Abstract
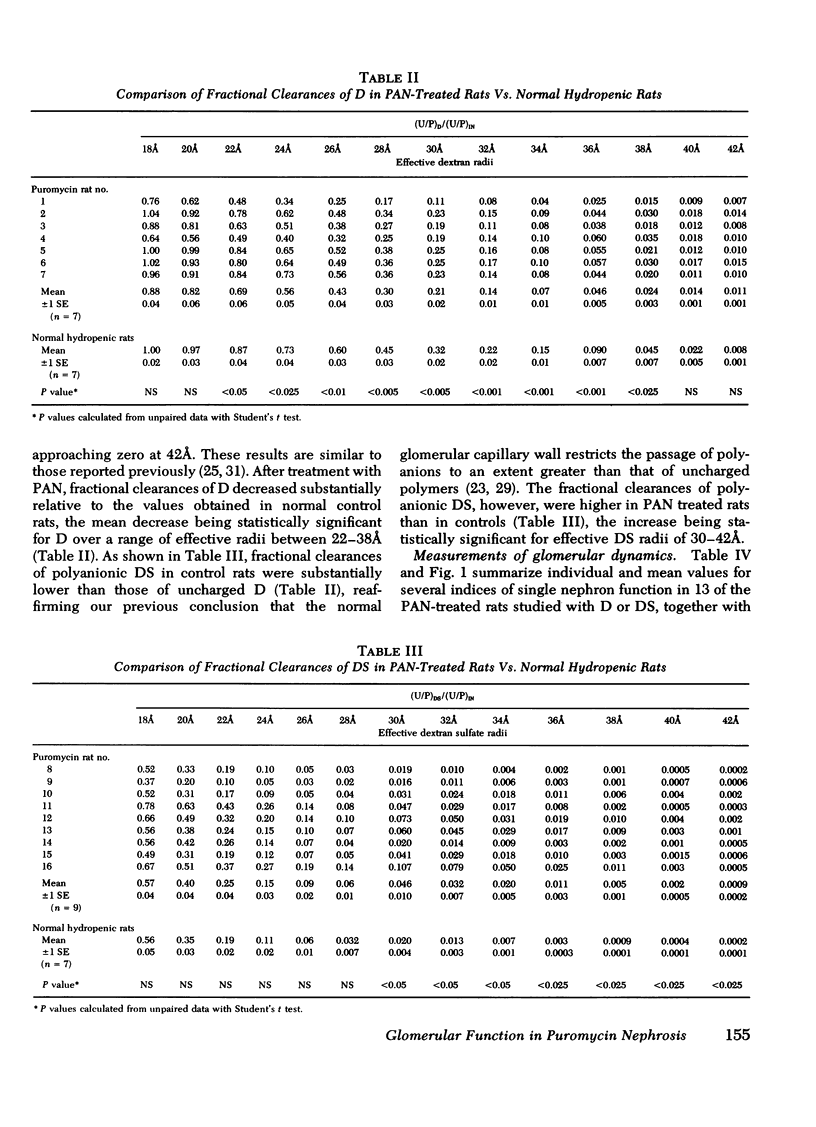
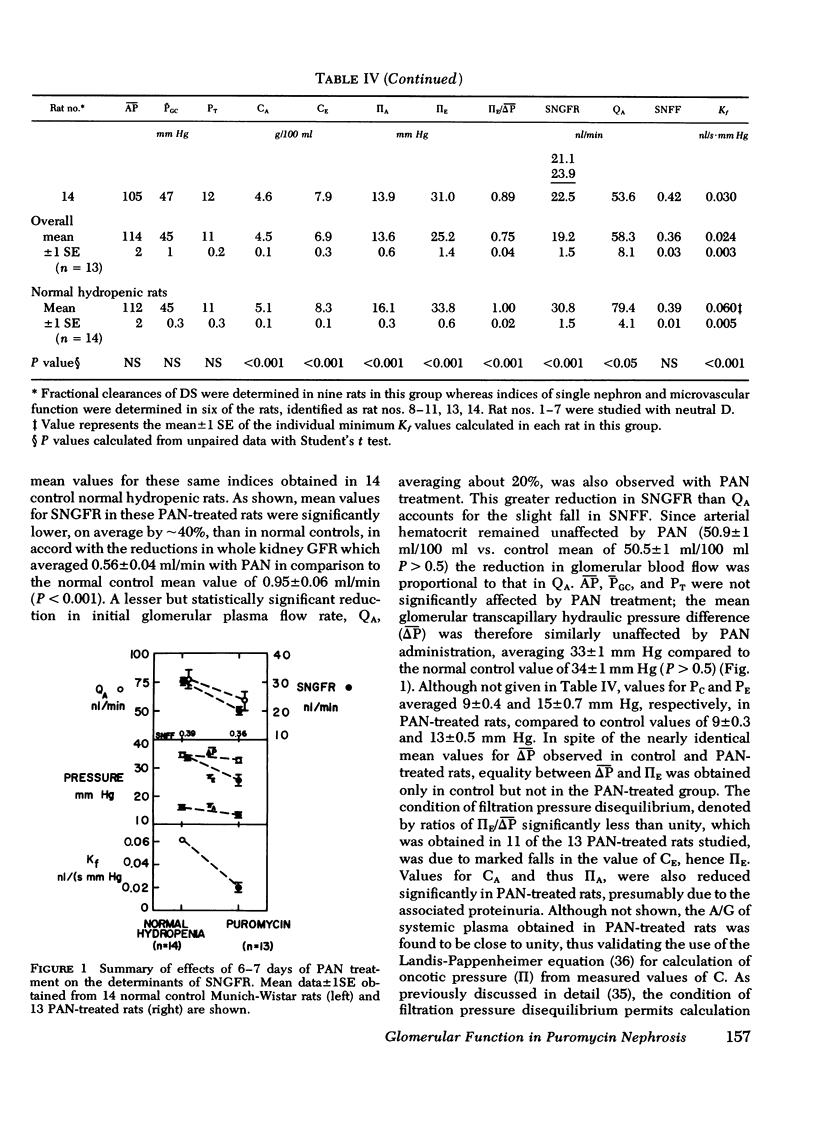
To investigate the mechanism(s) of increased filtration of serum proteins after glomerular injury, polydisperse samples of uncharged [3H]dextran (D) or anionic [3H]dextran sulfate (DS) were infused into 14 control and 16 puromycin aminonucleoside- (PAN) treated Munich-Wistar rats. Fractional clearances of D or DS ranging in radius from 18 to 42Å were determined in these rats, together with direct measurements of the forces governing the glomerular filtration rate of water. Whole kidney and single nephron glomerular filtration rates were ∼40% lower in PAN-treated rats, relative to controls, due mainly to a marked reduction in the glomerular capillary ultrafiltration coefficient and, to a lesser extent, to a small reduction in glomerular plasma flow rate as well. In PAN-treated rats, as in normal controls, inulin was found to permeate the glomerular capillary wall without measurable restriction, and both D and DS were shown to be neither secreted nor reabsorbed. Fractional clearances of uncharged D were reduced after PAN administration, falling significantly for effective D radii from 22 to 38Å. Utilizing a theory based on macromolecular transport through pores, these results indicate that in PAN-treated rats, effective pore radius is the same as in controls, ∼44Å. In PAN nephrosis, however, the ratio of total pore surface area/pore length, a measure of pore density, is reduced to approximately one-third that of control, due very likely to a reduction in filtration surface area. In contrast to the results with uncharged D, fractional clearances of DS were found to increase after PAN administration for all DS radii studied. These results with D and DS suggest that proteinuria in PAN nephrosis is due, not to an increase in effective pore radius or number of pores, but rather to a diminution of the electrostatic barrier function of the glomerular capillary wall, thereby allowing increased passage of polyanions such as DS and albumin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970 Nov;23(5):489–496. [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Blau E. B., Michael A. F. Rat glomerular glycoprotein composition and metabolism in aminonucleoside nephrosis. Proc Soc Exp Biol Med. 1972 Oct;141(1):164–172. doi: 10.3181/00379727-141-36737. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. The permeability of glomerular capillaries of aminonuceoside nephrotic rats to graded dextrans. J Exp Med. 1975 Jul 1;142(1):61–83. doi: 10.1084/jem.142.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Bennett C. M., Glassock R. J., Brenner B. M., Troy J. L., Ueki I. F., Rasmussen B. Permselectivity of of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using neutral dextran. J Clin Invest. 1976 May;57(5):1272–1286. doi: 10.1172/JCI108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975 Oct;8(4):212–218. doi: 10.1038/ki.1975.104. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Robertson C. R., Deen W. M., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. I. Theoretical considerations. Biophys J. 1975 Sep;15(9):861–886. doi: 10.1016/S0006-3495(75)85862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois R., Decoodt P., Gassée J. P., Verniory A., Lambert P. P. Determination of glomerular intracapillary and transcapillary pressure gradients from sieving data. I. A mathematical model. Pflugers Arch. 1975;356(4):299–316. doi: 10.1007/BF00580004. [DOI] [PubMed] [Google Scholar]
- FRENK S., ANTONOWICZ I., CRAIG J. M., METCOFF J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955 Jul;89(3):424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- Gaizutis M., Pesce A. J., Pollak V. E. Renal clearance of human and rat albumins in the rat. Proc Soc Exp Biol Med. 1975 Apr;148(4):947–952. doi: 10.3181/00379727-148-38666. [DOI] [PubMed] [Google Scholar]
- Gang N. F., Mautner W. Studies on the mechanism of the onset of proteinuria in aminonucleoside nephrosis. Lab Invest. 1972 Sep;27(3):310–316. [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- LAMBERT P. P., GREGOIRE F. Hémodynamique glomérulaire et excrétion de l'hémoglobine. Arch Int Physiol Biochim. 1955 Feb;63(1):7–34. doi: 10.3109/13813455509150857. [DOI] [PubMed] [Google Scholar]
- Lambert P. P., Du Bois R., Decoodt P., Gassée J. P., Verniory A. Determination of glomerular intracapillary and transcapillary pressure gradients from sieving data. II. A physiological study in the normal dog. Pflugers Arch. 1975 Aug 29;359(1-2):1–22. doi: 10.1007/BF00581274. [DOI] [PubMed] [Google Scholar]
- Latta H., Johnston W. H., Stanley T. M. Sialoglycoproteins and filtration barriers in the glomerular capillary wall. J Ultrastruct Res. 1975 Jun;51(3):354–376. doi: 10.1016/s0022-5320(75)80100-6. [DOI] [PubMed] [Google Scholar]
- Lui S., Kalant N. Carbohydrate of the glomerular basement membrane in normal and hephrotic rats. Exp Mol Pathol. 1974 Aug;21(1):52–62. doi: 10.1016/0014-4800(74)90078-1. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Fish A. J., Blau E. B., Michael A. F. The glomerular mesangium. I. Kinetic studies of macromolecular uptake in normal and nephrotic rats. J Clin Invest. 1972 May;51(5):1092–1101. doi: 10.1172/JCI106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Glomerular sialoprotein. Science. 1969 Jun 27;164(3887):1519–1521. doi: 10.1126/science.164.3887.1519. [DOI] [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Histochemical demonstration and localization of sialoproteins in the glomerulus. Exp Mol Pathol. 1970 Jun;12(3):316–323. doi: 10.1016/0014-4800(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Brenner B. M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975 Jul;37(1):101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VIII. Effects of hematocrit. Circ Res. 1975 Mar;36(3):425–435. doi: 10.1161/01.res.36.3.425. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Flamenbaum W. Micropuncture studies of proximal tubule albumin concentrations in normal and nephrotic rats. J Clin Invest. 1971 Jul;50(7):1498–1505. doi: 10.1172/JCI106635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R. Passage of molecules through capillary wals. Physiol Rev. 1953 Jul;33(3):387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- Pricam C., Humbert F., Perrelet A., Amherdt M., Orci L. Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest. 1975 Sep;33(3):209–218. [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson A. M., Giangiacomo J., Kienstra R. A., Naqvi S. T., Ingelfinger J. R. Normal glomerular permeability and its modification by minimal change nephrotic syndrmone. J Clin Invest. 1974 Nov;54(5):1190–1199. doi: 10.1172/JCI107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Cotran R. S., Karnovsky M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970 Dec 1;132(6):1168–1180. doi: 10.1084/jem.132.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Karnovsky M. J., Cotran R. S. Glomerular permeability. Ultrastructural studies in experimental nephrosis using horseradish peroxidase as a tracer. J Exp Med. 1969 Aug 1;130(2):381–399. doi: 10.1084/jem.130.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verniory A., Du Bois R., Decoodt P., Gassee J. P., Lambert P. P. Measurement of the permeability of biological membranes. Application to the glomerular wall. J Gen Physiol. 1973 Oct;62(4):489–507. doi: 10.1085/jgp.62.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEDERHIELM C. A., WOODBURY J. W., KIRK S., RUSHMER R. F. PULSATILE PRESSURES IN THE MICROCIRCULATION OF FROG'S MESENTERY. Am J Physiol. 1964 Jul;207:173–176. doi: 10.1152/ajplegacy.1964.207.1.173. [DOI] [PubMed] [Google Scholar]


