Summary
In addition to genetic alterations of gains and losses, epigenetic events of promoter methylation appear to further undermine a destabilized genomic repertoire in squamous head and neck carcinoma (HNSCC). This review provides an overview of frequently methylated tumor suppressor genes in benign head and neck papillomas, primary HNSCC tumors, and HNSCC cell lines and their relevance as epigenetic markers in head and neck tumorigenesis.
Keywords: promoter methylation, benign, malignant, primary, cell lines
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most prevalent cancers in the world with over 500,000 cases diagnosed annually. In the United States alone, it accounts for nearly 3.2% of all newly diagnosed cancers (1). Not only one of the most ubiquitous, HNSCC is also one of most lethal cancers responsible for 2.1% of all cancer deaths in the United States and is noted as the sixth most common malignant disease worldwide (1). HNSCC carries a high mortality rate despite advances in chemotherapy and radiation therapies. This is due mainly to the highly heterogeneous nature of the disease, both morphologically and genetically. A current shortcoming in the prognosis and treatment of HNSCC is a lack of methods and large study cohorts to adequately address the etiologic complexity and diversity of the disease.
The study of human disease has focused primarily on genetic mechanisms. Dispelling the belief that the only way to treat such conditions is by fixing or replacing damaged genes, scientists are instead focusing on the field of epigenetics--the study of changes in gene silencing that occur without changing the DNA sequence. Many types of epigenetic processes have been identified--they include methylation, acetylation, phosphorylation, ubiquitylation, and sumolyation. These processes are natural and essential to many organism functions, but if they occur improperly, there can be major adverse health and behavioral effects.
Epigenetic regulation is central to the biological function of all cells. Perhaps the best known epigenetic process, in part because it has been easiest to study with existing technology, is DNA methylation. This is the addition or removal of a methyl group (CH3). Hypermethylation is a well described DNA modification that has been implicated in normal mammalian development, (2, 3) imprinting (4) and X chromosome inactivation (5). However, recent studies have identified hypermethylation as a probable cause in the development of various cancers (6–8). Aberrant methylation by DNA-methyltransferases in the CpG islands of a gene’s promoter region can lead to transcriptional repression akin to other abnormalities such as a point mutation or deletion (9). Gene transcriptional inactivation via hypermethylation at the CpG islands within the promoter regions is an important mechanism (10). This anomalous hypermethylation has been noted in a variety of tumor-suppressor genes (TSGs), whose inactivation can lead many cells down the tumorigenesis continuum (10–12). In many cancers, aberrant DNA methylation of so called “CpG islands”, CpG-rich sequences frequently associated with promoters or first exons, is associated with the inappropriate transcriptional silencing of critical genes (13–15). These DNA methylation events represent an important tumor-specific marker occurring early in tumor progression and one that can be easily detected by PCR based methods in a manner that is minimally invasive to the patient.
Studies of sequential molecular alterations and genetic progression of pre-invasive HNSCC have not been clearly defined. A tissue field of somatic genetic alterations precedes the histopathological phenotypic changes of carcinoma (16). Genomic changes could be of potential use in the diagnosis and prognosis of pre-invasive squamous head and neck carcinoma (HNSCC) lesions and as markers for cancer risk assessment. A few studies have shown recurring alterations of chromosome 9p21 in the early stages of HNSCC (17–19). However, gene silencing via hypermethylation is still a relatively new idea in the development of HNSCC and little is known about the contribution of epigenetics to the development of neoplasia, its transformation, progression, and recurrence in HNSCC. Therefore, epigenetic events of promoter hypermethylation are emerging as one of the most promising molecular strategies for cancer detection and represent an important tumor-specific marker occurring early in tumor progression.
DNA methylation in HNSCC
Numerous tumor suppressor genes have been implicated as targets for methylation in other cancers (13–15). Promotor hypermethylation of genes in HNSCC have been reported for p16, p14, DAP-K, RASSF1A (20–26), RARβ2 (27–29), MGMT, a DNA repair gene that functions to remove mutagenic (O6-guanine) adducts from DNA (30), and E-cadherin, a Ca2+- dependent cell adhesion molecule that functions in cell-cell adhesion, cell polarity, and morphogenesis (31).
Historically, the molecular pathogenesis of cancer has been teased out one gene at a time. The development of several new high throughput analytical methods for the analysis of DNA, mRNA, and proteins within a cell (32–35) have permitted a more detailed molecular characterization of the cancer genome. In HNSCC, recent comprehensive high-throughput methods from our group and others have underscored the contribution of both genetic (36–38) and epigenetic events (26, 39–43), often working together (44), in the development and progression of HNSCC. In HNSCC, methylation of p16, RARβ, and MGMT suggested early events, with incidences of methylation in HNSCC cell lines and primary tumors being similar (27, 43–46).
Aberrant DNA methylation patterns in HNSCC have served as powerful diagnostic, prognostic, and risk assessment biomarkers. Promoter hypermethylation pattern of the p16, MGMT, GSTP1, and DAPK genes have been used as molecular markers for cancer cell detection in the paired serum DNA and almost half of the HNSCC patients with methylated tumors were found to display these epigenetic changes in the paired serum (26).
A: HNSCC Cell lines
The majority of published epigenetic data in HNSCC comes from methylation specific PCR following bisulfite treatment (MSP) (47). The success of MSP has been attributed to its increased sensitivity, however, it generally relies on a pre-selected number of genes, assessed one gene at a time, as opposed to high-throughput microarray based methylation analysis (48) and multi-candidate gene applications (44). Recently, using a multi-candidate gene approach, the Methylation Specific Multiplex Ligation-dependent Probe Amplification (MS-MLPA) assay (MS-MLPA, Figure 1) (44, 49), we identified nine genes, TIMP3, APC, KLK10, TP73, CDH13, IGSF4, FHIT, ESR1, and DAPK1 that were aberrantly methylated in paired HNSCC primary A) and recurrent or metastatic (B) UMSCC-11A/11B, UMSCC-17A/17B, and UMSCC-81A/81B cell lines (Figures 2–4)(44).
Figure 1.
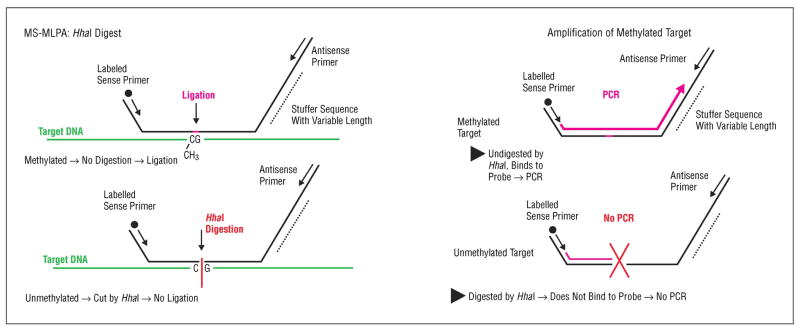
Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) with and without HhaI. CH3 indicates methyl group; PCR, polymerase chain reaction.
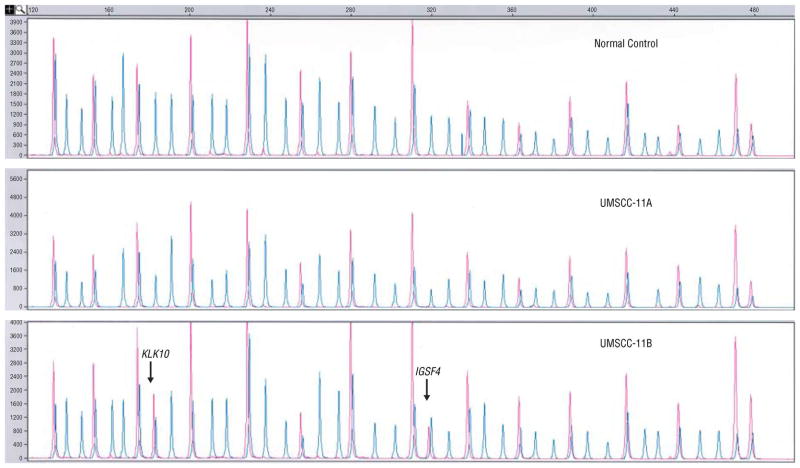
Figure 2.
Multiplex ligation-dependent probe amplification peaks with (red) and without (blue) HhaI for the normal DNA, UMSCC-11A, and UMSCC-11B.
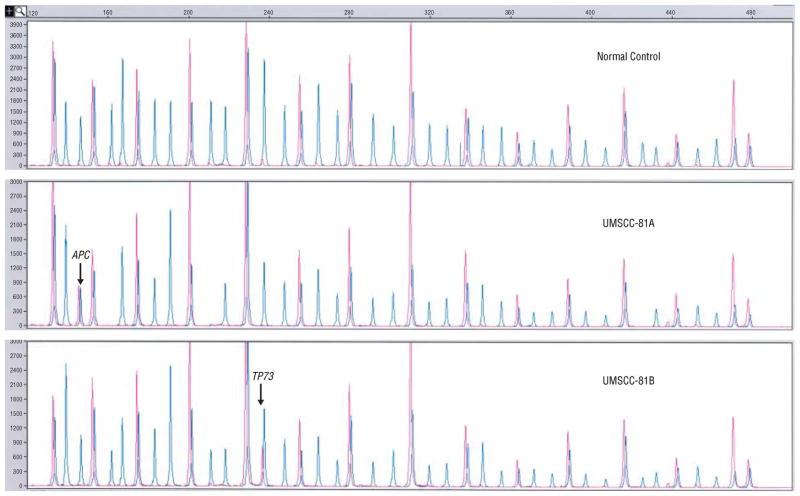
Figure 4.
Multiplex ligation-dependent probe amplification peaks with (red) and without (blue) HhaI for the normal DNA, UMSCC-81A, and UMSCC-81B.
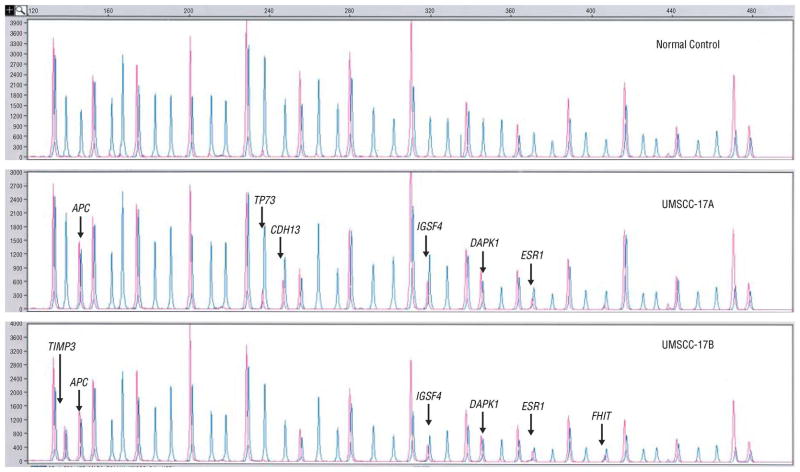
The most frequently hypermethylated genes were APC and IGSF4 observed in 3/6 cell lines, and TP73 and DAPK1 observed in 2/6. For KLK10 and IGSF4, TIMP3 and FHIT, and TP73, in recurrent/metastatic cell lines, promoter hypermethylation was a disease progression event, indicating complete abrogation of tumor suppressor function for KLK10, IGSF4, and TIMP3, and gene silencing of one of two copies of TP73. Hypermethylation of IGSF4, TP73, CDH13, ESR1, DAPK1, and APC were primary events in UMSCC-17A (Figure 3). Gene silencing through promoter hypermethylation was observed in 5/6 cell lines and contributed to primary and progressive events in HNSCC (44). In addition to genetic alterations of gains and losses, epigenetic events appear to further undermine a destabilized genomic repertoire in HNSCC.
Figure 3.
Multiplex ligation-dependent probe amplification peaks with (red) and without (blue) HhaI for the normal DNA, UMSCC-17A, and UMSCC-17B.
B: Primary HNSCC tissue
Subsequently (27), we evaluated aberrant methylation status in 28 primary HNSCC using MS-MLPA (Figures 5, 6) and confirmed aberrant promoter methylation using conventional Methylation Specific PCR (MSP) (47) (gel electrophoresis separation of products, Figures 7,8) and real time PCR following bisulfite treatment, Figure 9). MS-MLPA promoter methylation profiling of 22 tumor suppressor genes (Table 1), many of which are involved in head and neck cancer, identified RARβ, APC, and CHFR as frequent epigenetic events. These preliminary findings of promoter hypermethylation of RARβ and APC in both early and late stage tumors and of CHFR by MS-MLPA and MSP assays in only late stage tumors appear to suggest an epigenetic progression continuum, with CHFR as a late event and a putative diagnostic biomarker for late stage disease. The alterations of RARβ, APC, and CHFR via DNA hypermethylation have several implications in HNSCC. Decreased expression of RARβ has been associated with increased keratinizing squamous differentiation in HNSCC cells and pharmacological doses of retinoid ATRA (9-cis-RA) induced RARβ in HNSCC cells, resulting in restoration of a more normal differentiation (50). More importantly, RARβ2 silencing by promoter hypermethylation was shown to be an early event in head and neck carcinogenesis and 5-Aza-CdR restoredRARβ2 inducibility by ATRA in most cell lines (51). The examination of the prevalence and pattern of CHFR inactivation in human tumors found CpG methylation-dependent silencing of CHFR expression in 40%of primary colorectal cancers, 53% of colorectal adenomas, and 30% of primary HNSCC (52). We reported CHFR as a solely late stage 4 event, occurring in 7/28 HNSCC (27), suggesting a role for CHFR in tumor progression with potential utility as a biomarker of late stage disease. Treatment with the methyltransferase inhibitor 5-aza-2′-deoxycytidine induced re-expression of CHFR (52). Additionally because cancer cells that lack CHFR expression have shown to be more susceptible to the microtubule inhibitor taxol (52), silencing of CHFR by methylation can serve as a marker for predicting sensitivity to particular chemotherapeutic agents. APC (adenomatosis polyposis coli), a tumor suppressor gene, was originally implicated in colon cancer. Promoter hypermethylation of APC has been reported in 25% of oral cancers (53) and in Barrett’s metaplasia and dysplasia (54). In our primary HNSCC cohort (27), APC, like RARβ, was hypermethylated in early and late stage tumors, suggesting DNA methylation of APC and RARβ as earlier epigenetic events, when compared to CHFR. Validation of these findings in larger HNSCC cohorts would further support these genes as relevant epigenetic biomarkers of cancer therapy given the reversible nature of epigenetic gene silencing.
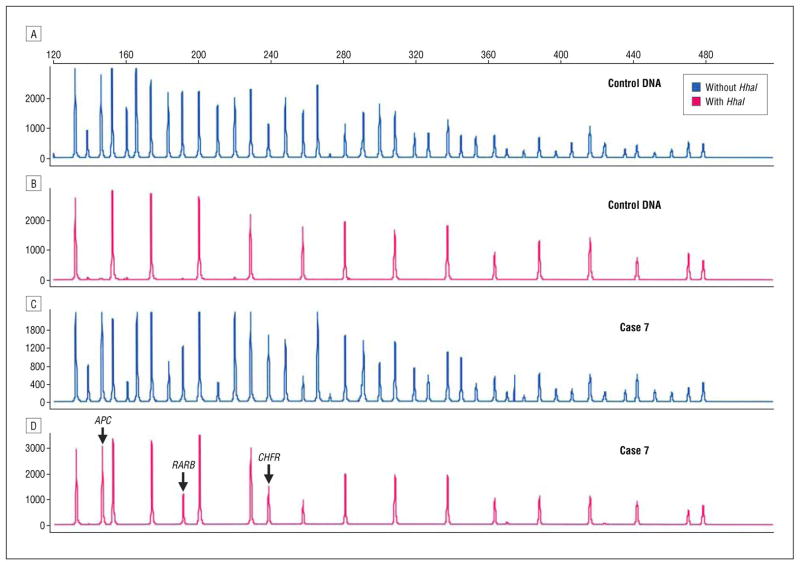
Figure 5.
Methylation-specific multiplex ligation-dependent probe amplification probe mix without (A and C) and with (B and D) HhaI enzyme. Fifteen peaks are seen in the control DNA sample (B). The methylation peaks in case 7 (D) that are not present in the control DNA (B) represent promoter hypermethylation APC, RARB, and CHFR.
Figure 6.
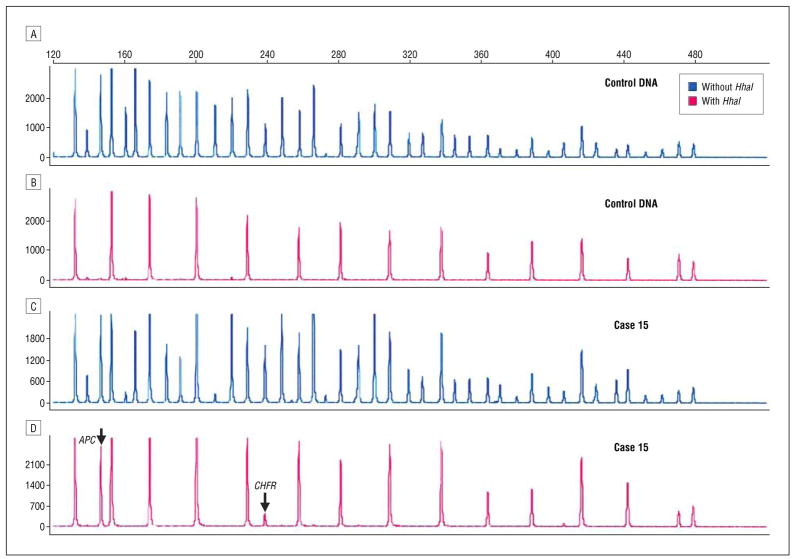
Methylation-specific multiplex ligation-dependent probe amplification probe mix without (A and C) and with HhaI enzyme (B and D) in DNA control DNA and DNA from case 15. Methylation of APC and CHFR is seen in case 15 with HhaI digestion (D).
Figure 7.
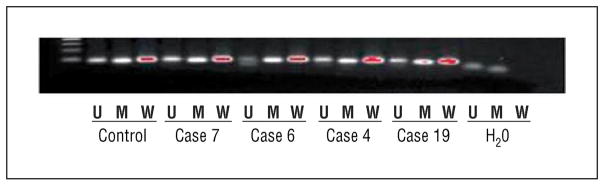
Gel electrophoresis methylation-specific polymerase chain reaction results for RARB. Note the presence of the 84–base pair (bp) methylation (M) band and the unmethylated (U) 94-bp product in cases 4, 6, 7, and 19; the latter indicates an admixture of normal and tumor cells. H2O indicates water; W, wild types.
Figure 8.
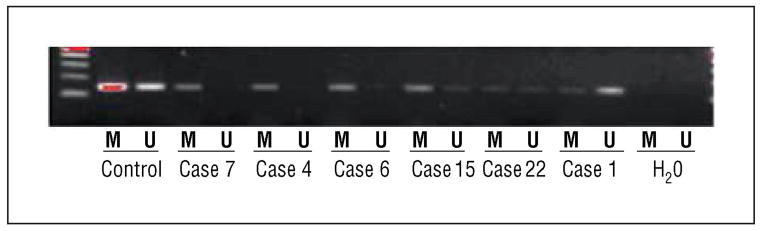
Gel electrophoresis methylation-specific polymerase chain reaction (MSP) results for CHFR. Note the presence of the 155–base pair (bp) methylation (M) band in cases 1, 4, 6, 7, 15, and 22 and the unmethylated (U) 155-bp product in cases 1, 15, and 22; the latter indicates an admixture of normal and tumor cells. H2O indicates water.
Figure 9.
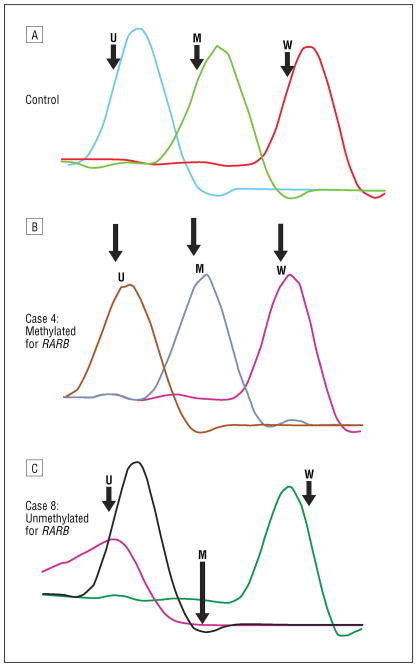
Real-time polymerase chain reaction (PCR) with methylation-specific PCR (MSP) methylated (M) and unmethylated (U) primers for RARB for the control specimen (A), case 4 (B), and case 8 (C). Specific melting temperature peaks are seen for control U, M, and wild-type (W) DNA. The presence of the M melting temperature peak in case 4 indicates promoter hypermethylation of RARB, supported by MSP gel electrophoresis (Figure 4). The absence of an M melting temperature peak in case 8 is supported by MSP gel electrophoresis and MS multiplex ligation-dependent probe amplification.
Table 1.
Methylation-Specific MLPA Probe Panel (ME001)
| # | Gene probe | Chrom Loc |
|---|---|---|
| 1 | TP73 | 01p36 |
| 2 | CASP8 | 02q22.3 |
| 3 | VHL | 03p25.3 |
| 4 | RARB | 03p24 |
| 5 | *MLH1 | 03p21.1 |
| 6 | MLH1 | 03p21.1 |
| CTNNB1 | 03p22 | |
| 7 | *RASSF1 | 03p21.3 |
| 8 | RASSF1 | 03p21.3 |
| 9 | FHIT | 03p14.2 |
| CASR | 03q21 | |
| 10 | APC | 05q21 |
| 11 | ESR1 | 06q25.1 |
| PARK2 | 06q26 | |
| CDK6 | 07q21.3 | |
| 12 | CDKN2A | 09p21 |
| 13 | CDKN2B | 09p21 |
| 14 | DAPK1 | 09q34.1 |
| AI651963 | 10p14 | |
| CREM | 10p12.1 | |
| 15 | PTEN | 10q23.3 |
| 16 | CD44 | 11p12 |
| 17 | GSTP1 | 11q13 |
| 18 | ATM | 11q23 |
| 19 | IGSF4 | 11q23 |
| TNFRSF1A | 12p13 | |
| TNFRSF7 | 12p13 | |
| 20 | CDKN1B | 12q13.1 |
| PAH | 12q23 | |
| 21 | CHFR | 12q24.33 |
| 22 | BRCA2 | 13q12.3 |
| BRCA2 | 13q12.3 | |
| MLH3 | 14q24.3 | |
| TSC2 | 16p13.3 | |
| CDH1 | 16q22.1 | |
| 23 | CDH13 | 16q24.2 |
| 24 | HIC1 | 17p13.3 |
| 25 | BRCA1 | 17q21 |
| BCL2 | 18q21.3 | |
| KLK3 | 19q13 | |
| 26 | TIMP3 | 22q12.3 |
Bolded=probes with HhaI site (n=26 probes);
genes with multiple probes in the promoter region
C. Delineating an epigenetic continuum in HNSCC
A tissue field of somatic genetic alterations precedes the histopathological phenotypic changes of carcinoma (16). Genomic changes could be of potential use in the diagnosis and prognosis of pre-invasive squamous head and neck carcinoma (HNSCC) lesions and as markers for cancer risk assessment. Studies of sequential molecular alterations and genetic progression of pre-invasive HNSCC have not been clearly defined. A few studies have shown recurring alterations of chromosome 9p21 in the early stages of HNSCC (17–19). However, gene silencing via hypermethylation is still a relatively new idea in the development of HNSCC and little is known about the contribution of epigenetics to the development of neoplasia, its transformation, progression, and recurrence in HNSCC.
Benign Papillomas
Papillomas are benign neoplasms of epithelium on a connective tissue core(55). They can involve the nose and sinuses (sinonasal papillomas - SP) as well as the respiratory tract (respiratory papillomatosis - RP) to include the larynx, trachea, and bronchi. Both SP and RP have a tendency to recur. Recurrent respiratory (laryngeal) papillomatosis (RRP) is an extremely rare condition (56). Inverted SP are associated with invasive squamous cell carcinoma (SCC)(57) and a small percentage of RRP cases also progress to malignancy (58).
Human papilloma virus (HPV) is frequently associated with sinonasal (59, 60) and laryngeal (61–63) papillomas. Most HPV-positive cases of SP are of the inverted type (64). Benign papillomas are preferentially associated with the low-risk HPV types 6 and 11, whereas their malignant counterparts are exclusively positive for HPV-16 DNA(65). Studies on HPV typing in benign laryngeal papillomas have demonstrated an association of HPV-11 with a more aggressive course of the disease(66, 67). HPV infection in inverted papillomas (68) and in particular HPV-11 infection in RRP(69) may be an early event in a multistep process of malignant transformation.
Sinonasal Papillomas
Sinonasal papillomas have been categorized histologically as inverted, fungiform (exophytic), and cylindrical cell papillomas (70). Inverted papillomas are the most commonly occurring sinonasal papillomas followed by exophytic(57). Inverted papillomas are benign, rare sinonasal lesions well known for their local recurrence, invasiveness and predisposition for malignant transformation. Recurrence rates vary widely, ranging from 6% to 33%, despite management by different surgical treatment options(71). Malignant transformation occurs in 7 to 10% of cases (57, 72). Morphology is not useful in determining if a lesion will recur or acquire malignant changes. A general belief is that once excised, and in the absence of malignancy in the excised specimen, a recurrence is unlikely to convert to malignancy(73).
Benign inverted papillomas were reported as monoclonal but lacking common genetic alterations associated with squamous head and neck cancer(73). Therefore we evaluated 7 patients with primary and recurrent sinonasal papillomas for aberrant promoter methylation status using MS-MLPA and confirmed aberrant methylation using conventional MSP. We found all 7 cases had at least one epigenetic event of aberrant DNA hypermethylation with 10 of the 22 methylation-prone genes being methylated (Table 2). Commonly methylated genes included CDKN2B, CDKN2A, TP73, and ESR1. The CDKN2B gene, detected by MS-MLPA (Figure 10), was a consistent target of aberrant methylation and was confirmed by MSP (Figure 11).
Table 2.
Clinical characteristics of cohort with MS-MLPA results
| Biopsies | Location | TIMP3 | APC | CDKN2A | MLH1 | CDKN2B | TP73 | FANCD2 | DAPK1 | ESR | GSTP1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1—IP | 1—reference | Nasal | M | M | M | M | M | M | ||||
| 2–10 months | Ethmoid and nasal maxillary-block 3 | M | M | M | ||||||||
| 2–10 months | Ethmoid and nasal maxillary-block 4 | M | ||||||||||
| Case 2—IP | 1—reference | Nasal cavity | M | M | M | M | ||||||
| 2–6 months | Ethmoid sinus | M | ||||||||||
| Case 3—IP | 1—reference | Ethmoid sinus | M | |||||||||
| Case 4—IP/EP | 1—reference | Nasal mucosa | M | M | ||||||||
| Case 5—IP/EP | 1—reference | Nasal vestibule | M | |||||||||
| Case 6—EP | 1—reference | Frontal sinus | M | M | ||||||||
| Case 7—EP | 1—reference | Nasal cavity | M | |||||||||
| Total | 1/7 | 1/7 | 2/7 | 1/7 | 6/7 | 2/7 | 1/7 | 1/7 | 2/7 | 1/7 |
EP, exophytic papilloma; IP/EP, inverted and exophytic papilloma; IP, inverted papilloma; M, methylated.
Figure 10.
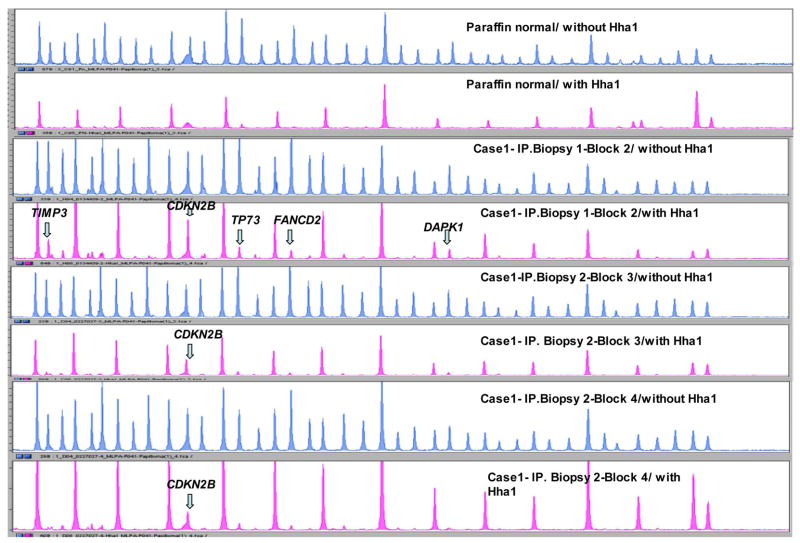
MS-MLPA probe mix with and without HhaI enzyme (DNA sequencer - ABI 3130). Results for Case 1 - biopsy 1 block 2 and biopsy 2 blocks 3 and 4. Note 15 peaks in the control DNA sample with HhaI. Presence of a peak in biopsies 1 and 2 (blocks 3 and 4) not present in the control DNA is that of aberrantly methylated CDKN2B gene. Presence of peak for aberrantly methylated DAPK1 in biopsy 1 block 2 and biopsy 2 block 3 not present in the control DNA.
Figure 11.
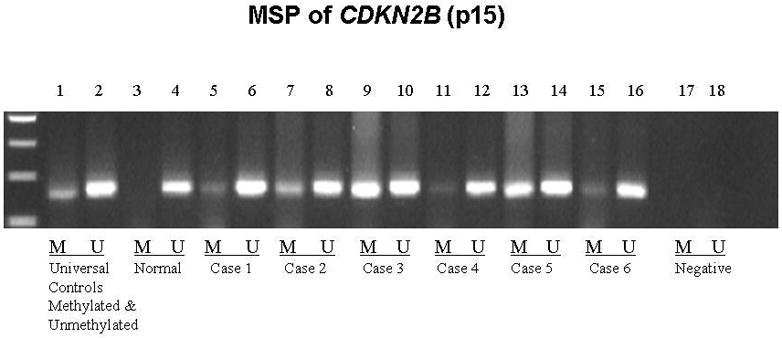
Methylation Specific PCR (MSP) confirmation of aberrant methylation detected by MS-MLPA for CDKN2B. Lane 1: universal methylated control; Lane 2: universal unmethylated control; Lanes 3 & 4: normal control, note presence of only unmethylated PCR product; Lanes 5–16 span Cases 1–6. Note presence of methylated and unmethylated product, the latter indicating admixture of normal and papilloma cells; Lanes 17 & 18: negative control.
Recurrent biopsies from 2 inverted papilloma cases had common epigenetic events: aberrant methylation of CDKN2B and DAPK1 in case 1, and CDKN2B in case 2, underscoring monoclonality for these lesions. Inactivation of the CDKN2B and CDKN2A genes at the genomic and epigenetic level is a frequent event in human oral SCCs (74) and in HNSCC (37, 44, 75). TP73 and ESR1 were aberrantly methylated in 2 of the 7 cases. TP73 is involved in cell cycle regulation and can activate TP53-responsive proteins, inhibit cell growth and induce apoptosis (76). We have reported TP73 hypermethylation in HNSCC to be a primary as well as a disease progression event (44). ESR1 has metastasis-suppressor properties in breast cancer cells (77), suggesting a tumor-suppressor role (78). ESR1 is methylated in Barrett’s metaplastic and dysplastic samples as well as in some adenocarcinoma samples suggesting that DNA hypermethylation is an early epigenetic event in the progression of esophageal adenocarcinomas (EAC) (54). These findings support a role for epigenetic events of promoter hypermethylation in the pathogenesis of benign inverted and exophytic papillomas. As a consistent target of aberrant promoter hypermethylation, CDKN2B may serve as a useful biomarker and a potential therapeutic target for gene reactivation studies and in disease monitoring for progression.
Recurrent respiratory (laryngeal) papillomas (RRP)
Recurrent respiratory (laryngeal) papillomas (RRP) present primarily as tiny warts on the vocal cords and can be potentially life-threatening due to airway obstruction(56). Human papillomavirus types 6 and 11 account for 80–90% of RRP(79). Laryngeal papillomas usually run a benign but recurrent course. In the spontaneous transformation of RP or RRP to SCC, a progression continuum to malignancy may not be histologically and clinically apparent, making these lesions difficult to diagnose early in the course of the transformation of the disease. Therefore we investigated alterations in DNA methylation in recurrent biopsies from patients with RRP to asses the contribution of promoter methylation-mediated epigenetic events in RRP tumorigenesis. Samples from 15 subjects who had 1 to 6 subsequent biopsies were interrogated by MS-MLPA. Aberrant methylation of CDKN2B and APC genes were most frequent, occurring in 8 of 14 cases, with dissimilar epigenetic events in the remaining cases (Table 3). There were 5 cases that had at least one abnormally methylated gene in a recurrent biopsy, of which the CDKN2B gene showed consistent hypermethylation in all 5 cases (Table 4). One case demonstrated aberrant methylation of APC and VHL promoter regions in all three biopsies.
Table 3.
Case Summary and Methylation Status
| Patient No.
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 2a | 3a | 4a | 5a | 6c | 7b | 8a | 9a | 10a | 11b | 12b | 13b | 14a | 15a | |
| No. of biopsies | 7 | 6 | 2 | 6 | 4 | 2 | 4 | 2 | 2 | 3 | 3 | 3 | 5 | 2 | 2 |
| TIMP3d | M | M | M | M | M | ||||||||||
| APCd | M | M | M | M | M | M | M | M | |||||||
| CDKN2Ad | M | M | M | M | M | ||||||||||
| MLH1 | M | M | |||||||||||||
| KLK10 | M | M | M | ||||||||||||
| MEN1 | M | ||||||||||||||
| CDKN2Bd | M | M | M | M | M | M | M | M | |||||||
| VHLd | M | M | M | M | M | ||||||||||
| TP73 | M | M | M | ||||||||||||
| FANCD26 | M | ||||||||||||||
| BRCA2 | M | M | |||||||||||||
| IGSF4 | M | M | |||||||||||||
| RASSF1 | M | ||||||||||||||
| DAPK1d | M | M | M | M | |||||||||||
| HIC1d | M | M | M | M | |||||||||||
| ESR1 | M | M | |||||||||||||
| CDKN1B | M | ||||||||||||||
| BRCA1 | M | M | |||||||||||||
| GSTP1d | M | M | M | M | |||||||||||
Abbreviation: M, methylated.
Cases with dissimilar epigenetic events in multiple biopsy specimens.
Cases with similar epigenetic events in multiple biopsy specimens.
Case with absence of M genes.
Commonly M genes (present in >3 cases).
Table 4.
Epigenetically Linked Recurrent Laryngeal Papilloma Cases
| Patient No. | Biopsy | APC | CDKN2B | VHL | TP73 | GSTP1 |
|---|---|---|---|---|---|---|
| 4 | 1 [Reference] | M | ||||
| 2 (10 mo) | M | M | ||||
| 3 (30 mo) | M | M | ||||
| 7 | 1 [Reference] | M | M | M | ||
| 2 (3 mo) | M | |||||
| 3 (6 mo) | M | M | M | |||
| 11 | 1 [Reference] | M | ||||
| 2 (15 mo) | M | |||||
| 12 | 1 [Reference] | M | ||||
| 2 (14 mo) | M | |||||
| 13 | 1 [Reference] | M | M | |||
| 2 (1 mo) | M | M | M | |||
| 3 (3 mo) | M | M | M |
Abbreviation: M, methylated.
In precancerous oral tissues(75) aberrant methylation of CDKN2B has been implicated as an early event in the pathogenesis of oral lesions. APC is a tumor suppressor gene originally implicated in colon cancer. Genetic and epigenetic alterations in this gene have since been recognized in other malignancies including oral squamous cell carcinomas(53). VHL is a tumor suppressor gene that is responsible for the Von Hippel-Lindau syndrome which is an inherited familial cancer syndrome that makes patients susceptible to a variety of cancers, malignant and benign. It has been found that treatment of methylated VHL tumors with a demethylating agent results in re-expression of the VHL transcripts(80). Persistence of the same aberrantly methylated gene in 36% of multiple recurrent biopsies (5/14) in our study supports a monoclonal origin for RRP and permits the tracing of an epigenetic continuum implicating key tumor suppressor genes in RRP. The high frequency of epigenetic events points to the utilization of gene silencing mechanisms as one of the driving forces behind the growth of recurrent laryngeal papillomas.
Conclusion
Epigenetic events of promoter hypermethylation are emerging as one of the most promising molecular strategies for cancer detection and represent an important tumor-specific marker occurring early in tumor progression.
Acknowledgments
This work was supported by NIH DE 15990 (MJW).
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 4.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer GP, Tanguay RL, Steigerwald SD, Riggs AD. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosomal DNA at the CpG island and promoter of human PGK-1. Genes Dev. 1990;4:1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 6.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, Su Huang H, Petrelli NJ, Zhang X, O’Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 7.Issa JP, Vertino PM, Wu J, Sazawal S, Celano P, Nelkin BD, Hamilton SR, Baylin SB. Increased cytosine DNA-methyltransferase activity during colon cancer progression. J Natl Cancer Inst. 1993;85:1235–1240. doi: 10.1093/jnci/85.15.1235. [DOI] [PubMed] [Google Scholar]
- 8.Lin SY, Yeh KT, Chen WT, Chen HC, Chen ST, Chang JG. Promoter CpG methylation of caveolin-1 in sporadic colorectal cancer. Anticancer Res. 2004;24:1645–1650. [PubMed] [Google Scholar]
- 9.Smiraglia DJ, Smith LT, Lang JC, Rush LJ, Dai Z, Schuller DE, Plass C. Differential targets of CpG island hypermethylation in primary and metastatic head and neck squamous cell carcinoma (HNSCC) J Med Genet. 2003;40:25–33. doi: 10.1136/jmg.40.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 11.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 12.Chan MF, Liang G, Jones PA. Relationship between transcription and DNA methylation. Curr Top Microbiol Immunol. 2000;249:75–86. doi: 10.1007/978-3-642-59696-4_5. [DOI] [PubMed] [Google Scholar]
- 13.Cairns P. Detection of promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Ann N Y Acad Sci. 2004;1022:40–43. doi: 10.1196/annals.1318.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Kwon YM, Kim JS, Lee H, Park JH, Shim YM, Han J, Park J, Kim DH. Tumor-specific methylation in bronchial lavage for the early detection of non-small-cell lung cancer. J Clin Oncol. 2004;22:2363–2370. doi: 10.1200/JCO.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 15.Roman-Gomez J, Jimenez-Velasco A, Castillejo JA, Agirre X, Barrios M, Navarro G, Molina FJ, Calasanz MJ, Prosper F, Heiniger A, Torres A. Promoter hypermethylation of cancer-related genes: a strong independent prognostic factor in acute lymphoblastic leukemia. Blood. 2004;104:2492–2498. doi: 10.1182/blood-2004-03-0954. [DOI] [PubMed] [Google Scholar]
- 16.Sanz-Ortega J, Saez MC, Sierra E, Torres A, Balibrea JL, Hernando F, Sanz-Esponera J, Merino MJ. 3p21, 5q21, and 9p21 allelic deletions are frequently found in normal bronchial cells adjacent to non-small-cell lung cancer, while they are unusual in patients with no evidence of malignancy. J Pathol. 2001;195:429–434. doi: 10.1002/path.987. [DOI] [PubMed] [Google Scholar]
- 17.Yoo WJ, Cho SH, Lee YS, Park GS, Kim MS, Kim BK, Park WS, Lee JY, Kang CS. Loss of heterozygosity on chromosomes 3p,8p,9p and 17p in the progression of squamous cell carcinoma of the larynx. J Korean Med Sci. 2004;19:345–351. doi: 10.3346/jkms.2004.19.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz-Ortega J, Valor C, Saez MC, Ortega L, Sierra E, Poch J, Hernandez S, Sanz-Esponera J. 3p21, 5q21, 9p21 and 17p13 allelic deletions accumulate in the dysplastic spectrum of laryngeal carcinogenesis and precede malignant transformation. Histol Histopathol. 2003;18:1053–1057. doi: 10.14670/HH-18.1053. [DOI] [PubMed] [Google Scholar]
- 19.Papadimitrakopoulou VA, Izzo J, Mao L, Keck J, Hamilton D, Shin DM, El-Naggar A, den Hollander P, Liu D, Hittelman WN, Hong WK. Cyclin D1 and p16 alterations in advanced premalignant lesions of the upper aerodigestive tract: role in response to chemoprevention and cancer development. Clin Cancer Res. 2001;7:3127–3134. [PubMed] [Google Scholar]
- 20.Miracca EC, Kowalski LP, Nagai MA. High prevalence of p16 genetic alterations in head and neck tumours. Br J Cancer. 1999;81:677–683. doi: 10.1038/sj.bjc.6690747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 22.Rosas SL, Koch W, da Costa Carvalho MG, Wu L, Califano J, Westra W, Jen J, Sidransky D. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–942. [PubMed] [Google Scholar]
- 23.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–4236. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- 24.Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003;105:41–46. doi: 10.1002/ijc.11028. [DOI] [PubMed] [Google Scholar]
- 25.El-Naggar AK, Lai S, Clayman G, Lee JK, Luna MA, Goepfert H, Batsakis JG. Methylation, a major mechanism of p16/CDKN2 gene inactivation in head and neck squamous carcinoma. Am J Pathol. 1997;151:1767–1774. [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Cespedes M, Esteller M, Wu L, Nawroz-Danish H, Yoo GH, Koch WM, Jen J, Herman JG, Sidransky D. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 2000;60:892–895. [PubMed] [Google Scholar]
- 27.Chen K, Sawhney R, Khan M, Benninger MS, Hou Z, Sethi S, Stephen JK, Worsham MJ. Methylation of multiple genes as diagnostic and therapeutic markers in primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1131–1138. doi: 10.1001/archotol.133.11.1131. [DOI] [PubMed] [Google Scholar]
- 28.Zou CP, Youssef EM, Zou CC, Carey TE, Lotan R. Differential effects of chromosome 3p deletion on the expression of the putative tumor suppressor RAR beta and on retinoid resistance in human squamous carcinoma cells. Oncogene. 2001;20:6820–6827. doi: 10.1038/sj.onc.1204846. [DOI] [PubMed] [Google Scholar]
- 29.Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–3587. [PubMed] [Google Scholar]
- 30.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50:6119–6129. [PubMed] [Google Scholar]
- 31.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, Jeffrey SS, Botstein D, Brown PO. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 33.Khan J, Saal LH, Bittner ML, Chen Y, Trent JM, Meltzer PS. Expression profiling in cancer using cDNA microarrays. Electrophoresis. 1999;20:223–229. doi: 10.1002/(SICI)1522-2683(19990201)20:2<223::AID-ELPS223>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Hanash SM, Teichroew D. Mining the human proteome: experience with the human lymphoid protein database. Electrophoresis. 1998;19:2004–2009. doi: 10.1002/elps.1150191119. [DOI] [PubMed] [Google Scholar]
- 35.Soltys SG, Le QT, Shi G, Tibshirani R, Giaccia AJ, Koong AC. The use of plasma surface-enhanced laser desorption/ionization time-of-flight mass spectrometry proteomic patterns for detection of head and neck squamous cell cancers. Clin Cancer Res. 2004;10:4806–4812. doi: 10.1158/1078-0432.CCR-03-0469. [DOI] [PubMed] [Google Scholar]
- 36.Smeets SJ, Braakhuis BJ, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, Meijer GA, Leemans CR, Brakenhoff RH. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25:2558–2564. doi: 10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 37.Worsham MJ, Pals G, Schouten JP, Van Spaendonk RM, Concus A, Carey TE, Benninger MS. Delineating genetic pathways of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:702–708. doi: 10.1001/archotol.129.7.702. [DOI] [PubMed] [Google Scholar]
- 38.Worsham MJ, Chen KM, Tiwari N, Pals G, Schouten JP, Sethi S, Benninger MS. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:409–415. doi: 10.1001/archotol.132.4.409. [DOI] [PubMed] [Google Scholar]
- 39.Shaw RJ, Liloglou T, Rogers SN, Brown JS, Vaughan ED, Lowe D, Field JK, Risk JM. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: quantitative evaluation using pyrosequencing. Br J Cancer. 2006;94:561–568. doi: 10.1038/sj.bjc.6602972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Cespedes M, Okami K, Cairns P, Sidransky D. Molecular analysis of the candidate tumor suppressor gene ING1 in human head and neck tumors with 13q deletions. Genes Chromosomes Cancer. 2000;27:319–322. doi: 10.1002/(sici)1098-2264(200003)27:3<319::aid-gcc13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 41.Shah SI, Yip L, Greenberg B, Califano JA, Chow J, Eisenberger CF, Lee DJ, Sewell DA, Reed AL, Lango M, Jen J, Koch WM, Sidransky D. Two distinct regions of loss on chromosome arm 4q in primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:1073–1076. doi: 10.1001/archotol.126.9.1073. [DOI] [PubMed] [Google Scholar]
- 42.Sidransky D. Circulating DNA. What we know and what we need to learn. Ann N Y Acad Sci. 2000;906:1–4. doi: 10.1111/j.1749-6632.2000.tb06579.x. [DOI] [PubMed] [Google Scholar]
- 43.Maruya S, Issa JP, Weber RS, Rosenthal DI, Haviland JC, Lotan R, El-Naggar AK. Differential methylation status of tumor-associated genes in head and neck squamous carcinoma: incidence and potential implications. Clin Cancer Res. 2004;10:3825–3830. doi: 10.1158/1078-0432.CCR-03-0370. [DOI] [PubMed] [Google Scholar]
- 44.Worsham MJ, Chen KM, Meduri V, Nygren AO, Errami A, Schouten JP, Benninger MS. Epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:668–677. doi: 10.1001/archotol.132.6.668. [DOI] [PubMed] [Google Scholar]
- 45.Stephen JK, Vaught LE, Chen KM, Sethi S, Shah V, Benninger MS, Gardner GM, Schweitzer VG, Khan M, Worsham MJ. Epigenetic events underlie the pathogenesis of sinonasal papillomas. Mod Pathol. 2007 doi: 10.1038/modpathol.3800944. [DOI] [PubMed] [Google Scholar]
- 46.Stephen JK, Vaught LE, Chen KM, Shah V, Schweitzer VG, Gardner G, Benninger MS, Worsham MJ. An epigenetically derived monoclonal origin for recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2007;133:684–692. doi: 10.1001/archotol.133.7.684. [DOI] [PubMed] [Google Scholar]
- 47.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang TH, Perry MR, Laux DE. Methylation profiling of CpG islands in human breast cancer cells. Hum Mol Genet. 1999;8:459–470. doi: 10.1093/hmg/8.3.459. [DOI] [PubMed] [Google Scholar]
- 49.Nygren AO, Ameziane N, Duarte HM, Vijzelaar RN, Waisfisz Q, Hess CJ, Schouten JP, Errami A. Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005;33:e128. doi: 10.1093/nar/gni127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan H, Oridate N, Lotan D, Hong WK, Lotan R. Overexpression of retinoic acid receptor beta in head and neck squamous cell carcinoma cells increases their sensitivity to retinoid-induced suppression of squamous differentiation by retinoids. Cancer Res. 1999;59:3518–3526. [PubMed] [Google Scholar]
- 51.Youssef EM, Lotan D, Issa JP, Wakasa K, Fan YH, Mao L, Hassan K, Feng L, Lee JJ, Lippman SM, Hong WK, Lotan R. Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res. 2004;10:1733–1742. doi: 10.1158/1078-0432.ccr-0989-3. [DOI] [PubMed] [Google Scholar]
- 52.Toyota M, Sasaki Y, Satoh A, Ogi K, Kikuchi T, Suzuki H, Mita H, Tanaka N, Itoh F, Issa JP, Jair KW, Schuebel KE, Imai K, Tokino T. Epigenetic inactivation of CHFR in human tumors. Proc Natl Acad Sci U S A. 2003;100:7818–7823. doi: 10.1073/pnas.1337066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uesugi H, Uzawa K, Kawasaki K, Shimada K, Moriya T, Tada A, Shiiba M, Tanzawa H. Status of reduced expression and hypermethylation of the APC tumor suppressor gene in human oral squamous cell carcinoma. Int J Mol Med. 2005;15:597–602. [PubMed] [Google Scholar]
- 54.Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI, Peters JH, DeMeester TR, Danenberg KD, Danenberg PV, Laird PW, Skinner KA. Fields of aberrant CpG island hypermethylation in Barrett’s esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- 55.Capper JW, Bailey CM, Michaels L. Squamous papillomas of the larynx in adults. A review of 63 cases. Clin Otolaryngol Allied Sci. 1983;8:109–119. doi: 10.1111/j.1365-2273.1983.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 56.Bauman NM, Smith RJ. Recurrent respiratory papillomatosis. Pediatr Clin North Am. 1996;43:1385–1401. doi: 10.1016/s0031-3955(05)70524-1. [DOI] [PubMed] [Google Scholar]
- 57.Batsakis JG, Suarez P. Schneiderian papillomas and carcinomas: a review. Adv Anat Pathol. 2001;8:53–64. doi: 10.1097/00125480-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Doyle DJ, Henderson LA, LeJeune FE, Jr, Miller RH. Changes in human papillomavirus typing of recurrent respiratory papillomatosis progressing to malignant neoplasm. Arch Otolaryngol Head Neck Surg. 1994;120:1273–1276. doi: 10.1001/archotol.1994.01880350079014. [DOI] [PubMed] [Google Scholar]
- 59.Buchwald C, Franzmann MB, Jacobsen GK, Lindeberg H. Human papillomavirus (HPV) in sinonasal papillomas: a study of 78 cases using in situ hybridization and polymerase chain reaction. Laryngoscope. 1995;105:66–71. doi: 10.1288/00005537-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Brandwein M, Steinberg B, Thung S, Biller H, Dilorenzo T, Galli R. Human papillomavirus 6/11 and 16/18 in Schneiderian inverted papillomas. In situ hybridization with human papillomavirus RNA probes. Cancer. 1989;63:1708–1713. doi: 10.1002/1097-0142(19900501)63:9<1708::aid-cncr2820630911>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 61.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79:5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabbott M, Cossart YE, Kan A, Konopka M, Chan R, Rose BR. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J Clin Microbiol. 1997;35:3098–3103. doi: 10.1128/jcm.35.12.3098-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syrjanen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174–181. doi: 10.1136/jcp.56.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Syrjanen S, Happonen RP, Virolainen E, Siivonen L, Syrjanen K. Detection of human papillomavirus (HPV) structural antigens and DNA types in inverted papillomas and squamous cell carcinomas of the nasal cavities and paranasal sinuses. Acta Otolaryngol. 1987;104:334–341. doi: 10.3109/00016488709107337. [DOI] [PubMed] [Google Scholar]
- 66.Hartley C, Hamilton J, Birzgalis AR, Farrington WT. Recurrent respiratory papillomatosis--the Manchester experience, 1974–1992. J Laryngol Otol. 1994;108:226–229. [PubMed] [Google Scholar]
- 67.Lie ES, Karlsen F, Holm R. Presence of human papillomavirus in squamous cell laryngeal carcinomas. A study of thirty-nine cases using polymerase chain reaction and in situ hybridization. Acta Otolaryngol. 1996;116:900–905. doi: 10.3109/00016489609137949. [DOI] [PubMed] [Google Scholar]
- 68.Katori H, Nozawa A, Tsukuda M. Markers of malignant transformation of sinonasal inverted papilloma. Eur J Surg Oncol. 2005;31:905–911. doi: 10.1016/j.ejso.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Lele SM, Pou AM, Ventura K, Gatalica Z, Payne D. Molecular events in the progression of recurrent respiratory papillomatosis to carcinoma. Arch Pathol Lab Med. 2002;126:1184–1188. doi: 10.5858/2002-126-1184-MEITPO. [DOI] [PubMed] [Google Scholar]
- 70.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 71.Wormald PJ, Ooi E, van Hasselt CA, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope. 2003;113:867–873. doi: 10.1097/00005537-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 72.Lawson W, Kaufman MR, Biller HF. Treatment outcomes in the management of inverted papilloma: an analysis of 160 cases. Laryngoscope. 2003;113:1548–1556. doi: 10.1097/00005537-200309000-00026. [DOI] [PubMed] [Google Scholar]
- 73.Califano J, Koch W, Sidransky D, Westra WH. Inverted sinonasal papilloma : a molecular genetic appraisal of its putative status as a Precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–337. doi: 10.1016/S0002-9440(10)64734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeh KT, Chang JG, Lin TH, Wang YF, Tien N, Chang JY, Chen JC, Shih MC. Epigenetic changes of tumor suppressor genes, P15, P16, VHL and P53 in oral cancer. Oncol Rep. 2003;10:659–663. [PubMed] [Google Scholar]
- 75.Shintani S, Nakahara Y, Mihara M, Ueyama Y, Matsumura T. Inactivation of the p14(ARF), p15(INK4B) and p16(INK4A) genes is a frequent event in human oral squamous cell carcinomas. Oral Oncol. 2001;37:498–504. doi: 10.1016/s1368-8375(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 76.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 77.Garcia M, Derocq D, Freiss G, Rochefort H. Activation of estrogen receptor transfected into a receptor-negative breast cancer cell line decreases the metastatic and invasive potential of the cells. Proc Natl Acad Sci U S A. 1992;89:11538–11542. doi: 10.1073/pnas.89.23.11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 79.Duggan MA, Lim M, Gill MJ, Inoue M. HPV DNA typing of adult-onset respiratory papillomatosis. Laryngoscope. 1990;100:639–642. doi: 10.1288/00005537-199006000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, et al. Proc Natl Acad Sci U S A. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]