of caspases as well as the percentage of apoptotic cells [38]. These findings indicate that OxLDL through its receptor LOX-1 modulates activity and expression of relevant players in apoptosis.
Fas is a death-receptor that triggers apoptosis when activated by its ligand FasL and is involved in OxLDL-induced apoptosis; in fact, OxLDL sensitizes vascular cells to Fas-mediated apoptosis, upregulating Fas surface expression, while OxLDL-induced apoptosis is reduced by FasL-neutralizing antibodies [37]. LOX-1 activation is involved in these effects, as neutralizing LOX-1 antibody prevents OxLDL-induced activation of Fas-mediated apoptosis and inhibits OxLDL-induced modulation of surface Fas expression in ECs [39].
2.5. OxLDL Increases Oxidative Stress through LOX-1 in ECs
High levels of ROS are produced in several disease conditions, including atherosclerosis, and contribute to endothelial dysfunction. ROS are involved in LDL oxidation, and, in turn, OxLDL mediates many of its biological effects by generating more intracellular ROS through the binding to LOX-1; anti-LOX-1 antibody markedly reduces OxLDL-induced ROS formation [23, 40]. OxLDL-induced increase of ROS also results in NF-κB activation [23] and in EC apoptosis [40]; both these effects are mediated by LOX-1, as pretreatment of the cells with LOX-1-blocking antibody significantly reduces ROS production, NF-κB activation, and apoptosis rate in response to OxLDL [23, 40].
Endothelial NADPH oxidase, a multisubunit enzymatic complex, is a major source of ROS in vascular ECs, and OxLDL induces a significant increase of NADPH oxidase-generated ROS in ECs [41]. The binding of OxLDL to LOX-1 activates NADPH oxidase by inducing translocation of specific subunits on the cell membrane, leading to a rapid increase in intracellular ROS such as hydrogen peroxide and superoxide; the latter reacts with intracellular NO, thereby causes intracellular NO level to decrease, and upregulates LOX-1 expression, thus resulting in further increase in ROS production [42].
p66Shc is a redox enzyme involved in mitochondrial ROS generation and the translation of oxidative signals into apoptosis; higher levels of p66Shc have been found under pathological conditions and well correlated with oxidative stress in cardiovascular disease [43]. When exposed to OxLDL, ECs increase phosphorylation of p66Shc, an effect that is prevented by blockade or molecular silencing of LOX-1 [43], supporting a role for LOX-1 also in this OxLDL-mediated effect. On the other hand, lysophosphatidylcholine (LPC), a relevant component of OxLDL, induced p66Shc activation independently of LOX-1 [43].
In HCAECs, OxLDL promotes intracellular ROS production and induces DNA oxidative damage [44], resulting in the regulation of several transcription factors, including NF-κB and octamer-binding transcription factor-1 (Oct-1). Oct-1 acts as a transcriptional repressor for endothelial enzymes involved in the production of vasoactive molecules, thus providing a link between oxidative DNA damage and impaired function of endothelium upon exposure to OxLDL. Oct-1 is also involved in OxLDL-induced LOX-1 promoter activation and gene expression [45]. Inhibition of LOX-1 attenuates OxLDL-mediated endothelial DNA damage, Oct-1/DNA binding, and reverses impaired production of vasoactive compound [44].
2.6. Other Effects of OxLDL Mediated by LOX-1 in ECs
Incubation of ECs with OxLDL modulates the expression of several other genes associated with atherosclerosis, and LOX-1 plays a crucial role in mediating such effects.
Metalloproteinases (MMPs) are a family of matrix degrading enzymes involved in vascular remodeling that contribute to the determination of atherosclerotic plaque stability; their expression and activity are increased in atherosclerotic plaques [46]. OxLDL increases the expression of MMP-1 and MMP-3 mRNA and protein in ECs, without affecting the expression of tissue inhibitor of metalloproteinases (TIMPs) [47], suggesting an OxLDL-induced imbalance between MMPs and TIMPs. LOX-1 activation mediates the modulation of MMPs by OxLDL: LOX-1-blocking antibody prevents the increase of MMPs in response to OxLDL [47]. Similarly, OxLDL enhances MMP-9 production in human aortic ECs, and anti-LOX-1 antibody inhibits this effect [48].
Angiogenesis is a highly regulated physiological process involved in several pathological conditions including inflammation and atherosclerosis and requires disruption of cell-cell contact, EC migration, proliferation, and capillary tube formation. Generation of small amount of ROS, as those induced by low concentrations of OxLDL (<5 µg/mL), seems to be involved in this process. Low concentrations of OxLDL stimulate tube formation from ECs in vitro. This effect is inhibited by anti-LOX-1 antibody, that also decreases OxLDL-induced ROS generation, NF-κB activation, and upregulation of vascular endothelial growth factor (VEGF) [49].
The plasminogen activator (PA) is involved in the control of fibrinolysis within the vascular lumen; plasminogen activator inhibitor-1 (PAI-1) attenuates fibrinolysis through inhibition of plasminogen activation and modulates cellular responses; increased expression of PAI-1 has been described in atherosclerosis [50]. OxLDL induces PAI-1 upregulation in cultured ECs through LOX-1, as showen by the inhibitory effect of anti-LOX-1-blocking antibody; this finding suggests an involvement of LOX-1 also in OxLDL-induced thrombotic process [51].
The LDL receptor regulates plasma LDL-cholesterol levels. OxLDL decreases the expression of LDL receptor in a concentration- and time-dependent manner in HCAECs [52]. This effect is mediated by LOX-1, as cell pretreatment with LOX-1-blocking antibody or with antisense to LOX-1 mRNA reduces the effect of OxLDL on LDL-receptor expression [52].
2.7. LOX-1 Mediates OxLDL-Induced Effects in Endothelial Progenitor Cells
Endothelial progenitor cells (EPCs) are involved in the regeneration of the injured endothelium and in the neovascularization process, which represents a compensatory mechanism in ischemic disease; atherosclerosis is associated with reduced numbers and impaired functionality of EPCs [53]. OxLDL has a negative effect on EPCs, induces EPC senescence, inhibits VEGF-induced EPC differentiation, decreases EPC number, and impairs their function [54–56]. LOX-1 is expressed in EPCs, and incubation of EPCs with OxLDL increases LOX-1 expression [57], an effect depending on the interaction between OxLDL and LOX-1, as confirmed by the use of an anti-LOX-1 antibody. OxLDL also induces EPC apoptosis in a dose-dependent manner, thus reducing their survival; moreover, OxLDL impairs EPC adhesive, migratory, and tube formation capacities [57]. All these effects are attenuated by pretreatment with a LOX-1 monoclonal antibody. Furthermore, OxLDL reduces eNOS expression and NO production, that may in part explain the inhibitory effect of OxLDL on EPC survival and function [57]. Pretreatment with anti-LOX-1 antibody inhibits all these OxLDL-induced effects.
At low concentrations (<10 μg/mL) OxLDL does not induce apoptosis but accelerates EPC senescence; this effect is significantly attenuated by LOX-1-blocking antibody or by atorvastatin (that reduces LOX-1 expression) [54]. OxLDL significantly reduces telomerase activity (which plays a critical role in cellular senescence) and impairs proliferation and network formation capacity, resulting in cellular dysfunction [54].
3. Role of LOX-1 in OxLDL-Induced Smooth Muscle Cell Proliferation and Apoptosis
LOX-1 is expressed also in smooth muscle cells (SMCs) [6]; in vitro, several stimuli, including OxLDL and Ang II, can upregulate LOX-1 expression in SMCs [58–60]; in vivo, LOX-1 protein is not detectable in the media of noninjured aorta but is present in SMCs two days after vascular injury or after balloon-angioplasty [61]. OxLDL and LOX-1 colocalize with SMCs of human restenotic lesions, suggesting a role for LOX-1 in OxLDL-induced SMC proliferation and restenosis [61].
MicroRNAs are small noncoding RNAs that negatively modulate gene expression through the binding with their mRNA and play a role also in atherosclerosis [60]. MicroRNA let-7g targets LOX-1 gene and inhibits its expression; OxLDL, by inducing the transcription factor Oct-1, reduces let-7g expression. Let-7g mimic reduces OxLDL-induced LOX-1 and Oct-1 upregulation as well as OxLDL-enhanced SMC proliferation and migration [60].
At higher concentrations, OxLDL upregulates LOX-1 expression and induces apoptosis of vascular SMCs [62] (Figure 3), a process that may contribute to atherosclerotic plaque destabilization. OxLDL-induced apoptosis is a consequence of LOX-1 upregulation, as apoptosis is inhibited by a neutralizing anti-LOX-1 antibody. Furthermore, OxLDL increases the expression of the proapoptotic protein Bcl-2-associated X protein (Bax) and inhibits the expression of the antiapoptotic Bcl2. This effect is mediated by LOX-1, as anti-LOX-1 antibody markedly inhibits OxLDL-induced modulation of these two proteins [62]. LOX-1 colocalizes with Bax in human atherosclerotic plaques, particularly in rupture-prone shoulder region, suggesting a role for LOX-1 in the mechanisms that contribute to plaque destabilization [62].
Since SMCs can transform into foam cells, LOX-1 might be a potential player in this process (Figure 3). Treatment of SMCs with LPC increases LOX-1 expression [63], with consequent LOX-1-mediated increase of OxLDL uptake; anti-LOX-1 antibody markedly inhibits the LPC-enhanced OxLDL uptake [63].
Bone marrow cells can potentially originate smooth muscle progenitor cells (SMPCs) that may differentiate into smooth muscle-like cells (SMLCs) in the damaged vessels [64], thus contributing to the atherogenic process. In vitro, long-term culture with platelet-derived growth factor PDGF-BB induces the differentiation of SMPCs into SMLCs that express SMC-specific markers [65]; OxLDL inhibits PDGF-BB-induced differentiation of SMLCs, as revealed by the decrease of SMC marker expression in the presence of OxLDL. Furthermore, OxLDL incubation induces lipid droplet accumulation in the cytoplasm and LOX-1 surface expression [65]. Anti-LOX-1 antibody significantly reduces OxLDL uptake by SMLCs, suggesting a role for LOX-1 in the transdifferentiation of SMLCs into foam-like cells [65].
4. Role of LOX-1 in OxLDL-Induced Effects in Macrophages
Macrophages internalize OxLDL by several SRs including SR-AI/II, SR-BI, cluster of differentiation 36 (CD36), and LOX-1, resulting in lipid accumulation and transformation into foam cells. LOX-1 is not detectable in freshly isolated human monocytes, but its expression increases in differentiated macrophages [66]. LOX-1 expression in macrophages can be upregulated by several stimuli, including OxLDL, LPC, high-glucose levels, and proinflammatory cytokines [8]. The contribution of LOX-1 in macrophage uptake and degradation of OxLDL under normal conditions is small. No significant differences are observed between wild-type and LOX-1 deficient macrophages [67], probably due to the high expression of other SRs that could mask the contribution of LOX-1. However, in cells stimulated with LPC, LOX-1 expression and OxLDL uptake and degradation increase in wilt type cells but not in LOX-1-deficient cells [67]. These observations suggest that LOX-1 gene inactivation does not markedly modify OxLDL uptake in unstimulated macrophages, as LOX-1 accounts for 5–10% of OxLDL uptake by these cells, but, when LOX-1 is upregulated, internalization of OxLDL increases by more than 40% [67] (Figure 2). Proinflammatory cytokines upregulate LOX-1 and downregulate other SRs (SR-AI/II and CD36), suggesting that, in inflamed microenvironments, where these cytokines are relatively abundant (such as in atherosclerotic lesions), LOX-1 might play a significant role in macrophage OxLDL uptake.
Monocytes may also differentiate into dendritic cells (DCs), a specific type of leukocytes that play a key role in the initiation of innate and adaptive immune responses, and OxLDL affects both DC maturation and migration [68, 69]. LOX-1 is highly expressed on mature DCs and significantly contributes to OxLDL uptake, as anti-LOX-1 antibody reduced OxLDL uptake by 48% [70].
5. Role of LOX-1 in Platelet Activation
Platelets express several SRs, some of which are constitutively expressed (including CD36), while LOX-1 appears on the surface of platelets on activation [71]; thus, in resting platelets, CD36 mediates OxLDL binding to platelets, while, in activated platelets, in which OxLDL binding is increased compared to resting cells, binding of OxLDL is mediated by both CD36 and LOX-1 [71]. Activated platelets internalize significant amount of OxLDL compared with resting platelets [72] (Figure 3); OxLDL induces platelet activation, increases their ability to adhere to ECs, induces an inflammatory response, and leads to platelet accumulation after vascular injury. In fact, OxLDL-positive platelets induce adhesion molecule expression on ECs, reduce regeneration of ECs, and induce foam cell formation [72], suggesting that OxLDL-activated platelets may contribute to vascular inflammation by several mechanisms.
Dysfunctional endothelium exhibits procoagulant and adhesive properties to platelets, and endothelial LOX-1 plays a major role in the platelet-endothelium interaction thus enhancing endothelial dysfunction. In fact LOX-1 binds also anionic phospholipids, including those present on the surface of apoptotic cells or activated platelets [73], thus working as an adhesion molecule for platelets. OxLDL partially inhibits the binding of platelets to LOX-1, indicating a high affinity of platelets for this receptor [73]. The binding of activated platelets to endothelial LOX-1 induces the release of ET-1, further supporting the induction of endothelial dysfunction [73]. Furthermore, the binding of activated platelet to endothelial LOX-1 induces ROS generation followed by a reduction of NO bioavailability; all effects are prevented by anti-LOX-1 antibody [74].
6. LOX-1 and Atherosclerosis: Experimental Evidences
As previously described, LOX-1 mediates many of the effects of OxLDL, that is, EC growth, dysfunction, adhesion, and activation of monocytes/macrophages, and all critical features of atherosclerosis. LOX-1 mRNA has also been found in human atherosclerotic plaques, while negligible amounts are detectable in unaffected aortas [11]. The key relevance of LOX-1 in atherosclerosis has been demonstrated by key experiments in LOX-1 KO mice crossed to a mouse model prone to develop atherosclerosis such as the LDL-R KO mouse. Feeding these mice with a cholesterol-rich diet results in a reduced binding of OxLDL to the aortic endothelium and a preservation of endothelium-dependent vasorelaxation after treatment with OxLDL compared to wild type animals [12]. More importantly, LOX-1 KO/LDL-R KO animals show a significant reduction in atherosclerosis compared with LDL-R KO mice [12]. This is associated with reduced NF-κB expression as well as decreased inflammatory markers and also with an increased eNOS expression, suggesting an improved endothelial function [12]. Conversely, mice overexpressing LOX-1 on ApoE KO background show a dramatic increase in atherosclerosis compared to the nontransgenic mice [13]. In hypercholesterolemic rabbits, LOX-1 expression is detected mainly in atherosclerotic plaques with a thinner fibromuscular cap and is localized to the lipid core where it correlates with tissue factor expression and apoptosis [75], potentially connecting LOX-1 with plaque destabilization and rupture. In line with this, LOX-1 expression is increased in unstable plaques when the AMI-prone Watanabe heritable hyperlipidemic rabbit and control rabbits are injected with an antibody tracing LOX-1 [76].
Among the critical player in atherogenesis, the activation of the rennin-angiotensin system and the consequent generation of Ang II are thought to be critical factors. Ang II via its type 1 receptor (AT1 receptor) also upregulates the expression of LOX-1 mRNA, and OxLDL via LOX-1 upregulates the expression of AT1 receptor [77]. Of note hypertensive rats present an upregulation of LOX-1 expression mainly in vascular ECs [78–80]. The mutually facilitatory cross-talk between LOX-1 and AT1 receptors may explain the coexistence of multiple risk factors in the same patient and the increase in atherosclerosis risk with the presence of multiple risk factors. In agreement with this assumption, LOX-1 expression is increased in several animal models of cardiometabolic disorders, including streptozotocin-induced diabetes [81] or ischemia-reperfusion (I/R) injury [82]. LOX-1 deficiency is associated with a more preserved left ventricular function following I/R injury which resulted in a reduced oxidative stress, collagen deposition, and fibronectin expression thus resulting in a significant decrease in myocardial injury as well as in the accumulation of inflammatory cells [83, 84].
Altogether, the results from experimental atherosclerosis support a proatherogenic role for LOX-1.
7. Genetics of LOX-1 and Atherosclerosis
LOX-1 is encoded by the oxidized low-density lipoprotein (lectin-like) receptor 1 (OLR1) gene, mapped to chromosome 12p13 [85]. The association of polymorphisms in the human OLR1 gene with the susceptibility to myocardial infarction (MI) has been reported [85, 86]. In particular, six single nucleotide polymorphisms (SNPs), located within introns 4, 5, and 3′ UTR (untranslated region), that are comprised in a linkage disequilibrium (LD) block are strongly associated with the elevated risk to develop MI [86]. The observation that the SNPs related to an increased risk of MI do not affect the coding sequence of the gene suggests the possibility that the SNPs could give rise to a functional product such as messenger RNA (mRNA) isoforms as a consequence of alternative splicing. Indeed it has been shown that the SNPs located in the LD block regulate the level of the new fully functional transcript by modulating the retention of exon 5 of the OLR1 gene [85]. The alternative splicing of OLR1 mRNA leads to different ratios of LOX-1 full receptor and LOXIN (lack of exon 5), an isoform lacking part of the C-terminus lectin-like domain which is a functional domain. LOXIN, through heterooligomerization with LOX-1 [87], blocks the negative effects of LOX-1 activation, and this variant has a functional role on plaque instability and therefore in the pathogenesis of MI [86]. Ex vivo data show that macrophages from subjects carrying the “nonrisk” allele at OLR1 gene display increased expression of LOXIN resulting in protection against OxLDL-mediated apoptosis [87].
Later, an SNP on exon 4, rs11053646 (G501C), which leads to an amino acidic substitution (lysine to asparagine at position 167, K167N) has been studied. Of note, basic residues in the lectin domain are important for strengthening the ligand binding; substitution of this residue (K167N) causes a change on the positive isopotential surface and thereby results in reduced binding and internalization of OxLDL [88]. This study, performed on CV-1 (simian) in Origin and carrying the SV40 (COS-1) cells overexpressing either GG (KK) or CC (NN) LOX-1, showed that GG (KK) COS-1 cells bind and internalize less OxLDL than CC (NN) [88].
The K167N SNP has been identified among others in the ORL-1 gene to be associated with acute MI and coronary artery disease (CAD) [89–91]. The frequencies of the KK genotype and the K allele are higher in the CAD group than in controls (P < 0.05), while the opposite is true for NN genotype (P < 0.05) [92]. The relevance of LOX-1 SNPs has been tested also in relation to markers of atherosclerosis. Among them, ultrasound detection and quantification of the common carotid artery wall thickness (intima-media thickness, IMT) are considered a surrogate marker of subclinical atherosclerosis [93, 94]. The association between the nonsynonymous substitution K167N (rs11053646) and IMT has been tested in 2,141 samples from the Progression of Lesions in the Intima of the Carotid (PLIC) study (a prospective population-based study) [95]. Significantly increased IMT has been observed in male carriers of the minor C (N) allele compared to GC and GG (KN and KK) genotypes [95]. A gender-specific association has been also described between the C (N) allele and prevalence of carotid plaque also in a cohort of Dominican-Hispanic origin [96].
Functional analysis on macrophages suggests a decreased association to OxLDL in NN carriers compared to KN and KK carriers which is also associated with a reduced OLR1 mRNA expression [95]. Macrophages from NN carriers present also a specific inflammatory gene expression pattern compared to cells from KN and KK carriers [95]. How these data, obtained with human primary macrophages, relate with those obtained in a transfected fibroblast-like cell line derived from monkey kidney tissue is unclear, and further studies are warranted to clarify this issue [88].
In summary, genetic alterations favoring LOXIN isoform production coupled to the observation that this isoform exerts a dominant-negative effect on LOX-1 function make it an attractive new target for prevention and treatment of initiation, progression, and clinical consequences of atherosclerosis such as plaque instability, acute myocardial infarction, and ischemia reperfusion injury.
8. Concluding Remarks
OxLDL plays multiple roles in atherosclerosis; LOX-1 scavenger receptor mediates many of the OxLDL-induced effects, and the OxLDL-LOX-1 interaction can alter the expression of several genes, resulting in the induction of cellular dysfunction, proliferation, and apoptosis. A recent study showed that LOX-1 inhibition in ApoE KO mice using a schizophyllan-based antisense oligonucleotide therapy resulted in reduced LOX-1 expression in the arterial wall [97]. Blocking the expression and/or function of LOX-1 results in the improvement of cellular functions and reduction of atherosclerotic lesion formation, suggesting that LOX-1 might be an attractive therapeutic target for the management and the prevention of atherosclerosis.
References
- 1.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annual Review of Pathology. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 3.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxidants and Redox Signaling. 2010;13(1):39–75. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochemical Journal. 2008;409(2):349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- 5.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 6.Draude G, Hrboticky N, Lorenz RL. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochemical Pharmacology. 1999;57(4):383–386. doi: 10.1016/s0006-2952(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 7.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovascular Research. 2006;69(1):36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cellular and Molecular Life Sciences. 2012 doi: 10.1007/s00018-012-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirillo A, Reduzzi A, Ferri N, Kuhn H, Corsini A, Catapano AL. Upregulation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) by 15-lipoxygenase-modified LDL in endothelial cells. Atherosclerosis. 2011;214(2):331–337. doi: 10.1016/j.atherosclerosis.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Pirillo A, Uboldi P, Ferri N, Corsini A, Kuhn H, Catapano AL. Upregulation of lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human endothelial cells by modified high density lipoproteins. Biochemical and Biophysical Research Communications. 2012;428(2):230–233. doi: 10.1016/j.bbrc.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka H, Kume N, Miyamoto S, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99(24):3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 12.Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circulation Research. 2007;100(11):1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circulation Research. 2005;97(2):176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- 14.Twigg MW, Freestone K, Homer-Vanniasinkam S, Ponnambalam S. The LOX-1 scavenger receptor and its implications in the treatment of vascular disease. Cardiology Research and Practice. 2012;2012:6 pages. doi: 10.1155/2012/632408.632408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matarazzo S, Quitadamo MC, Mango R, Ciccone S, Novelli G, Biocca S. Cholesterol-lowering drugs inhibit lectin-like oxidized low-density lipoprotein-1 receptor function by membrane raft disruption. Molecular Pharmacology. 2012;82(2):246–254. doi: 10.1124/mol.112.078915. [DOI] [PubMed] [Google Scholar]
- 16.Mehta JL, Li DY. Identification and autoregulation of receptor for ox-LDL in cultured human coronary artery endothelial cells. Biochemical and Biophysical Research Communications. 1998;248(3):511–514. doi: 10.1006/bbrc.1998.9004. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(4):1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101(25):2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 19.Mattaliano MD, Huard C, Cao W, et al. LOX-1-dependent transcriptional regulation in response to oxidized LDL treatment of human aortic endothelial cells. American Journal of Physiology. 2009;296(6):C1329–C1337. doi: 10.1152/ajpcell.00513.2008. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. Journal of Pharmacology and Experimental Therapeutics. 2002;302(2):601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Xia M, Hou M, et al. Ox-LDL plays dual effect in modulating expression of inflammatory molecles through LOX-1 pathway in human umbilical vein endothelial cells. Frontiers in Bioscience. 2005;10(2):2585–2594. doi: 10.2741/1722. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6) doi: 10.1101/cshperspect.a001651.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cominacini L, Fratta Pasini A, Garbin U, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-κB through an increased production of intracellular reactive oxygen species. Journal of Biological Chemistry. 2000;275(17):12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 24.Lievens D, Eijgelaar WJ, Biessen EAL, Daemen MJAP, Lutgens E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thrombosis and Haemostasis. 2009;102(2):206–214. doi: 10.1160/TH09-01-0029. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Liu L, Chen H, Sawamura T, Mehta JL. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(5):816–821. doi: 10.1161/01.ATV.0000066685.13434.FA. [DOI] [PubMed] [Google Scholar]
- 26.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. European Heart Journal. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. Journal of Biological Chemistry. 1999;274(45):32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 28.Cominacini L, Rigoni A, Pasini AF, et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. Journal of Biological Chemistry. 2001;276(17):13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 29.Ryoo S, Lemmon CA, Soucy KG, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circulation Research. 2006;99(9):951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 30.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214(2):279–287. doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Gao X, Potter BJ, Cao J-M, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(4):871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 32.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sciences. 2012;91(13-14):507–516. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai K, Cominacini L, Garbin U, et al. Induction of endothelin-1 production in endothelial cells via co-operative action between CD40 and lectin-like oxidized LDL receptor (LOX-1) Journal of Cardiovascular Pharmacology. 2004;44(supplement 1):S173–S180. doi: 10.1097/01.fjc.0000166243.43616.8b. [DOI] [PubMed] [Google Scholar]
- 34.Montecucco F, Pende A, Mach F. The renin-angiotensin system modulates inflammatory processes in atherosclerosis: evidence from basic research and clinical studies. Mediators of Inflammation. 2009;2009:13 pages. doi: 10.1155/2009/752406.752406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morawietz H, Rueckschloss U, Niemann B, et al. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation. 1999;100(9):899–902. doi: 10.1161/01.cir.100.9.899. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Singh RM, Liu L, et al. Oxidized-LDL through LOX-1 increases the expression of angiotensin converting enzyme in human coronary artery endothelial cells. Cardiovascular Research. 2003;57(1):238–243. doi: 10.1016/s0008-6363(02)00674-0. [DOI] [PubMed] [Google Scholar]
- 37.Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochimica et Biophysica Acta. 2002;1585(2-3):213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circulation Research. 2004;94(3):370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 39.Imanishi T, Hano T, Sawamura T, Takarada S, Nishio I. Oxidized low density lipoprotein potentiation of Fas-induced apoptosis through lectin-like oxidized-low density lipoprotein receptor-1 in human umbilical vascular endothelial cells. Circulation Journal. 2002;66(11):1060–1064. doi: 10.1253/circj.66.1060. [DOI] [PubMed] [Google Scholar]
- 40.Chen X-P, Xun K-L, Wu Q, Zhang T-T, Shi J-S, Du G-H. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascular Pharmacology. 2007;47(1):1–9. doi: 10.1016/j.vph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Rueckschloss U, Galle J, Holtz J, Zerkowski H-R, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104(15):1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 42.Ou H-C, Song T-Y, Yeh Y-C, et al. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. Journal of Applied Physiology. 2010;108(6):1745–1756. doi: 10.1152/japplphysiol.00879.2009. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Cosentino F, Camici GG, et al. Oxidized low-density lipoprotein activates p66shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase c-β, and c-jun n-terminal kinase kinase in human endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(9):2090–2097. doi: 10.1161/ATVBAHA.111.229260. [DOI] [PubMed] [Google Scholar]
- 44.Thum T, Borlak J. LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. Journal of Biological Chemistry. 2008;283(28):19456–19464. doi: 10.1074/jbc.M708309200. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Liu Y, Liu H, Hermonat PL, Mehta JL. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) transcriptional regulation by Oct-1 in human endothelial cells: implications for atherosclerosis. Biochemical Journal. 2006;393(part 1):255–265. doi: 10.1042/BJ20050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. The Canadian Journal of Cardiology. 2006;22(supplement B):25B–30B. doi: 10.1016/s0828-282x(06)70983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107(4):612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Renier G. The oral anti-diabetic agent, gliclazide, inhibits oxidized LDL-mediated LOX-1 expression, metalloproteinase-9 secretion and apoptosis in human aortic endothelial cells. Atherosclerosis. 2009;204(1):40–46. doi: 10.1016/j.atherosclerosis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of OXLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(11):2435–2442. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 50.Diebold I, Kraicun D, Bonello S, Görlach A. The ‘PAI-1 paradox’ in vascular remodelling. Thrombosis and Haemostasis. 2008;100(6):984–991. [PubMed] [Google Scholar]
- 51.Sangle GV, Zhao R, Shen GX. Transmembrane signaling pathway mediates oxidized low-density lipoprotein-induced expression of plasminogen activator inhibitor-1 in vascular endothelial cells. American Journal of Physiology. 2008;295(5):E1243–E1254. doi: 10.1152/ajpendo.90415.2008. [DOI] [PubMed] [Google Scholar]
- 52.Hu B, Li D, Sawamura T, Mehta JL. Oxidized LDL through LOX-1 modulates LDL-receptor expression in human coronary artery endothelial cells. Biochemical and Biophysical Research Communications. 2003;307(4):1008–1012. doi: 10.1016/s0006-291x(03)01295-6. [DOI] [PubMed] [Google Scholar]
- 53.Du F, Zhou J, Gong R, et al. Endothelial progenitor cells in atherosclerosis. Frontiers in Bioscience. 2011;17(6):2327–2349. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clinical and Experimental Pharmacology and Physiology. 2004;31(7):407–413. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 55.Imanishi T, Hano T, Matsuo Y, Nishio I. Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clinical and Experimental Pharmacology and Physiology. 2003;30(9):665–670. doi: 10.1046/j.1440-1681.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Chen J, Tao Q, Zhu J, Shang Y. Effects of ox-LDL on number and activity of circulating endothelial progenitor cells. Drug and Chemical Toxicology. 2004;27(3):243–255. doi: 10.1081/dct-120037505. [DOI] [PubMed] [Google Scholar]
- 57.Feng XM, Zhou B, Chen Z, et al. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. Journal of Lipid Research. 2006;47(6):1227–1237. doi: 10.1194/jlr.M500507-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y, Chen X. Ox-LDL-induced LOX-1 expression in vascular smooth muscle cells: role of reactive oxygen species. Fundamental and Clinical Pharmacology. 2011;25(5):572–579. doi: 10.1111/j.1472-8206.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 59.Limor R, Kaplan M, Sawamura T, et al. Angiotensin II increases the expression of lectin-like oxidized low-density lipoprotein receptor-1 in human vascular smooth muscle cells via a lipoxygenase-dependent pathway. American Journal of Hypertension. 2005;18(3):299–307. doi: 10.1016/j.amjhyper.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Chen K-C, Hsieh I-C, Hsi E, et al. Negative feedback regulation between microRNA let-7g and the oxLDL receptor LOX-1. Journal of Cell Science. 2011;124(23):4115–4124. doi: 10.1242/jcs.092767. [DOI] [PubMed] [Google Scholar]
- 61.Eto H, Miyata M, Kume N, et al. Expression of lectin-like oxidized LDL receptor-1 in smooth muscle cells after vascular injury. Biochemical and Biophysical Research Communications. 2006;341(2):591–598. doi: 10.1016/j.bbrc.2005.12.211. [DOI] [PubMed] [Google Scholar]
- 62.Kataoka H, Kume N, Miyamoto S, et al. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(6):955–960. doi: 10.1161/01.atv.21.6.955. [DOI] [PubMed] [Google Scholar]
- 63.Aoyama T, Chen M, Fujiwara H, Masaki T, Sawamura T. LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Letters. 2000;467(2-3):217–220. doi: 10.1016/s0014-5793(00)01154-6. [DOI] [PubMed] [Google Scholar]
- 64.Caplice NM, Bunch TJ, Stalboerger PG, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu J, Li Y, Li M, Qu Z, Ruan Q. Oxidized low density lipoprotein-induced transdifferentiation of bone marrow-derived smooth muscle-like cells into foam-like cells in vitro. International Journal of Experimental Pathology. 2010;91(1):24–33. doi: 10.1111/j.1365-2613.2009.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochemical Journal. 1998;334(part 1):9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaeffer DF, Riazy M, Parhar KS, et al. LOX-1 augments oxLDL uptake by lysoPC-stimulated murine macrophages but is not required for oxLDL clearance from plasma. Journal of Lipid Research. 2009;50(8):1676–1684. doi: 10.1194/jlr.M900167-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perrin-Cocon L, Coutant F, Agaugué S, Deforges S, André P, Lotteau V. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. Journal of Immunology. 2001;167(7):3785–3891. doi: 10.4049/jimmunol.167.7.3785. [DOI] [PubMed] [Google Scholar]
- 69.Nickel T, Pfeiler S, Summo C, et al. oxLDL downregulates the dendritic cell homing factors CCR7 and CCL21. Mediators of Inflammation. 2012;2012:10 pages. doi: 10.1155/2012/320953.320953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nickel T, Schmauss D, Hanssen H, et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205(2):442–450. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Chen M, Kakutani M, Naruko T, et al. Activation-dependent surface expression of LOX-1 in human platelets. Biochemical and Biophysical Research Communications. 2001;282(1):153–158. doi: 10.1006/bbrc.2001.4516. [DOI] [PubMed] [Google Scholar]
- 72.Daub K, Seizer P, Stellos K, et al. Oxidized LDL-activated platelets induce vascular inflammation. Seminars in Thrombosis and Hemostasis. 2010;36(2):146–156. doi: 10.1055/s-0030-1251498. [DOI] [PubMed] [Google Scholar]
- 73.Kakutani M, Masaki T, Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(1):360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cominacini L, Fratta Pasini A, Garbin U, et al. The platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells. Journal of the American College of Cardiology. 2003;41(3):499–507. doi: 10.1016/s0735-1097(02)02811-5. [DOI] [PubMed] [Google Scholar]
- 75.Kuge Y, Kume N, Ishino S, et al. Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biological and Pharmaceutical Bulletin. 2008;31(8):1475–1482. doi: 10.1248/bpb.31.1475. [DOI] [PubMed] [Google Scholar]
- 76.Ishino S, Mukai T, Kuge Y, et al. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. Journal of Nuclear Medicine. 2008;49(10):1677–1685. doi: 10.2967/jnumed.107.049536. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Phillips MI, Mehta JL. LOX-1 and angiotensin receptors, and their interplay. Cardiovascular Drugs and Therapy. 2011;25(5):401–417. doi: 10.1007/s10557-011-6331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagase M, Hirose S, Fujita T. Unique repetitive sequence and unexpected regulation of expression of rat endothelial receptor for oxidized low-density lipoprotein (LOX-1) Biochemical Journal. 1998;330(3):1417–1422. doi: 10.1042/bj3301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ando K, Fujita T. Role of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in the development of hypertensive organ damage. Clinical and Experimental Nephrology. 2004;8(3):178–182. doi: 10.1007/s10157-004-0288-9. [DOI] [PubMed] [Google Scholar]
- 80.Nagase M, Ando K, Nagase T, Kaname S, Sawamura T, Fujita T. Redox-sensitive regulation of LOX-1 gene expression in vascular endothelium. Biochemical and Biophysical Research Communications. 2001;281(3):720–725. doi: 10.1006/bbrc.2001.4374. [DOI] [PubMed] [Google Scholar]
- 81.Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochemical and Biophysical Research Communications. 2001;287(4):962–968. doi: 10.1006/bbrc.2001.5674. [DOI] [PubMed] [Google Scholar]
- 82.Li D, Williams V, Liu L, et al. Expression of lectin-like oxidized low-density lipoprotein receptors during ischemia-reperfusion and its role in determination of apoptosis and left ventricular dysfunction. Journal of the American College of Cardiology. 2003;41(6):1048–1055. doi: 10.1016/s0735-1097(02)02966-2. [DOI] [PubMed] [Google Scholar]
- 83.Hu C, Dandapat A, Chen J, et al. LOX-1 deletion alters signals of myocardial remodeling immediately after ischemia-reperfusion. Cardiovascular Research. 2007;76(2):292–302. doi: 10.1016/j.cardiores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Hu C, Chen J, Dandapat A, et al. LOX-1 abrogation reduces myocardial ischemia-reperfusion injury in mice. Journal of Molecular and Cellular Cardiology. 2008;44(1):76–83. doi: 10.1016/j.yjmcc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Mango R, Predazzi IM, Romeo F, Novelli G. LOX-1/LOXIN: the Yin/Yang of atheroscleorosis. Cardiovascular Drugs and Therapy. 2011;25(5):489–494. doi: 10.1007/s10557-011-6333-5. [DOI] [PubMed] [Google Scholar]
- 86.Mango R, Biocca S, del Vecchio F, et al. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circulation Research. 2005;97(2):152–158. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]
- 87.Biocca S, Filesi I, Mango R, et al. The splice variant LOXIN inhibits LOX-1 receptor function through hetero-oligomerization. Journal of Molecular and Cellular Cardiology. 2008;44(3):561–570. doi: 10.1016/j.yjmcc.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Biocca S, Falconi M, Filesi I, et al. Functional analysis and molecular dynamics simulation of LOX-1 K167N polymorphism reveal alteration of receptor activity. PLoS One. 2009;4(2, article e4648) doi: 10.1371/journal.pone.0004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mango R, Clementi F, Borgiani P, et al. Association of single nucleotide polymorphisms in the oxidised LDL receptor 1 (OLR1) gene in patients with acute myocardial infarction. Journal of Medical Genetics. 2003;40(12):933–936. doi: 10.1136/jmg.40.12.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohmori R, Momiyama Y, Nagano M, et al. An oxidized low-density lipoprotein receptor gene variant is inversely associated with the severity of coronary artery disease. Clinical Cardiology. 2004;27(11):641–644. doi: 10.1002/clc.4960271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tatsuguchi M, Furutani M, Hinagata J-I, et al. Oxidized LDL receptor gene (OLR1) is associated with the risk of myocardial infarction. Biochemical and Biophysical Research Communications. 2003;303(1):247–250. doi: 10.1016/s0006-291x(03)00326-7. [DOI] [PubMed] [Google Scholar]
- 92.Kurnaz Ö, Aydoğan HY, Isbir CS, Tekeli A, Isbir T. Is LOX-1 K167N polymorphism protective for coronary artery disease? In Vivo. 2009;23(6):969–973. [PubMed] [Google Scholar]
- 93.Norata GD, Garlaschelli K, Grigore L, et al. Effects of PCSK9 variants on common carotid artery intima media thickness and relation to ApoE alleles. Atherosclerosis. 2010;208(1):177–182. doi: 10.1016/j.atherosclerosis.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Norata GD, Raselli S, Grigore L, et al. Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis. 2009;206(2):556–562. doi: 10.1016/j.atherosclerosis.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Predazzi IM, Norata GD, Vecchione L, et al. Association between OLR1 K167N SNP and intima media thickness of the common carotid artery in the general population. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031086.e31086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Yanuck D, Beecham A, et al. A candidate gene study revealed sex-specific association between the OLR1 gene and carotid plaque. Stroke. 2011;42(3):588–592. doi: 10.1161/STROKEAHA.110.596841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amati F, Diano L, Vecchione L, et al. LOX-1 inhibition in ApoE KO mice using a schizophyllan-based antisense oligonucleotide therapy. Molecular Therapy. Nucleic Acids. 2012;1(12, article e58) doi: 10.1038/mtna.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


