Abstract
To evaluate the contribution of genetic influences on the individual variation in plateau serum salicylate levels, salicylate metabolism was studied in seven pairs of identical and six pairs of fraternal twins.
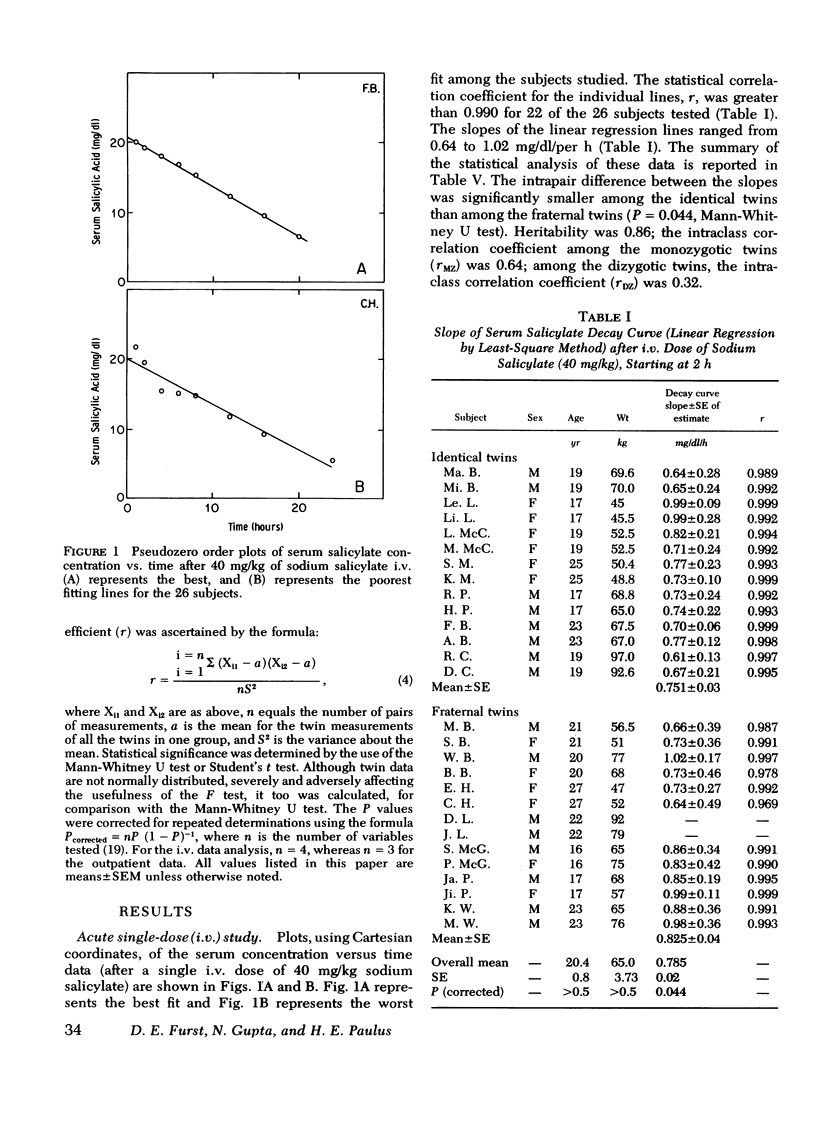
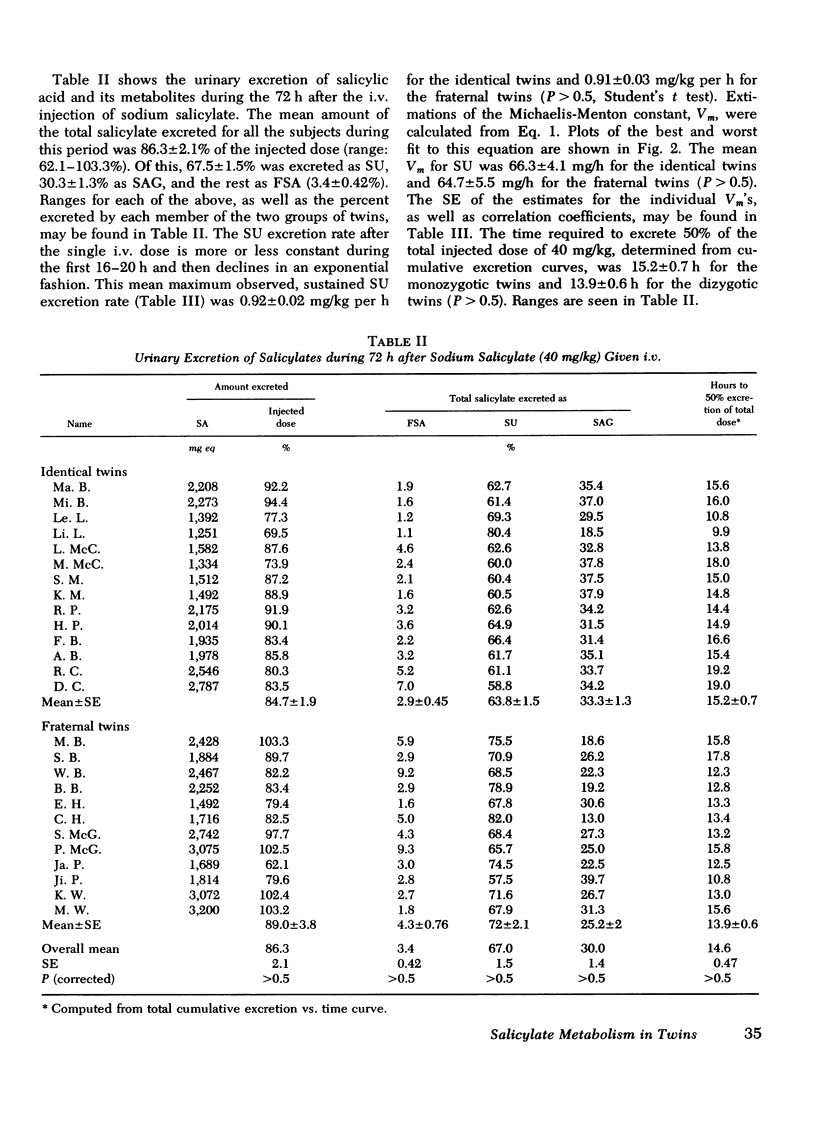
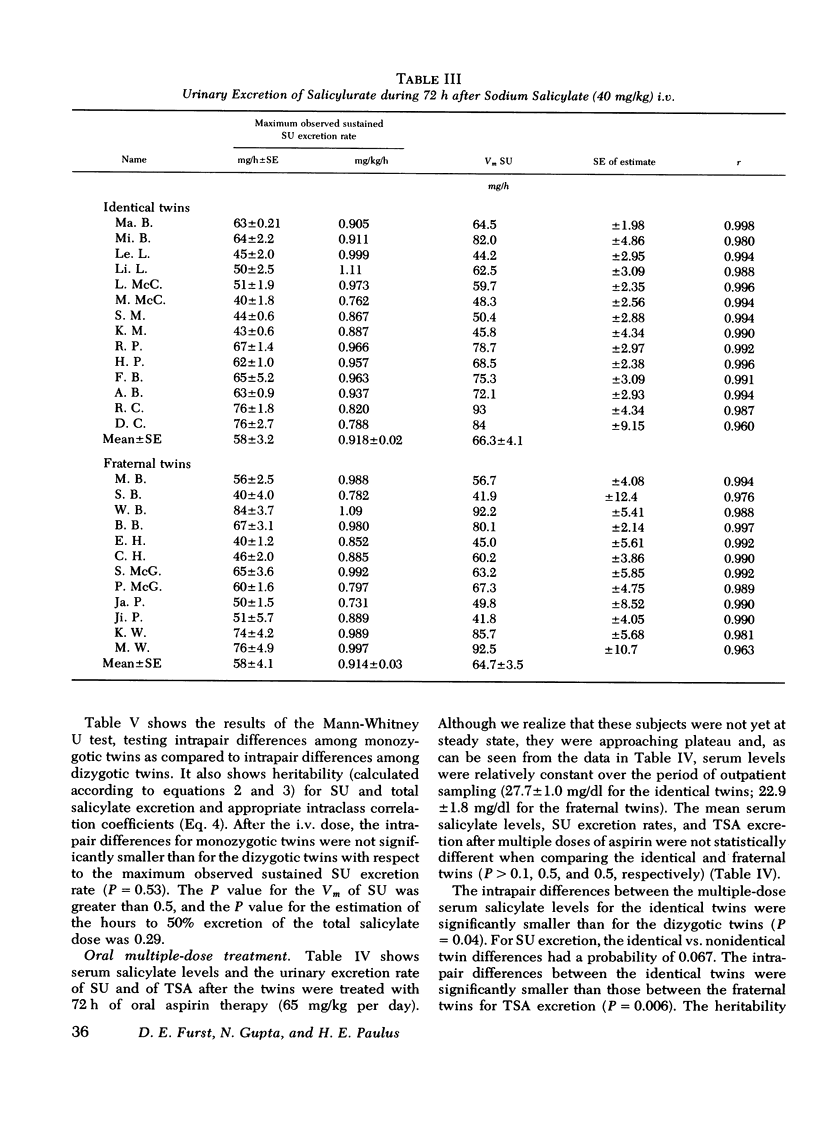
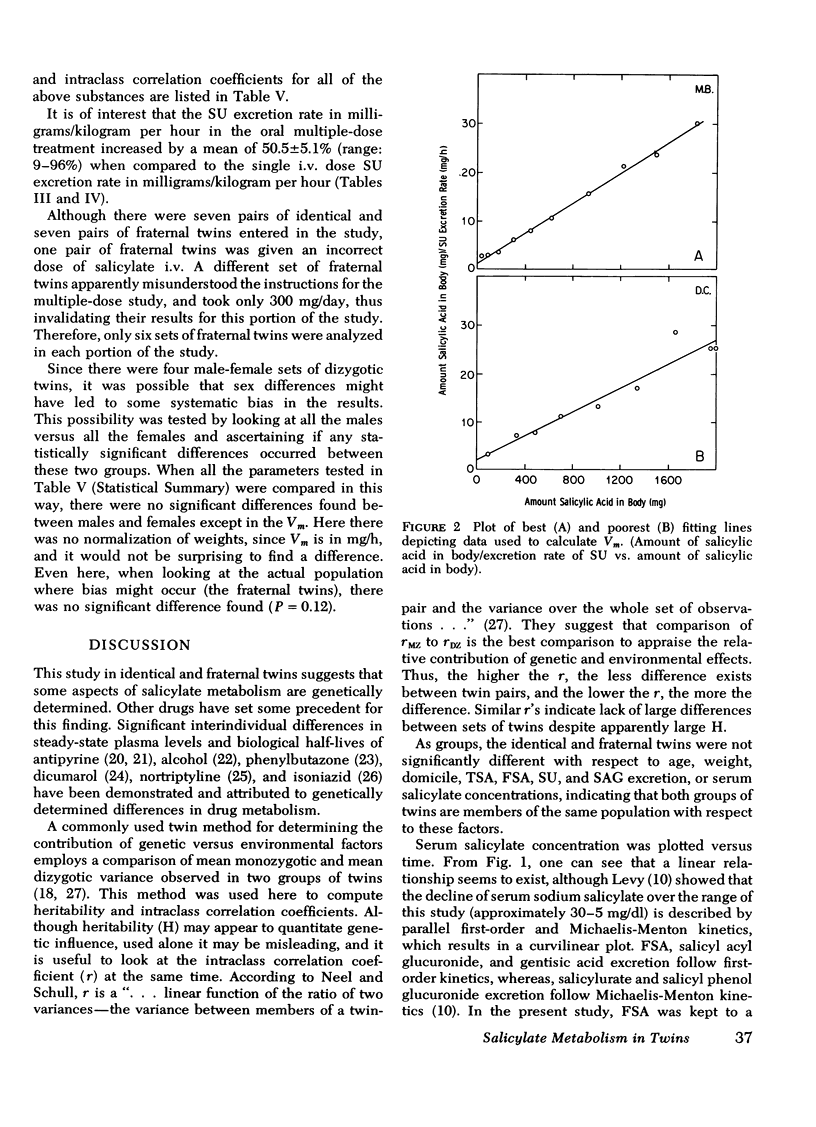
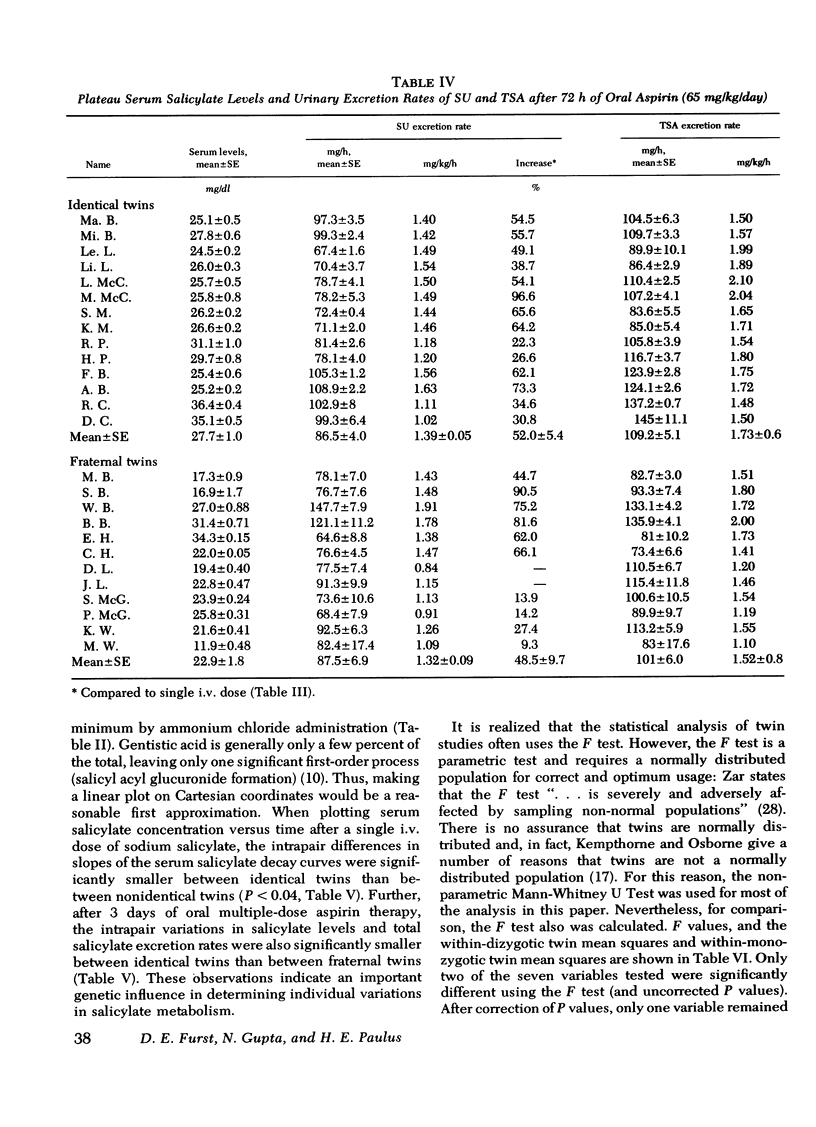
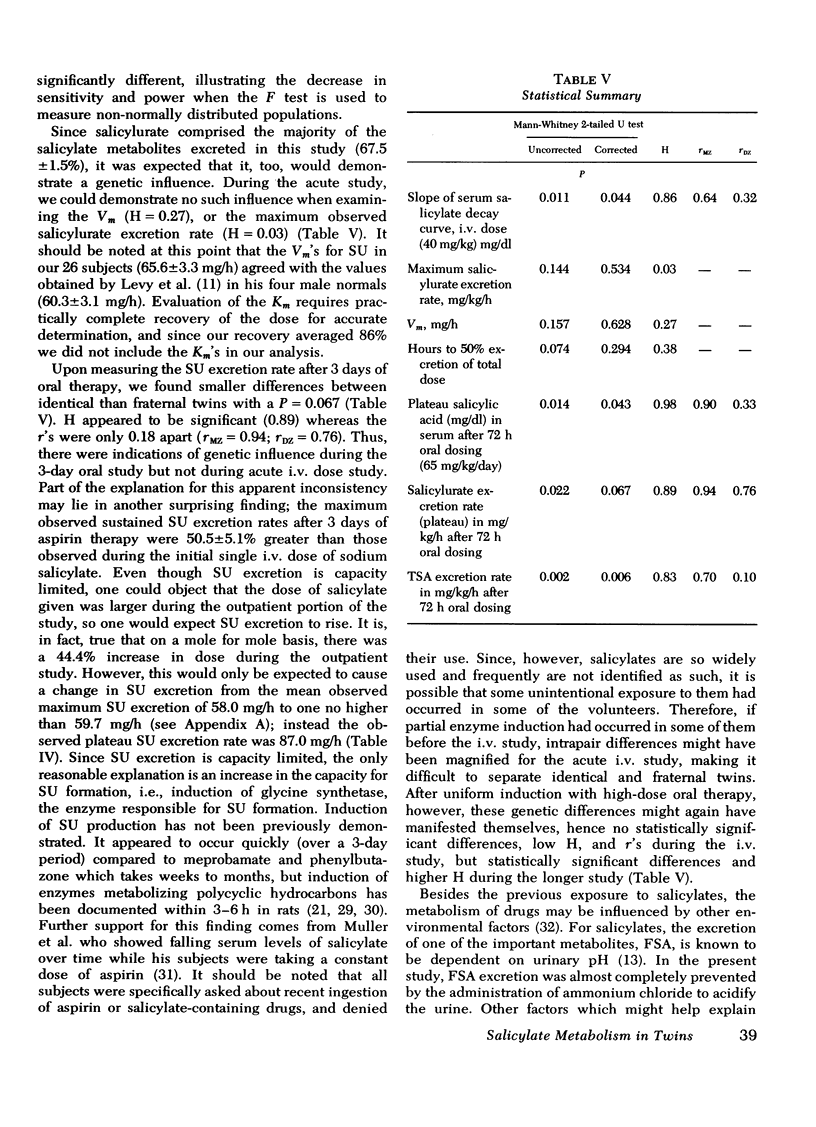
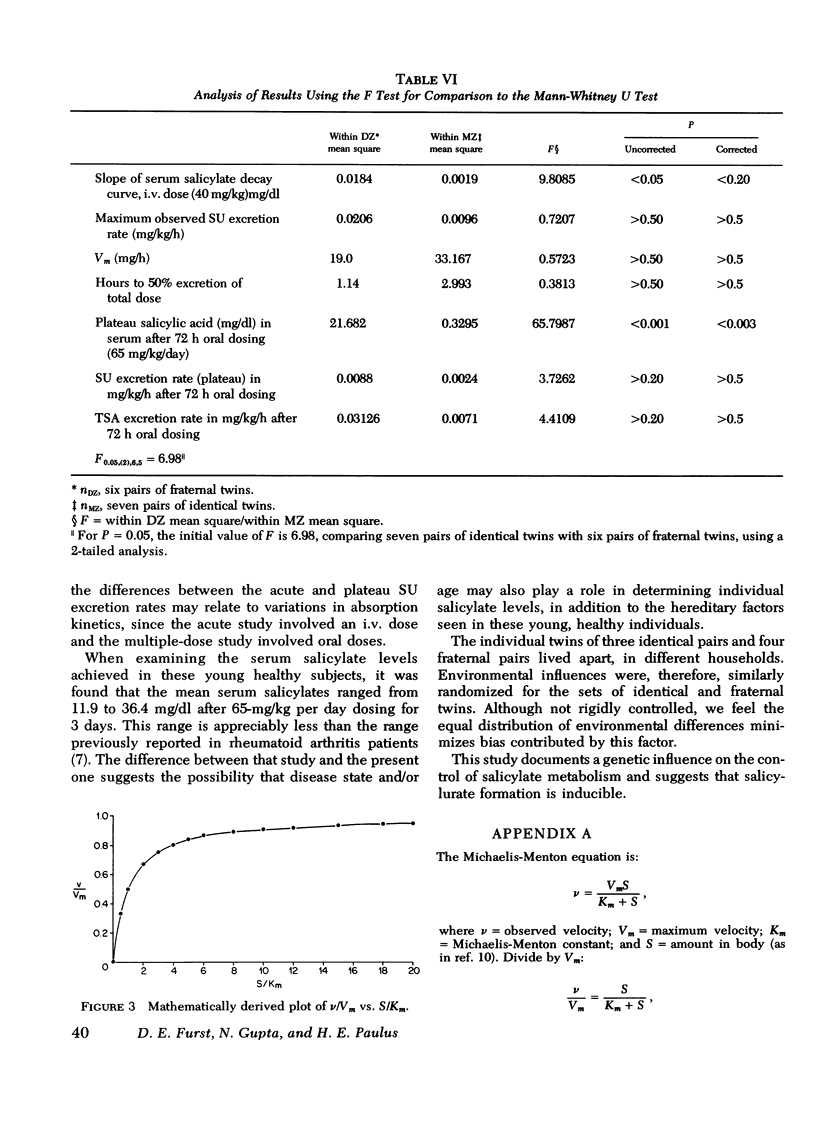
Under the conditions of this study, after a single i.v. dose (40 mg/kg) of sodium salicylate, the serum salicylate concentration versus time curve approximated a straight line on linear coordinates (appeared approximately zero order). The slopes of the decay curves ranged between 0.64 and 1.02. The intrapair variation for identical twin pairs was significantly less than for fraternal twin pairs (P = 0.044). Likewise pleateau serum salicylic acid concentrations (milligrams/deciliter) and total salicylic acid excretion rate after multiple doses demonstrated significantly less intrapair variation for identical twins than for fraternal twins (P = 0.043 and 0.006). Plateau salicylurate excretion (milligram/kilogram per hour) differences after multiple dosing had a P = 0.067. Michaelis-Menton constant for salicylurate formation and hours to 50% excretion after the i.v. dose were not different when comparing identical and nonidentical twins.
Salicylurate formation rates were increased after 3 days of oral therapy, and this induction phenomenon may account for much of the apparent discrepancy between genetic influences on salicylurate formation rates observed after single and multiple dose salicylate administration.
This study suggests that the plateau concentration of serum salicylate varies among individuals given the same weight-adjusted dose in part because of genetically determined variations in their metabolism of salicylate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexanderson B., Evans D. A., Sjöqvist F. Steady-state plasma levels of nortriptyline in twins: influence of genetic factors and drug therapy. Br Med J. 1969 Dec 27;4(5686):764–768. doi: 10.1136/bmj.4.5686.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDFORD C., CUMMINGS A. J., MARTIN B. K. A KINETIC STUDY OF THE ELIMINATION OF SALICYLATE IN MAN. Br J Pharmacol Chemother. 1965 Apr;24:418–431. doi: 10.1111/j.1476-5381.1965.tb01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Craig J. O., Ferguson I. C., Syme J. Infants, toddlers, and aspirin. Br Med J. 1966 Mar 26;1(5490):757–761. doi: 10.1136/bmj.1.5490.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings A. J., Martin B. K., Renton R. The elimination of salicylic acid in man: serum concentrations and urinary excretion rates. Br J Pharmacol Chemother. 1966 Feb;26(2):461–467. doi: 10.1111/j.1476-5381.1966.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- EVANS D. A., CLARKE C. A. Pharmacogenetics. Br Med Bull. 1961 Sep;17:234–240. doi: 10.1093/oxfordjournals.bmb.a069915. [DOI] [PubMed] [Google Scholar]
- KEMPTHORNE O., OSBORNE R. H. The interpretation of twin data. Am J Hum Genet. 1961 Sep;13:320–339. [PMC free article] [PubMed] [Google Scholar]
- LEVY G., GAGLIARDI B. A. GASTROINTESTINAL ABSORPTION OF ASPIRIN ANHYDRIDE. J Pharm Sci. 1963 Aug;52:730–732. doi: 10.1002/jps.2600520804. [DOI] [PubMed] [Google Scholar]
- LEVY G., HOLLISTER L. E. VARIATION IN RATE OF SALICYLATE ELIMINATION BY HUMANS. Br Med J. 1964 Aug 1;2(5404):286–288. doi: 10.1136/bmj.2.5404.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G., Leonards J. R. Urine pH and salicylate therapy. JAMA. 1971 Jul 5;217(1):81–81. doi: 10.1001/jama.217.1.81c. [DOI] [PubMed] [Google Scholar]
- Levy G. Pharmacokinetics of salicylate elimination in man. J Pharm Sci. 1965 Jul;54(7):959–967. doi: 10.1002/jps.2600540703. [DOI] [PubMed] [Google Scholar]
- Levy G., Procknal J. A. Drug biotransformation interactions in man. I. Mutual inhibition in glucuronide formation of salicylic acid and salicylamide in man. J Pharm Sci. 1968 Aug;57(8):1330–1335. doi: 10.1002/jps.2600570811. [DOI] [PubMed] [Google Scholar]
- Levy G., Tsuchiya T., Amsel L. P. Limited capacity for salicyl phenolic glucuronide formation and its effect on the kinetics of salicylate elimination in man. Clin Pharmacol Ther. 1972 Mar-Apr;13(2):258–268. doi: 10.1002/cpt1972132258. [DOI] [PubMed] [Google Scholar]
- Levy G., Tsuchiya T. Salicylate accumulation kinetics in man. N Engl J Med. 1972 Aug 31;287(9):430–432. doi: 10.1056/NEJM197208312870903. [DOI] [PubMed] [Google Scholar]
- Levy G., Vogel A. W., Amsel L. P. Capacity-limited salicylurate formation during prolonged administration of aspirin to healthy human subjects. J Pharm Sci. 1969 Apr;58(4):503–504. doi: 10.1002/jps.2600580432. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Siegel M., Mongan E., Okun R., Calabro J. J. Variations of serum concentrations and half-life of salicylate in patients with rheumatoid arthritis. Arthritis Rheum. 1971 Jul-Aug;14(4):527–532. doi: 10.1002/art.1780140412. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. Genetic and environmental factors affecting hexobarbital metabolism in mice. Ann N Y Acad Sci. 1968 Jul 31;151(2):900–912. doi: 10.1111/j.1749-6632.1968.tb48275.x. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of dicumarol levels in man. J Clin Invest. 1968 Dec;47(12):2657–2663. doi: 10.1172/JCI105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of drug levels in man: antipyrine. Science. 1968 Jul 5;161(3836):72–73. doi: 10.1126/science.161.3836.72. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of drug levels in man: phenylbutazone. Science. 1968 Mar 29;159(3822):1479–1480. doi: 10.1126/science.159.3822.1479. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of the phenobarbital-induced shortening of plasma antipyrine half-lives in man. J Clin Invest. 1969 Dec;48(12):2202–2209. doi: 10.1172/JCI106186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G., Passananti G. T. Genetic and environmental factors affecting ethanol metabolism in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):192–201. doi: 10.1002/cpt1971122part1192. [DOI] [PubMed] [Google Scholar]


