Abstract
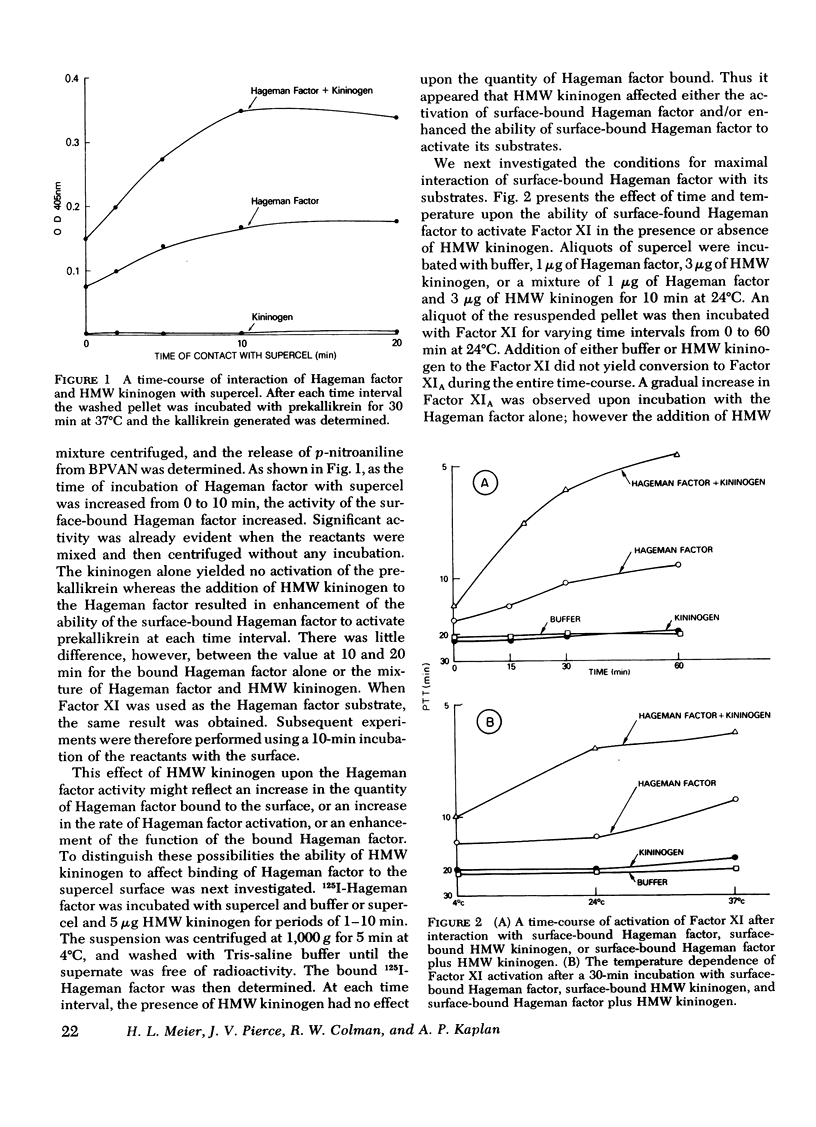
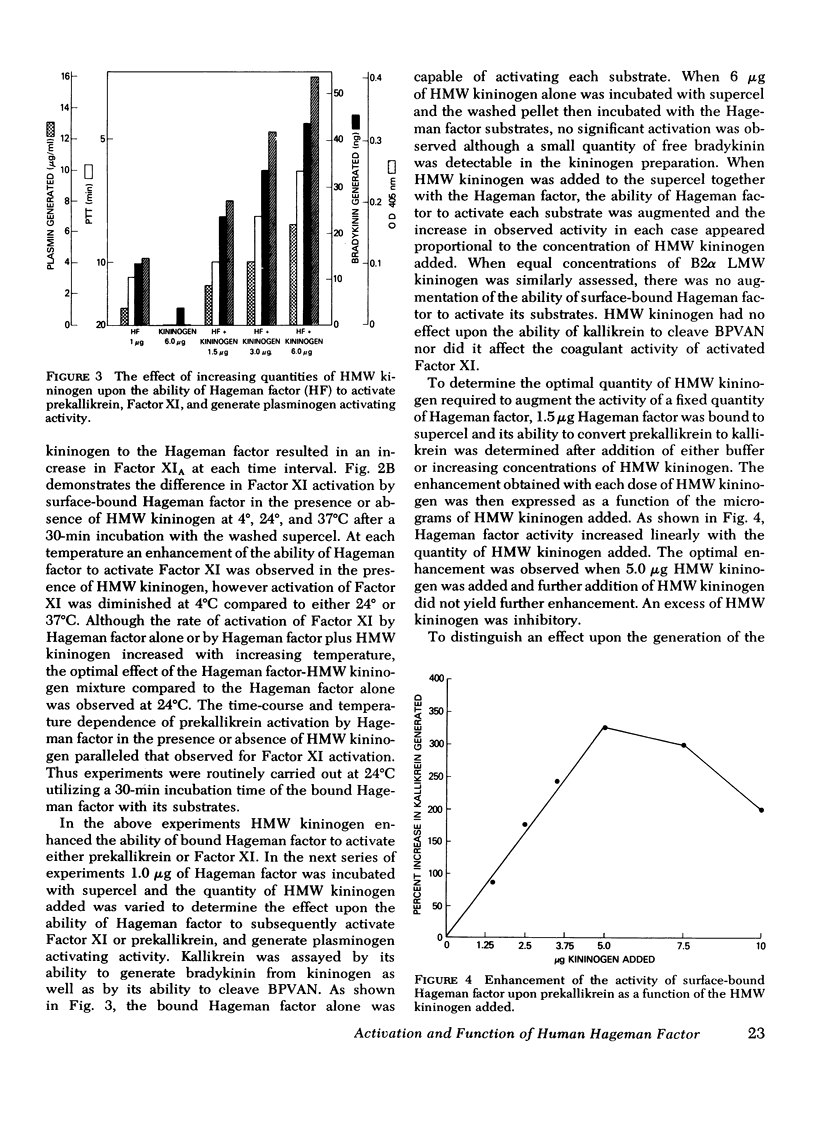
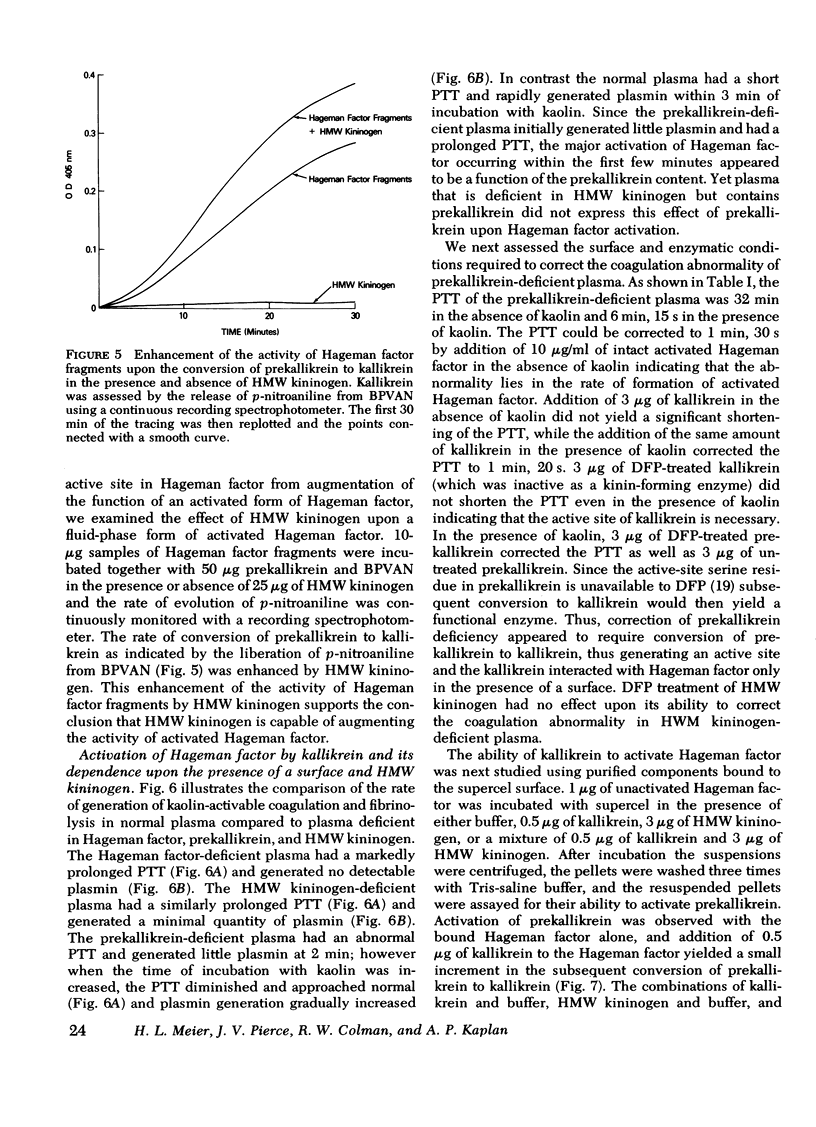
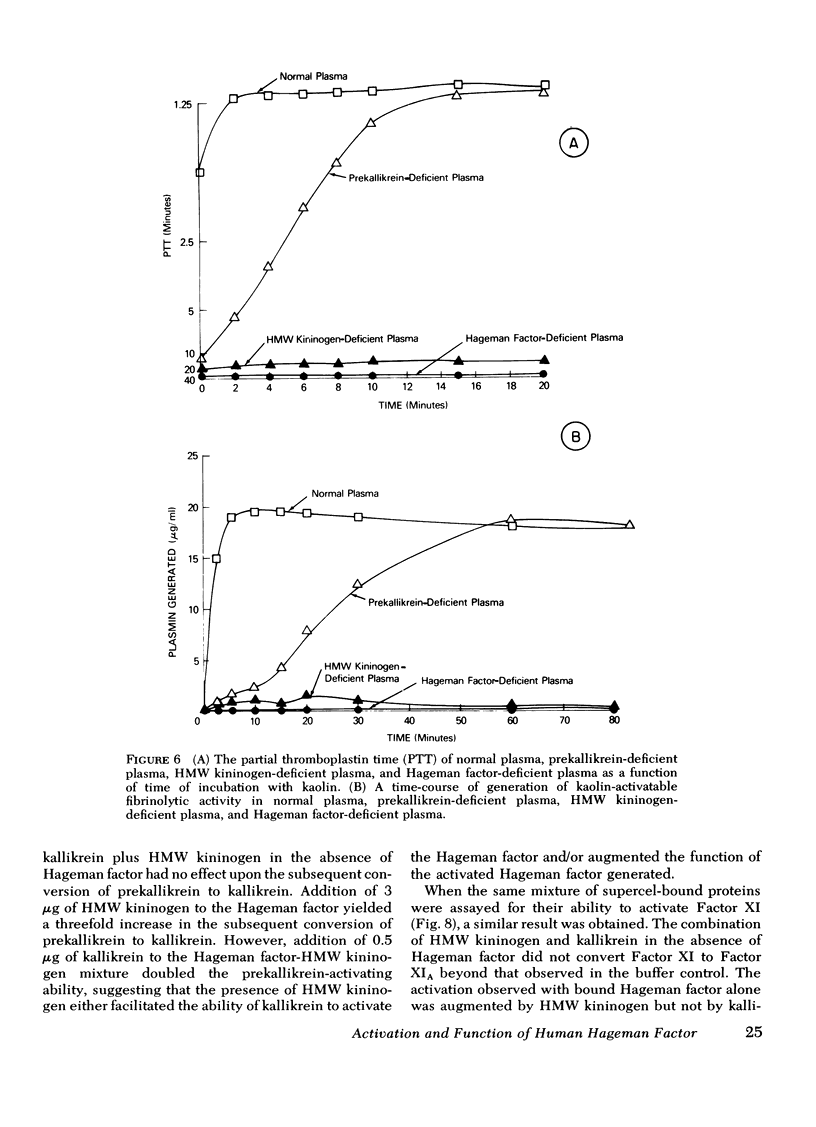
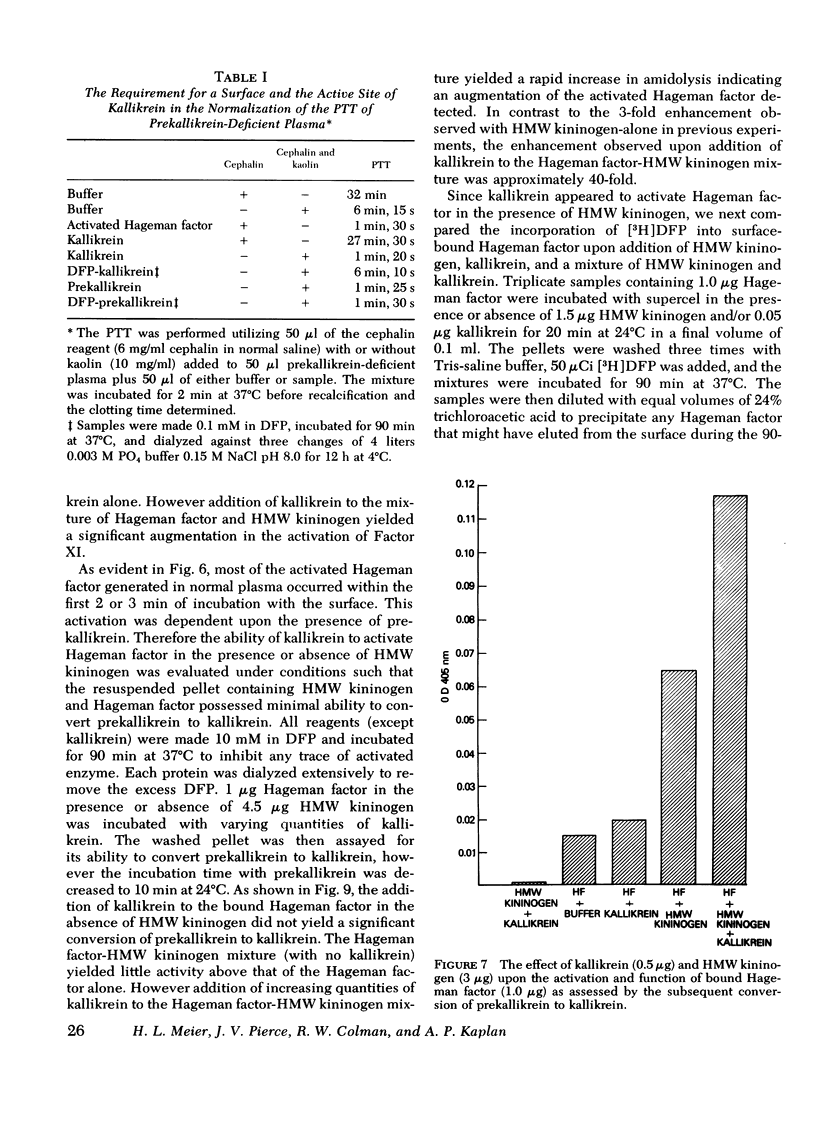
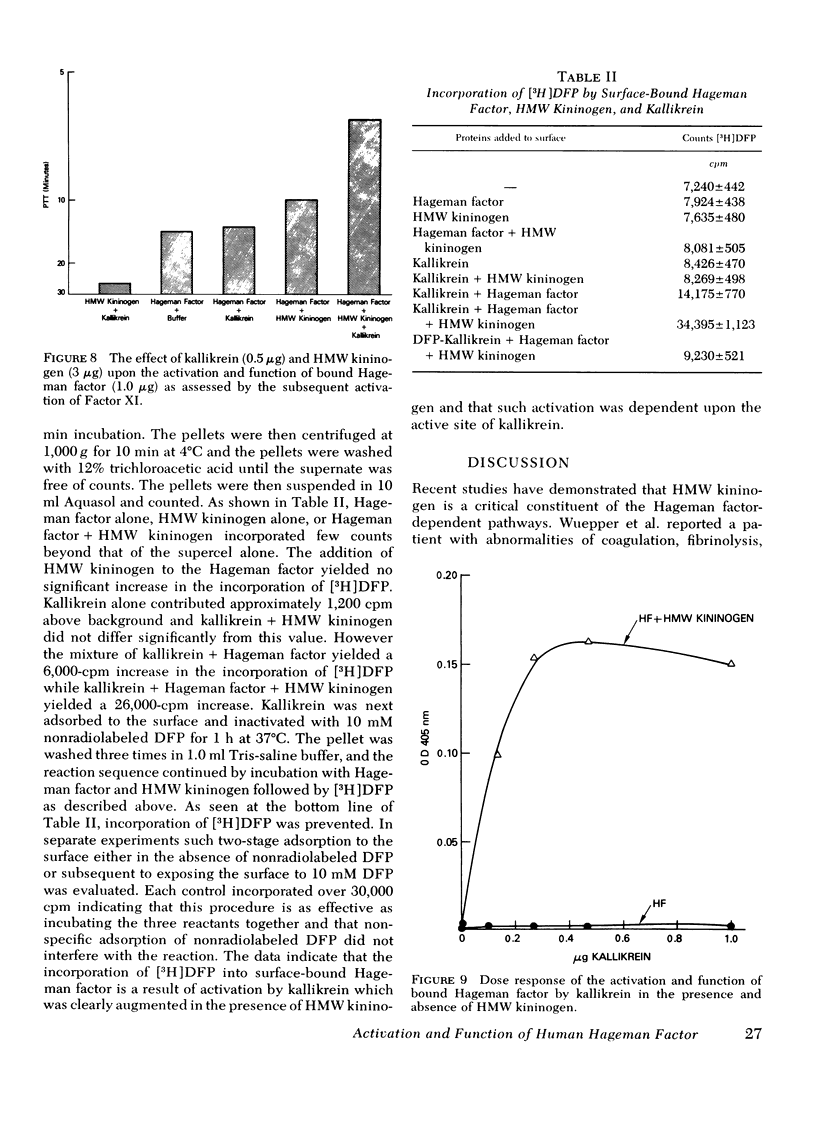
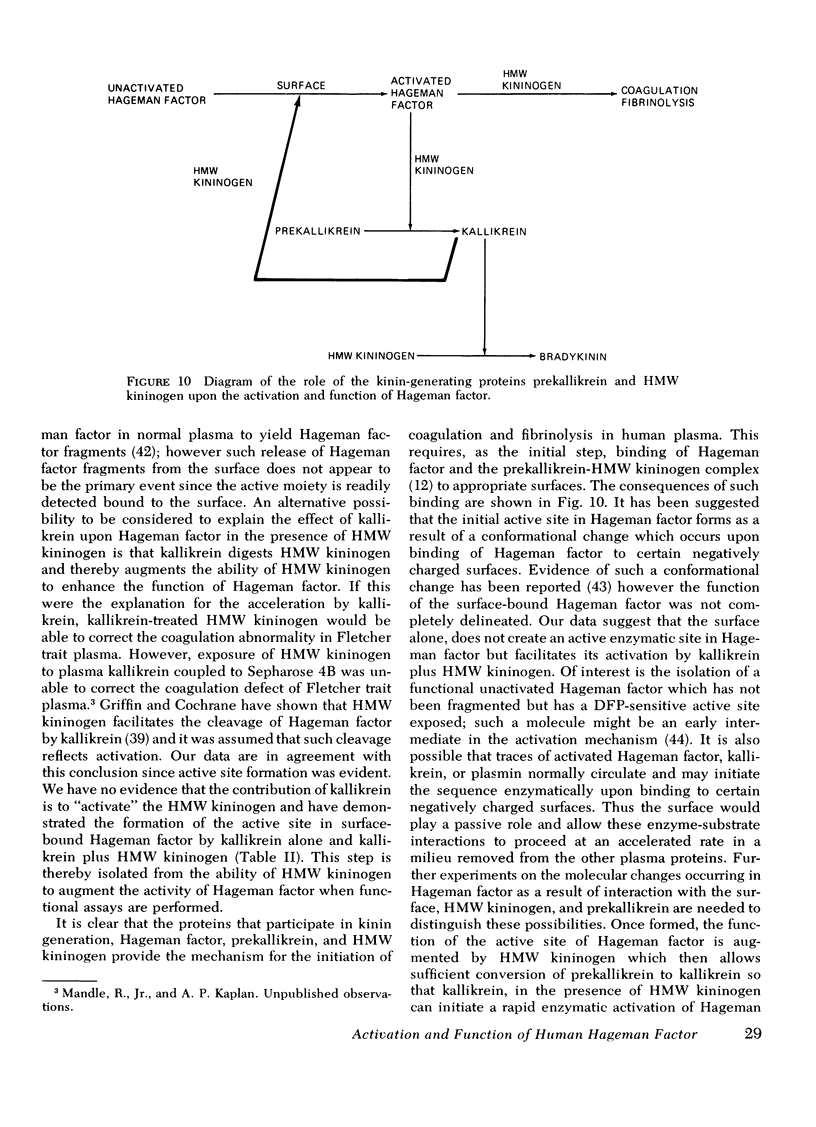
The activation and function of surface-bound Hageman factor in human plasma are dependent upon both high molecular weight (HMW) kininogen and prekallikrein. HMW kininogen does not affect the binding of Hageman factor to surfaces, but it enhances the function of surface-bound Hageman factor as assessed by its ability to activate prekallikrein and Factor XI. The initial conversion of prekallikrein to kallikrein by the surface-bound Hageman factor in the presence of HMW kininogen is followed by a rapid enzymatic activation of Hageman factor by kallikrein. The latter interaction is also facilitated by HMW kininogen. Kallikrein therefore functions as an activator of Hageman factor by a positive feedback mechanism and generates most of the activated Hageman factor during brief exposure of plasma to activating surfaces. HMW kininogen is a cofactor in the enzymatic activation of Hageman factor by kallikrein and it also augments the function of the activated Hageman factor generated. The stoichiometry of the Hagman factor interaction with HMW kininogen suggests that it enhances the activity of the active site of Hageman factor. Since HMW kininogen and prekallikrein circulate as a complex, HMW kininogen may also place the prekallikrein in an optimal position for its reciprocal interaction with Hageman factor to proceed. The surface appears to play a passive role upon which bound Hageman factor and the prekallikrein-HMW kininogen complex can interact.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bagdasarian A., Lahiri B., Colman R. W. Origin of the high molecular weight activator of prekallikrein. J Biol Chem. 1973 Nov 25;248(22):7742–7747. [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A., Talamo R. C., Scott C. F., Seavey M., Guimaraes J. A., Pierce J. V., Kaplan A. P. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H., Glueck H. I., Miller M. A., Movat H. Z., Habal F. Kininogen deficiency in Fitzgerald trait: role of high molecular weight kininogen in clotting and fibrinolysis. J Lab Clin Med. 1976 Feb;87(2):327–337. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Kaplan A. P. Substrates of Hageman factor. I. Isolation and characterization of human factor XI (PTA) and inhibition of the activated enzyme by alpha 1-antitrypsin. J Exp Med. 1974 Dec 1;140(6):1615–1630. doi: 10.1084/jem.140.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A pre-albumin activator of prekallikrein. J Immunol. 1970 Oct;105(4):802–811. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. The fibrinolytic pathway of human plasma. Isolation and characterization of the plasminogen proactivator. J Exp Med. 1972 Dec 1;136(6):1378–1393. doi: 10.1084/jem.136.6.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Kay A. B., Austen K. F. A prealbumin activator of prekallikrein. 3. Appearance of chemotactic activity for human neutrophils by the conversion of human prekallikrein to kallikrein. J Exp Med. 1972 Jan;135(1):81–97. doi: 10.1084/jem.135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lincoln T. M., Hall C. L., Park C. R., Corbin J. D. Guanosine 3':5'-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2559–2563. doi: 10.1073/pnas.73.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandle R. J., Colman R. W., Kaplan A. P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin C. R., Saito H., Ratnoff O. D., Walton A. G. The secondary structure of human Hageman factor (factor XII) and its alteration by activating agents. J Clin Invest. 1974 Dec;54(6):1312–1322. doi: 10.1172/JCI107877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston D., Bennett N. B., Ogston C. M., Ratnoff O. D. The assay of a plasma component necessary for the generation of a plasminogen activator in the presence of Hageman factor (Hageman factor co-factor). Br J Haematol. 1971 Feb;20(2):209–216. doi: 10.1111/j.1365-2141.1971.tb07029.x. [DOI] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., COLOPY J. E. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955 Apr;34(4):602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W. The purification of activated Hageman factor (activated factor XII). Biochemistry. 1962 Nov;1:967–975. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Saito H. Evidence that Fitzgerald factor counteracts inhibition by kaolin or ellagic acid of the amidolytic properties of a plasma kallikrein. Blood. 1976 Feb;47(2):243–251. [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Donaldson V. H. Defective activation of clotting, fibrinolytic, and permeability-enhancing systems in human Fletcher trait plasma. Circ Res. 1974 May;34(5):641–651. doi: 10.1161/01.res.34.5.641. [DOI] [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Waldmann R., Abraham J. P. Fitzgerald Trait: Deficiency of a Hitherto Unrecognized Agent, Fitzgerald Factor, Participating in Surface-Mediated Reactions of Clotting, Fibrinolysis, Generation of Kinins, and the Property of Diluted Plasma Enhancing Vascular Permeability (PF/Dil). J Clin Invest. 1975 May;55(5):1082–1089. doi: 10.1172/JCI108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S., Lee P. Partial purification and characterization of contact activation cofactor. J Clin Invest. 1975 Nov;56(5):1082–1092. doi: 10.1172/JCI108182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S., Lee P. Preparation, characterization, and activation of a highly purified factor XI: evidence that a hitherto unrecognized plasma activity participates in the interaction of factors XI and XII. Br J Haematol. 1974 May;27(1):101–114. doi: 10.1111/j.1365-2141.1974.tb06778.x. [DOI] [PubMed] [Google Scholar]
- Stead N., Kaplan A. P., Rosenberg R. D. Inhibition of activated factor XII by antithrombin-heparin cofactor. J Biol Chem. 1976 Nov 10;251(21):6481–6488. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss A. S., Gallin J. I., Kaplan A. P. Fletcher factor deficiency. A diminished rate of Hageman factor activation caused by absence of prekallikrein with abnormalities of coagulation, fibrinolysis, chemotactic activity, and kinin generation. J Clin Invest. 1974 Feb;53(2):622–633. doi: 10.1172/JCI107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuepper K. D., Miller D. R., Lacombe M. J. Flaujeac trait. Deficiency of human plasma kininogen. J Clin Invest. 1975 Dec;56(6):1663–1672. doi: 10.1172/JCI108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuepper K. D. Prekallikrein deficiency in man. J Exp Med. 1973 Dec 1;138(6):1345–1355. doi: 10.1084/jem.138.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]


