The receptor-like protein ReMAX/RLP1 of Arabidopsis functions as a pattern recognition receptor for eMax, a microbe-associated molecular pattern (MAMP) from Xanthomonas. Nicotiana benthamiana has no detection system for eMax but gains responsiveness to this MAMP when expressing a hybrid receptor with the extracellular domain of ReMAX and the cytoplasmic part of the related Eix2 from tomato.
Abstract
As part of their immune system, plants have pattern recognition receptors (PRRs) that can detect a broad range of microbe-associated molecular patterns (MAMPs). Here, we identified a PRR of Arabidopsis thaliana with specificity for the bacterial MAMP eMax from xanthomonads. Response to eMax seems to be restricted to the Brassicaceae family and also varied among different accessions of Arabidopsis. In crosses between sensitive accessions and the insensitive accession Shakhdara, eMax perception mapped to RECEPTOR-LIKE PROTEIN1 (RLP1). Functional complementation of rlp1 mutants required gene constructs that code for a longer version of RLP1 that we termed ReMAX (for receptor of eMax). ReMAX/RLP1 is a typical RLP with structural similarity to the tomato (Solanum lycopersicum) RLP Eix2, which detects fungal xylanase as a MAMP. Attempts to demonstrate receptor function by interfamily transfer of ReMAX to Nicotiana benthamiana were successful after using hybrid receptors with the C-terminal part of ReMAX replaced by that of Eix2. These results show that ReMAX determines specificity for eMax. They also demonstrate hybrid receptor technology as a promising tool to overcome problems that impede interfamily transfer of PRRs to enhance pathogen detection in crop plants.
INTRODUCTION
As part of their innate immune systems, higher plants and animals have surface receptors, also referred to as pattern recognition receptors (PRRs), which recognize specific microbe-associated molecular patterns (MAMPs). In higher vertebrates, which also have an adaptive immune system, the PRR family includes a dozen Toll-like receptors and a few additional, structurally unrelated, receptors (Akira et al., 2006). Higher plants rely on innate immunity alone and seem to have vast arrays of PRRs, as suggested by the increasing number of different MAMPs that have been reported to trigger defense responses in various plant species (Boller and Felix, 2009). These molecular patterns include peptides, proteins, carbohydrates, lipids, and lipopolysaccharides (LPSs) that are typical for whole classes of microbes but do not occur in plant hosts.
Compared with the number of MAMPs, the number of PRRs with known ligand specificity is still small. Most of the these identified PRRs belong to the receptor-like kinase (RLK) or the receptor-like protein (RLP) families. Examples of defined ligand/receptor pairs of MAMPs and PRRs include flagellin/FLAGELLIN SENSING2 (FLS2) (Gómez-Gómez and Boller, 2000), EF-Tu/EF-Tu RECEPTOR (EFR) (Zipfel et al., 2006), and peptidoglycan/LYSIN-MOTIF1 (LYM1) and LYM3 (Willmann et al., 2011) with MAMPs from bacteria as well as xylanase/ETHYLENE INDUCING XYLANASE2 (Eix2) (Ron and Avni, 2004), avirulence gene Ave1/VERTICILIUM1 (Ve1) (de Jonge et al., 2012), and chitin/CHITIN ELICITOR RECEPTOR KINASE1 (Miya et al., 2007) with MAMPs from fungi, respectively.
MAMP perception in plants characteristically leads to a general state of resistance, also termed pattern-triggered immunity (PTI) (Jones and Dangl, 2006). Early symptoms characteristically associated with MAMP perception include altered ion fluxes across the plasma membrane, leading to extracellular alkalinization and increased Ca2+ concentration in the cytoplasm, induction of an oxidative burst, and enhanced biosynthesis of the stress hormone ethylene (Boller and Felix, 2009).
Many phytopathogenic microorganisms have evolved specific effector proteins that can block various steps of the plant defense response pathway (Jones and Dangl, 2006). In addition, some pathogens have evolved mechanisms to avoid immunodetection. For example, Agrobacteria and some species of Xanthomonas have changes in the flg22 epitope of flagellin that render this MAMP nondetectable by FLS2, the flagellin receptor of plants (Felix et al., 1999; Sun et al., 2006). In turn, recognition of a given type of microbe does not depend on a single MAMP. Apart from flagellin, Arabidopsis thaliana detects bacteria also via MAMPs like LPS (Dow et al., 2000), peptidoglycan (Gust et al., 2007), and EF-Tu (Kunze et al., 2004). Currently, the number of distinct perception systems with specificity for bacterial MAMPs is difficult to estimate. Identification of novel MAMPs and their corresponding PRRs remains an important issue to assess the repertoire of PRRs that collectively provide basal immunity to the plant.
As a species, Arabidopsis is widespread in the northern hemisphere in Europe, central Asia, and North America where it is exposed to a broad range of environmental and climatic conditions (Hoffmann, 2002). More than 700 Arabidopsis accessions, previously also called ecotypes, have been collected from various habitats for studying natural variation of this plant species. As part of the 1001 Arabidopsis genome project (www.1001genomes.org), almost 500 of these accessions have been sequenced to date. Natural variation in these accessions also affects the repertoire of functional PRRs. For example, the accession Wassilewskija-0 carries a mutation in FLS2 and is insensitive to flagellin (Gómez-Gómez et al., 1999; Zipfel et al., 2004). A more recent screen of 56 accessions revealed insensitivity to flagellin in three additional accessions as well as insensitivity to EF-Tu in two other accessions (Vetter et al., 2012). This opens the possibility that accessions with defects in the perception of other MAMPs might exist. Natural variation might thus be exploited as genetic tools to map and identify further PRRs.
Here, we report that accession Shakhdara (Sha), in contrast with many other accessions of Arabidopsis, lacks the ability to recognize eMAX, a proteinaceous MAMP occurring in xanthomonads. This allowed efficient mapping and identification of the corresponding PRR as a longer form of RECEPTOR-LIKE PROTEIN1 (RLP1), which we termed ReMAX (for receptor of eMax). The predicted structure of ReMAX/RLP1 resembles the tomato (Solanum lycopersicum) RLP Eix2, which detects fungal xylanase as a MAMP (Ron and Avni, 2004). A hybrid receptor with the ectodomain from ReMAX and the C-terminal part of Eix2 proved functional in eMAX perception when expressed in a non-native system like Nicotiana benthamiana. This identifies ReMAX as the PRR for eMax and suggests that a hybrid receptor strategy might prove useful for interfamily transfer of PRR function to enhance crop disease resistance.
RESULTS
Arabidopsis and Other Brassicaceae Detect a MAMP from Xanthomonas
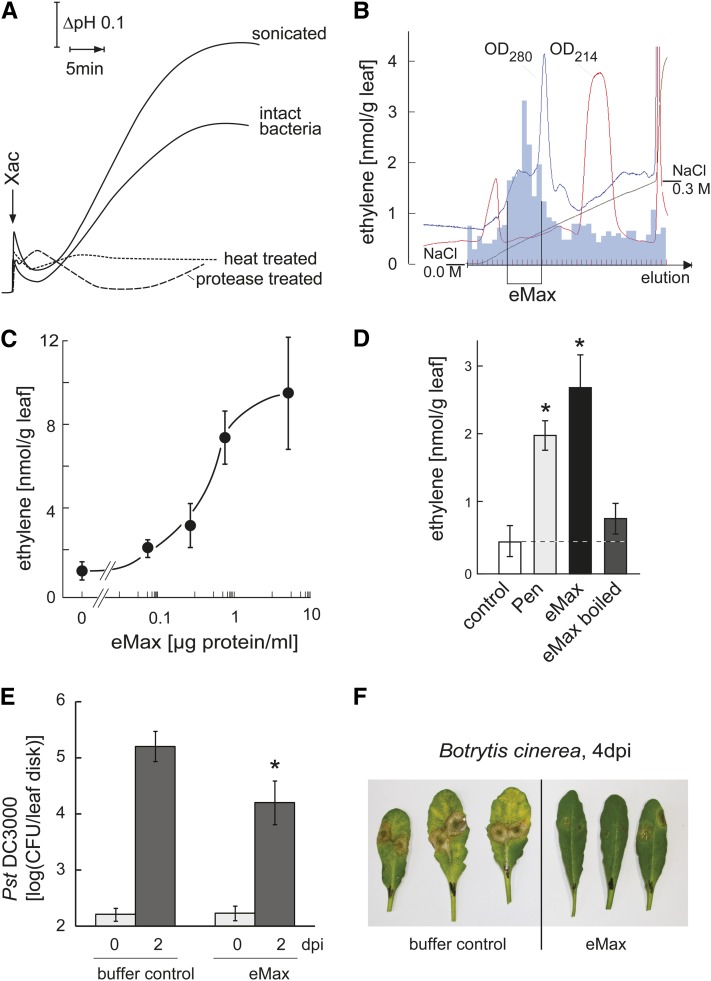
To search for novel bacterial MAMPs and identify additional PRRs, we used an Arabidopsis double mutant for the two major known PRRs, FLS2 and EFR; this double mutant line is unable to detect the flagellin and EF-Tu MAMPs. A cell culture system derived from these double mutant plants responded with a characteristic MAMP response, rapid extracellular alkalinization, when treated with preparations of Xanthomonas axonopodis pv citri strain 306 (Xac) (Figure 1A), indicating the presence of a MAMP that is not flagellin or EF-Tu. This response was triggered by treatment with intact bacteria, but responses were even stronger after sonication of the bacteria (Figure 1A). Further characterization showed that the eliciting activity was strongly reduced or abolished by heat treatment and digestion with proteases (Figure 1A), two features that distinguish the activity of Xac from peptidoglycan and LPS, two other, nonproteinaceous, bacterial MAMPs known to be recognized by Arabidopsis (Dow et al., 2000; Gust et al., 2007). A previous report identified superoxide dismutase SodM from either Xanthomonas campestris campestris or Escherichia coli (Watt et al., 2006) as a MAMP recognized by cells of tobacco (Nicotiana tabacum; Watt et al., 2006). However, the Arabidopsis cells used in our study showed no response to SodM (see Supplemental Figure 1A online). In summary, these results indicate that the activity in extracts of Xac represents a proteinaceous MAMP that does not correspond to one of the previously identified MAMPs flagellin, EF-Tu, peptidoglycan, LPS, or SodM. An activity similar to the one observed in Xac could be detected in sonicated preparations obtained from other xanthomonads, including strains of X. campestris pv vesicatoria and Xanthomonas arboricola pv juglandis but not in extracts prepared from other bacteria like Agrobacterium tumefaciens or Pseudomonas syringae pv tomato (see Supplemental Figure 1B online).
Figure 1.
(A) Preparations of living Xac bacteria or bacteria after sonication induce extracellular alkalinization in cultured cells of the Arabidopsis double mutant fls2 efr (Col-0 background). The activity is strongly impaired by heating (95°, 10 min) or by digestion with Proteinase K. The data show continuous pH tracings of representative examples from n > 5 repetitions of these experiments. The pH at the start of the experiment was 5.3, and scaling of time (x axis) and pH (y axis) were as indicated by the arrows.
(B) Partial purification of the activity in the Xac extract by anion exchange chromatography at pH 8.0. Eluate obtained by increasing NaCl concentration (x axis) was analyzed for OD280 and OD215. Fractions with highest activity (measured by ethylene emission from leaf pieces of fls2 efr plants; y axis) were pooled and denominated eMax as indicated.
(C) Dose dependency for ethylene induction. Leaf pieces of fls2 efr plants were treated with different concentrations of eMax. Values represent mean and sd of three replicates.
(D) Ethylene production of Col-0 leaf pieces treated with eMax (2 μg/mL), heat-treated eMax (2 μg/mL), or the fungal preparation Pen (90 µg/mL) as positive control. Bars and error bars represent mean and sd of n = 3 replicates. Asterisks show results that differ significantly from control treatment (Student's t test, P < 0.01).
(E) PTI against P. syringae pv tomato strain DC3000 (Pst DC3000). Arabidopsis fls2 efr leaves were pretreated by pressure infiltration of leaves with 100 μL of buffer (0.4 mM Tris, pH 8.0, with 5 mM NaCl) or buffer with eMax (0.6 µg protein) for 12 h before pressure infiltration with P. syringae pv tomato strain DC3000. Bars and error bars show mean and sd of bacterial numbers (measured as colony-forming units [CFU], at the indicated days after infection [dpi]) from n = 8 replicates. Asterisks mark significant induction over control based on a Student’s t test, P value < 0.01.
(F) PTI against B. cinerea. Arabidopsis fls2 efr plants were sprayed with eMax or buffer and 12 h later infected by spotting 5 μL drops containing ∼500 spores of B. cinerea. Photographs were taken 4 d after infection.
The MAMP activity in the Xac extract was partially purified by anion exchange chromatography (Figure 1B). To monitor for MAMP activity, eluates from the column were assayed for induction of ethylene biosynthesis in leaf pieces of fls2 efr double mutant plants. Fractions with highest activity were combined, resulting in a preparation that induced half-maximal induction of ethylene biosynthesis at a concentration of ∼1 µg protein/mL (Figure 1C). This MAMP was termed eMax for “enigmatic MAMP of Xanthomonas” since, so far, our attempts to further purify and determine its molecular identity have not been successful.
Heat treatment abolished most of the MAMP activity of eMax when tested in wild-type Columbia-0 (Col-0) Arabidopsis (Figure 1D). This indicates that the single purification step via anion exchange chromatography resulted in a preparation essentially free of heat stable MAMPs such as the known bacterial MAMPs flagellin, EF-Tu, peptidoglycan, and LPS (Felix et al., 1999; Dow et al., 2000; Kunze et al., 2004; Gust et al., 2007). Testing eMax for induction of ethylene biosynthesis in various plant species revealed responsiveness in several species of the Brassicaceae family but not in tomato, N. benthamiana, pea (Pisum sativum), and other species belonging to different plant families (see Supplemental Figure 2 online).
MAMP perception in plants characteristically leads to a general state of resistance, also termed PTI (Jones and Dangl, 2006). Arabidopsis leaves pretreated with eMax similarly showed a significant increase in general resistance to infection with the bacterial pathogen P. syringae pv tomato and the fungal pathogen Botrytis cinerea (Figures 1E and 1F).
Responsiveness to eMax Maps to RLP1
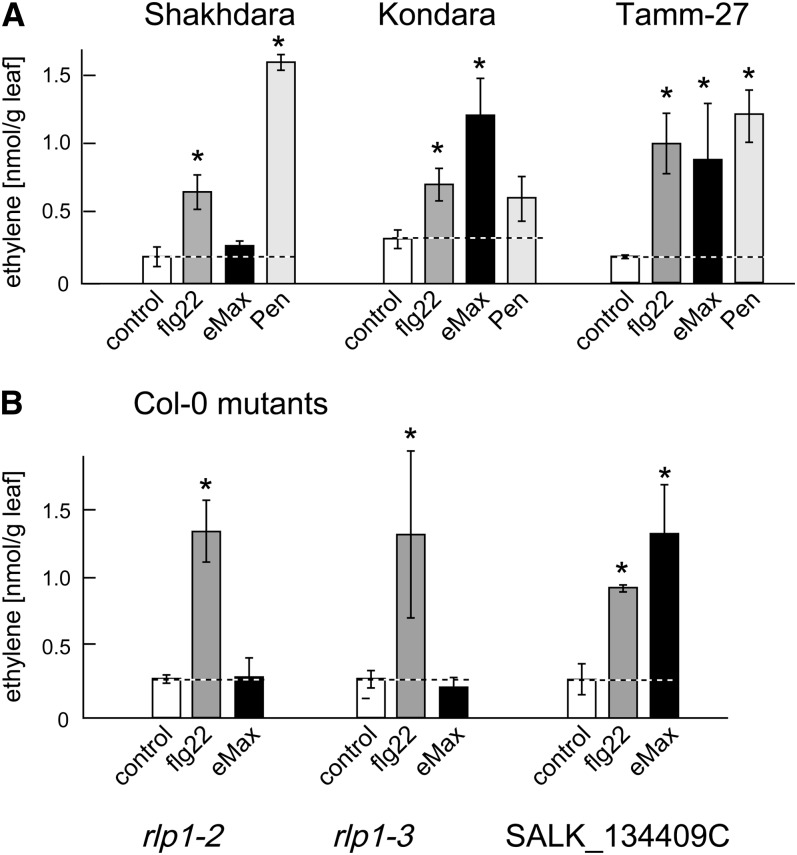
In a screen including 61 different accessions of Arabidopsis, we detected that leaves from most of these accessions reacted to eMax with a significant increase in production of ethylene, as exemplified for the accessions Kondara and Tamm-27 in Figure 2 and summarized in Supplemental Table 1 online. Accession Sha was an exception and did not respond with increased ethylene biosynthesis when treated with eMax (Figure 2). Since Sha responded to other MAMPs like bacterial flg22 and the fungal extract Penicillium (Pen), it seemed specifically affected in the recognition of eMax.
Figure 2.
Response to eMax in Leaves from Different Arabidopsis Accessions and Mutants.
(A) Ethylene biosynthesis in response to eMax (2 μg/mL), flg22 (100 nM), and the fungal preparation Pen (90 μg/mL) in the accessions Sha, Kondara, and Tamm-27. Responses in the accessions Kondara and Tamm-27 are representative for the majority of the 61 accessions tested.
(B) Response to eMax and flg22 in Col-0 plants with T-DNA insertions in the RLK gene At1g06840 (SALK_134409C) or the RLP1 gene At1g07390, lines rlp1-2 (SALK_116923) and rlp1-3 (SALK_049403C), respectively. The bars and error bars show means and sd of three replicates.
Asterisks mark significant induction over control based on a Student’s t test, P value < 0.01.
Well-characterized recombinant inbred lines (RILs) of accession Sha with Landsberg erecta (Ler) (Clerkx et al., 2004) and Bayreuth (Bay-0) (Loudet et al., 2002) have been established and are available for mapping approaches. We tested the 114 RILs of Ler × Sha collection (Clerkx et al., 2004) for ethylene biosynthesis in response to eMax and fungal Pen as a positive control. Only markers for the very top of chromosome 1 were linked with higher than 50% to responsiveness to eMax (see Supplemental Figure 3A online). The two additional markers T7I23 and T1G11 allowed to narrow the locus to a position between the markers T1G11 and F21M12.
For finer mapping, the 13 RILs from a cross between Bay-0 and Sha (Loudet et al., 2002; West et al., 2006) with recombinations between T1G11 and F21M12 were assayed for response to eMax. This allowed location of the trait to the 0.7 Mb between the genes At1g06460 and At1g08540 (see Supplemental Figure 3B online). This region comprises ∼200 annotated genes in the reference genome, among them the two RLKs At1g06840 and At1g07650 and the RLP, At1g07390, respectively. We speculated that a defect in one of these receptor-type genes might cause insensitivity in Sha. The accession Sha is fully sequenced and can be compared with accessions that show responsiveness to eMax (polymorph.weigelworld.org; Clark et al., 2007). According to this database, At1g07390 and At1g07650 display no single nucleotide polymorphisms (SNPs) that are unique for Sha and would lead to alterations in their amino acid sequences. At1g06840 has one SNP that causes a change from Val to Ile in its amino acid sequence. However, a mutant line with a T-DNA insertion in At1g06840 in the Col-0 background proved as responsive to eMax as the wild type (Figure 2B), excluding this gene from the list of candidates. Transcriptome data of Sha (West et al., 2006) revealed normal expression of At1g07650 but showed a strongly reduced transcription level for At1g07390 encoding RLP1. This was somewhat surprising since At1g07390 appeared to be free of SNPs also within >2 kb of its promoter region. We reexamined the genomic region encoding this gene in Sha and Col-0 and found a 7336-bp deletion in Sha covering the entire gene At1g07390 and also parts of the flanking genes At1g07380 and At1g07400 (see Supplemental Figure 4 online). A closer inspection of the polymorph database for the occurrence of deletions (SV Deletion; MPICao2010 version) indeed confirms this deletion in the Sha genome albeit only when sequences are searched region-wise rather than with the Atg numbers.
To corroborate the importance of RLP1 for eMax perception, two independent T-DNA insertion mutants in At1g07390 of Col-0, rlp1-2 and rlp1-3, were obtained. Like Sha, both mutant lines lacked responsiveness to eMax (Figure 2B).
A Version of RLP1 Transcribed from an Upstream Start Site Encodes ReMAX
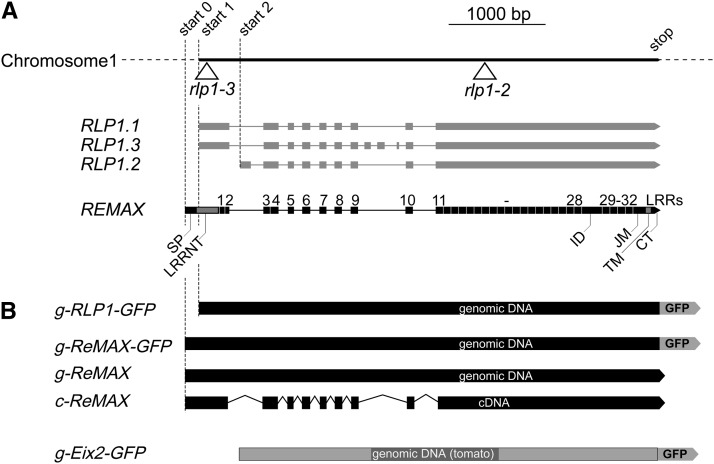
Three gene models with two distinct start sites have been proposed for RLP1 (www.tair.de; Figure 3A). However, a closer inspection of the genomic sequence indicated that the open reading extends further to the 5′ end, comprising a potential start codon 129 bp further upstream. We produced cDNA from Col-0 plants and demonstrated the presence of this longer transcript by amplifying with a primer encompassing this upstream start site. This cDNA predicts a longer form of RLP1, with nine exons encoding a protein of 1077 amino acids (Figure 3; see Supplemental Figure 5 online) that we tentatively termed ReMAX (for receptor of eMax).
Figure 3.
Gene Models for RLP1/ReMAX and Tomato Eix2.
(A) Three different gene models with start positions 1 at 2,269,893 or start 2 at 2,270,336 and different splicing options have been proposed for RLP1/At1g07390 (www.tair.org). The mutant lines rlp1-2 (SALK_116923) and rlp1-3 (SALK_049403C) have T-DNA insertions in predicted exons as indicated. Sequencing of Col-0 cDNAs resulted in a gene model with a start at position 2,269,764 (start 0) that we define as ReMAX. SP, signal peptide; LRR-NT, N terminus of the LRR domain; ID, island domain; JM, juxtamembrane domain; TM, transmembrane domain; CT, cytoplasmic tail.
(B) Gene constructs used for complementation experiments: Genomic and cDNA with the start positions of ReMAX (start 0) and RLP1.1/RLP1.3 (start 1) were cloned under the control of the 35S promoter. Where indicated, constructs were C-terminally fused in frame with a GFP tag. A similar construct with a GFP tag was produced for the RLP Eix2 (Ron and Avni, 2004) based on genomic DNA from tomato.
ReMAX/RLP1 is a typical RLP (Jones et al., 1994) and resembles in its primary structure the well-characterized RLP Eix2 from tomato (Ron and Avni, 2004) (see Supplemental Figure 5 online). Eix2 detects fungal xylanase as a ligand and triggers typical MAMP responses, including induction of ethylene biosynthesis. Both RLPs have large extracellular domains with 32 and 31 LRRs, respectively, that are interrupted with island domains inserted before the last four of the LRRs. The LRR domains, flanked by characteristic pairs of Cys residues (Li and Chory, 1997), are followed by juxtamembrane domains with characteristic series of acidic residues, single membrane spanning transmembrane domains, and short cytoplasmic tails. While related with respect to the number of LRRs and the presence and position of island domains, the overall sequence identity between Eix2 and ReMAX/RLP1 is only ∼29%. The proteins are most divergent in the amino acid residues that form the surface of LRR domain thought to be involved in ligand binding (see Supplemental Figure 5 online).
ReMAX Restores eMax Response in rlp1 Mutants of Arabidopsis
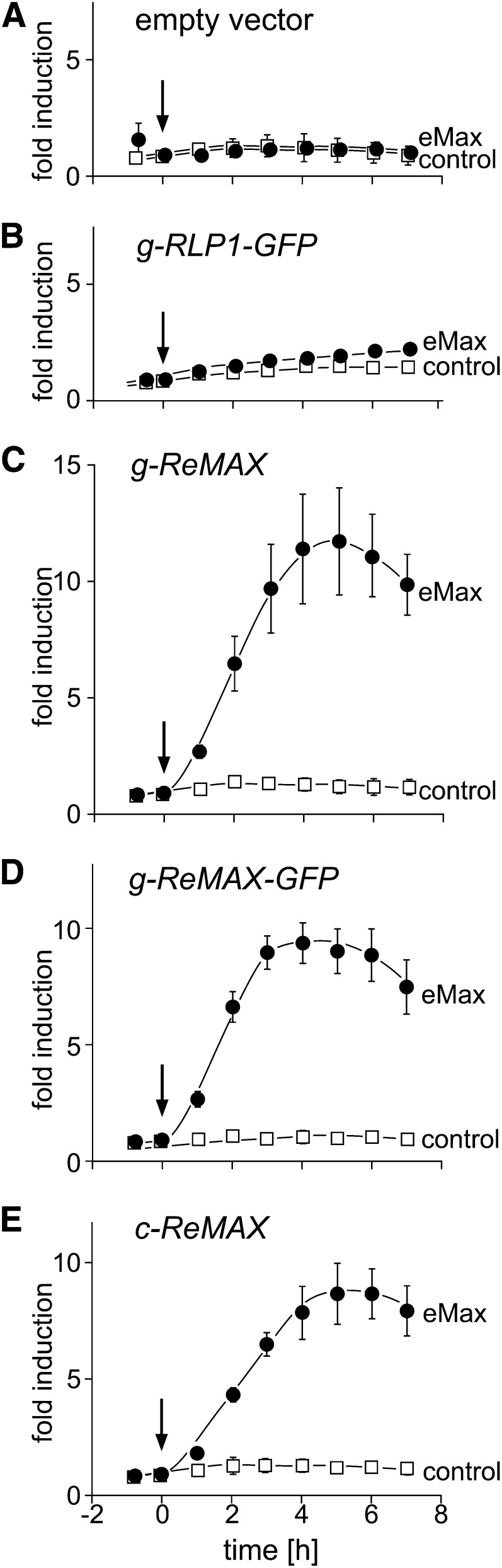
To confirm the role of ReMAX, different gene constructs were cloned from the genomic and cDNA, either with or without a C-terminal green fluorescent protein (GFP) tag, and used for complementation experiments (Figure 3B). Similarly, genomic DNA of tomato was used for a gene construct encoding the RLP Eix2 (Ron and Avni, 2004) fused in frame to a C-terminal GFP tag (Figure 3B). These constructs were tested in complementation assays with mesophyll protoplasts from leaves of the mutants rlp1-2, rlp1-3, and Sha (Figure 4; see Supplemental Figure 6 online). For monitoring the MAMP response, protoplasts were cotransfected with pFRK1:Luc. This reporter construct with luciferase under the FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1) promoter was used for testing MAMP response in several prior studies (Asai et al., 2002; Yoo et al., 2007; Albert et al., 2010; Mueller et al., 2012). When transfected with the reporter pFRK1:Luc alone, protoplasts from rlp1-2, rlp1-3, and Sha showed no changes in luciferase activity following treatment with eMax; by contrast, protoplasts from wild-type Col-0 showed an increase in luciferase activity (Figure 4A; see Supplemental Figures 6B and 6D online). Similarly, no response to eMax was observed in protoplasts of rlp1-2 after cotransformation of the reporter, and RLP1-GFP did not respond to eMax (Figure 4B). The rlp1-2, rlp1-3, and Sha protoplasts, however, gained responsiveness to eMax when transformed with the constructs g-ReMAX, c-ReMAX, or g-ReMAX-GFP that extend to the start codon on the full length cDNA (Figures 4C to 4E; see Supplemental Figures 6C and 6E online). These results demonstrated that ReMAX is indeed required for eMax perception. They also show that functional ReMAX requires the transcriptional start site upstream of the start sites proposed for RLP1. Furthermore, the GFP tag fused to the short cytoplasmic tail of ReMAX seems not to compromise functionality of perception.
Figure 4.
Perception of eMax in Protoplasts of rlp1-2 (Col-0) Plants Expressing RLP1/ReMAX Constructs.
Protoplasts were cotransformed with the reporter construct pFRK1:luc and the different constructs of RLP1/ReMAX as indicated. At time t = 0, the protoplasts were treated with eMax (5 μg/mL) or with the inactive peptide flg22A.tum (100 nM) as a control. Fold induction of luciferase activity was calculated with respect to values at time 0. Values, except for results in (B), represent means and sd of three replicates. Results shown are representative for n ≥ 3 independent repetitions of the experiments.
Interestingly, Arabidopsis protoplasts transformed with the construct encoding tomato Eix2-GFP showed reproducible and significant induction of the reporter gene after treatment with fungal xylanase (see Supplemental Figure 7 online). Although somewhat lower than the response to flg22 used as a positive control, this result showed that tomato Eix2 can functionally interact with all elements required for transmembrane signaling and induction of downstream responses in cells of a plant from a different order.
A Hybrid RLP Made from ReMAX and Eix2 Confers Perception of eMax to N. benthamiana
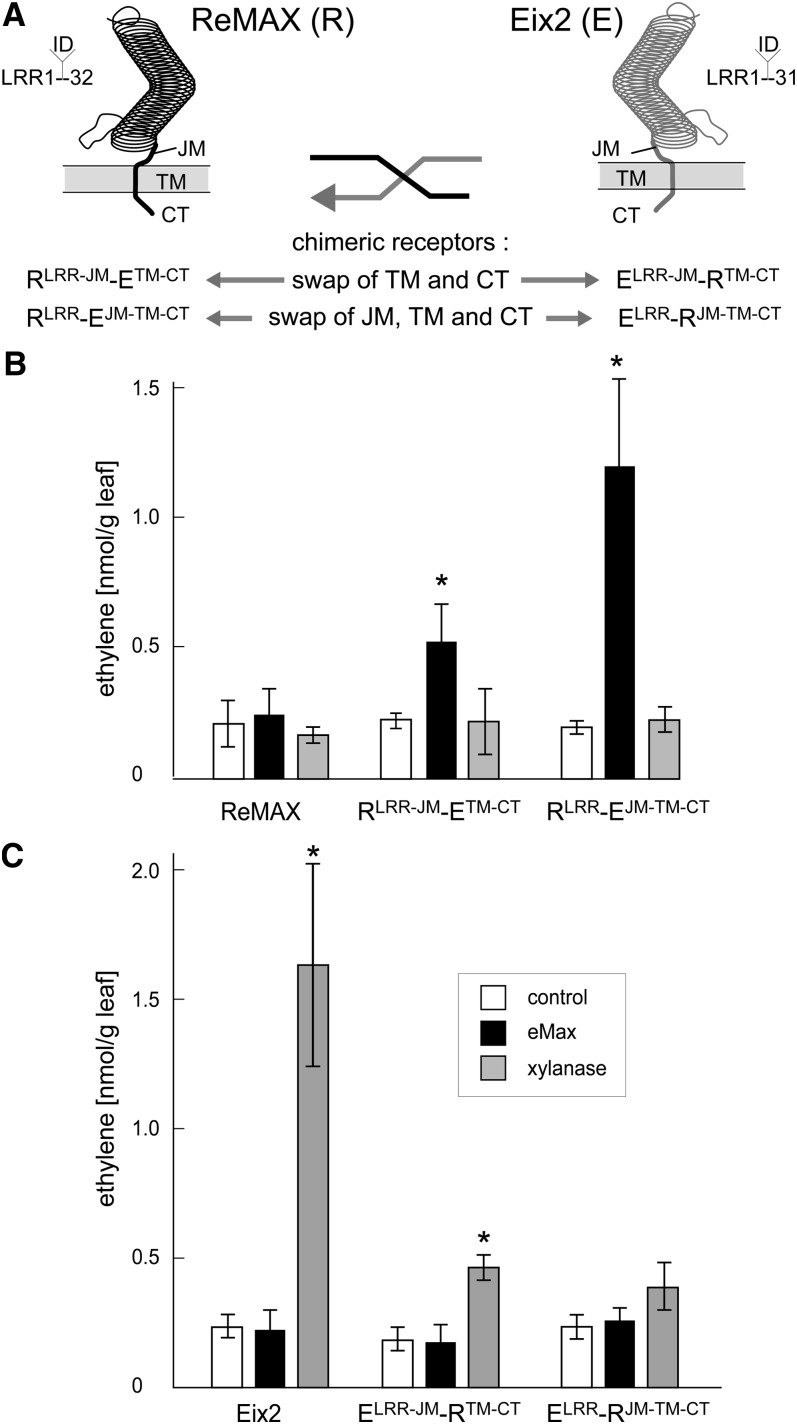
N. benthamiana lacks endogenous perception systems for xylanase and eMax (see Supplemental Figure 7 online). Confirming an earlier report (Ron and Avni, 2004), we observed that N. benthamiana leaves transformed with Eix2-GFP gain responsiveness to xylanase and respond with increased production of the stress hormone ethylene (see Supplemental Figure 8 online). By contrast, however, transfection of g-ReMAX-GFP was not sufficient to render N. benthamiana responsive to eMax. This could indicate that ReMAX alone does not form the genuine receptor site determining specificity for eMax. Alternatively, ReMAX might function as receptor site for eMax but encounter compatibility problems with adaptor proteins required for receptor activation in the heterologous cell context of N. benthamiana. We addressed the second possibility by constructing chimeric forms of the RLPs ReMAX and Eix2 with defined, reciprocal, swaps of their C-terminal parts, as summarized in Figure 5A.
Figure 5.
Functionality of ReMAX, Eix2, and Chimeric Forms of These RLPs in N. benthamiana.
(A) Schematic representation of ReMAX, Eix2, and the chimeric forms with reciprocal swaps of the C-terminal domains.
(B) and (C) Ethylene response of N. benthamiana transformed with the different constructs after treatment with Pen (90 μg/mL) as positive control, eMax (2 μg/mL), or xylanase (2 μg/mL) for 4 h. The bars show means and sd of three replicates. Asterisks mark significant induction over control based on a Student’s t test, P value < 0.05.
When expressed in N. benthamiana leaves, all constructs led to accumulation of tagged proteins with the expected size (see Supplemental Figure 9 online). Compared with Eix2, the construct ELRR-JM-RTM-CT, Eix2 with the transmembrane and C-terminal domains of ReMAX, lost much of its functionality as a xylanase receptor in N. benthamiana cells (Figure 5C), and the reciprocal construct RLRR-JM-ETM-CT did gain some responsiveness to eMax (Figure 5B). However, in eight repetitions of this experiment responsiveness to eMax was either faint, 4 times, or not detectable, 4 times, respectively. Apparently, transmembrane and C-terminal domains of Eix2 are not sufficient for robust functionality in N. benthamiana. The results with the extended swaps, including also the juxtamembrane domain, were unequivocal and reproducible. Expression of RLRR-EJM-TM-CT in N. benthamiana resulted in clear and significant response to eMax (Figure 5B) in all repetitions (n > 6). ELRR-RJM-TM-CT, in turn, was barely functional as xylanase receptor in N. benthamiana (Figure 5C). Thus, the C-terminal part including the apoplastic juxtamembrane domain determines functionality of ReMAX in different plant species. Equally important, this experiment also shows that the LRR ectodomain of ReMAX indeed specifies the specificity for the recognition of eMax, indicating that ReMAX is the bona fide receptor for eMax.
DISCUSSION
In this report, we started out from the observation that Arabidopsis has a sensitive detection system specific for a proteinaceous MAMP present in xanthomonads. MAMP activity of eMax is heat labile, indicating that the plant perception system is specific for the native configuration of the protein, similar to the perception of fungal xylanase by the tomato RLP Eix2 (Enkerli et al., 1999; Furman-Matarasso et al., 1999). Although remaining an important aim for future work, the molecular identification of the eMax protein was not a prerequisite to identify the corresponding PRR. A preparation essentially free of other types of MAMP was sufficient for a genetic approach to screen for genes involved in the perception of eMax. Identification of a particular PRR in plants can still present a big challenge, since these surface receptors occur in low abundance, rendering straightforward biochemical approaches difficult. Identification of most of the plant PRRs known to date relied on forward or reverse genetic approaches (Jones et al., 1994; Gómez-Gómez and Boller, 2000; Ron and Avni, 2004; Zipfel et al., 2006; Miya et al., 2007; Boller and Felix, 2009; Willmann et al., 2011; de Jonge et al., 2012), often starting out from natural variation observed between different varieties or strains of a species.
Natural variation with respect to presence or absence of particular PRRs is surprisingly high among different accessions of Arabidopsis. Accessions that lack either the PRR FLS2 for flagellin or the PRR EFR for EF-Tu have previously been reported (Gómez-Gómez et al., 1999; Vetter et al., 2012). In this work, we made use of this natural variation and screening of <70 accessions revealed a particular accession that was insensitive to eMax. Facilitated by the sets of genetic tools and comprehensive databases available for the model plant Arabidopsis, this perception system could be attributed to a particular member of the RLP family. In Arabidopsis, the family of RLPs with apoplastic LRR domains comprises 57 members (Fritz-Laylin et al., 2005; Wang et al., 2010b). With the exceptions of the RLPs CLAVATA2 (CLV2; RLP10) and Too Many Mouths (TMM; RLP17), which play roles in developmental processes (Jeong et al., 1999; Nadeau and Sack, 2002), and RLP41 involved in abscisic acid–induced senescence (Wang et al., 2008), the functions of the other members of this family remain unknown. Here, we show that ReMAX/RLP1 specifically functions as a PRR, sensing a specific proteinaceous signal occurring in Xanthomonas.
Lack of responsiveness to bacterial eMax in the accession Sha could be attributed to a deletion of >7 kb that includes the entire locus encoding RLP1. Independent mutant lines with T-DNA insertions in RLP1 also lacked responsiveness to eMax, corroborating the importance of this gene for eMax perception. Restoring responsiveness to eMax in rlp1 mutants and Sha plants required transfection with gene constructs encompassing a translational start site upstream of the ones proposed for RLP1. We termed this extended version of the RLP1 protein ReMAX.
The presence of intact ReMAX is a prerequisite for perception of eMax, but these genetic results cannot solve the question of whether ReMAX acts as the genuine receptor site for eMax. Without a clearly defined ligand at hand, interaction studies to demonstrate direct, specific interaction between eMax and ReMAX were not possible. Convincing evidence for the functionality and specificity of a receptor can also be obtained by expression in heterologous systems that lack an endogenous perception system for the particular ligand. Examples where this line of evidence successfully demonstrated receptor function include the Arabidopsis receptor kinase EFR expressed in N. benthamiana and tomato (Zipfel et al., 2006; Lacombe et al., 2010), the rice (Oryza sativa) receptor kinase XA21 expressed in Citrus sinensis (Cardoso et al., 2010), the tomato RLP Eix2 in N. benthamiana (Ron and Avni, 2004), and the tomato RLP Ve1 in Arabidopsis (Fradin et al., 2011). In our study, we found that Eix2 also functions in protoplasts of Arabidopsis, but Arabidopsis ReMAX, in turn, was nonfunctional as receptor of eMax in N. benthamiana. Functional perception of eMax was achieved by swapping the LRR domain of ReMAX to the C-terminal part of Eix2, thus demonstrating that the ectodomain of ReMAX was indeed determining specificity for the response to eMax (Figure 5).
Chimeric RLPs have previously been used to identify sites important for the ligand specificity of closely related Cf resistance proteins from tomato (van der Hoorn et al., 2005; Wulff et al., 2009). By contrast, in our study, chimeras of two RLPs exhibiting only 29% sequence identity were used to investigate subdomains important for downstream signaling in cells of heterologous plant species. Unlike receptor kinases, RLPs have only short cytoplasmic tails, and activation of downstream signaling is likely to depend on adaptors or coreceptors. A current theme for signaling via RLPs is heterodimerization with RLKs, as convincingly demonstrated for the RLP CLV2 and the receptor kinase CORYNE (Bleckmann et al., 2010; Wang et al., 2010a). The results with chimeric ReMAX and Eix2 suggests that the C-terminal part of these RLPs, including the extracellular juxtamembrane domain, the transmembrane domain, and the cytoplasmic tail, is important for interaction with adaptors and other receptor components essential for activation of downstream signaling. There is an apparent asymmetry between tomato Eix2, which functionally interacts with such components in cells of both Arabidopsis and N. benthamiana, and Arabidopsis ReMAX, which was not compatible in N. benthamiana. Chimeric forms also provide tools to further map the epitopes of RLPs that are important for the compatibility with these adaptors and to investigate the molecular mechanism that leads to receptor activation in this class of PRRs.
The presence of ReMAX/RLP1 appears to be restricted to species of Brassicales, and BLAST searches revealed no apparent orthologs in species outside this plant family. In turn, RLPs like Cf1 to Cf9, Ve1, and Eix2 found in Solanales have no functional counterpart in Arabidopsis (Fradin et al., 2011). More generally, apart from CLV2 and TMM, which are important for developmental processes common to all higher plants, all other RLPs seem not to be conserved among different plant families. RLPs with functions as PRRs seem to have evolved and diversified rather recently in evolution (Wang et al., 2010b) (e.g., by reduplication and shuffling of LRR subdomains). The genomic organization of ReMAX/RLP1, with several of the LRR subunits precisely separated by introns, might still reflect such a recent origin (Figure 3). The diversification of RLPs and RLKs in different species suggests that the variety of MAMPs detected by higher plants might greatly exceed the number of ligands currently known. Thus, species-specific repertoires of PRRs, including that of the model plant Arabidopsis, can serve as valuable resources to enlarge and enhance the immunodetection systems of agronomically important plants. This was successfully demonstrated recently for the receptor kinase EFR of Arabidopsis, which conferred increased resistance against bacteria to tomato (Lacombe et al., 2010).
In a very comprehensive survey, a collection of individual T-DNA insertions in the RLPs of Arabidopsis was assessed for changes in susceptibility to various microbial pathogens. This investigation also included infection with X. campestris pv campestris via application to wound sites or by infiltration into leaves. None of the knockout lines, including one of RLP1/ReMAX, showed a significant change in susceptibility to this pathogen (Wang et al., 2008). However, considering the redundancy of recognition systems, the loss of a single PRR might be limiting for plant defense only under specific conditions. Future experiments will have to include plants with multiple knockouts of PRRs for assessing the role of a particular RLP like ReMAX. Also, experimental conditions of infection are relevant, as observed for the flagellin receptor FLS2, which can restrict infection by P. syringae pv tomato after spray inoculation but not after pressure infiltration (Zipfel et al., 2004). Thus, more experiments with different strains of Xanthomonas, different initial doses and routes of pathogen application, as well as different growth conditions of the host plants will be required to assess the function of ReMAX for host defense. Alternatively, using chimeric forms of ReMAX as described in this article, it will be possible to test gain of eMax perception for resistance against xanthomonads in Solanaceaous plants.
In summary, in this study, we could attribute ReMAX of Arabidopsis with the specific perception of a MAMP from Xanthomonas. Chimeric constructs that combine functional elements from the RLPs ReMAX and Eix2 demonstrate that ReMAX acts as the genuine receptor for eMax. This chimeric approach provides a new option for transferring functional PRRs to rather distantly related plants, thus allowing researchers to equip agronomically important crop species with novel specificities for pathogen recognition.
METHODS
Materials
Xylanase from Trichoderma viride (Sigma-Aldrich) was purified by ion-exchange chromatography as described (Enkerli et al., 1999). The fungal preparation Pen from Penicillium chrysogenum (Thuerig et al., 2005) and the peptides flg22 and flg22A.tum (Felix et al., 1999) were used as described before. Xanthomonas strains used were Xanthomonas axonopodis pv citri strain 306 (Xac) (da Silva et al., 2002), Xanthomonas arboricola pv juglandis strain DSM-1049 (Xaj) (DSMZ), and Xanthomonas campestris pv vesicatoria strain 85-10 Bonas et al., 1989).
Plant Material
Arabidopsis thaliana accessions, the RILs of Ler × Sha and Bay × Sha, the lines SALK_116923 (rlp1-2), SALK_049403C (rlp1-3), and SALK_134409C (T-DNA insertion in At1g06840) were from the Nottingham Arabidopsis Stock Centre. The double mutant fls2 efr (SAIL_691C4 × SALK_044334) was obtained from V. Nekrasov (Nekrasov et al., 2009).
Medium Alkalinization and Ethylene Measurement
Medium alkalinization in suspension cultured cells and ethylene biosynthesis in leaf tissue as assays for MAMP responses were performed as described before (Felix et al., 1999). To test for general responsiveness to MAMPs preparations of Pen (Thuerig et al., 2005), elf18 (Zipfel et al., 2006) and flg22 were used as positive controls and flg22A.tum as negative control, respectively (Felix et al., 1999).
Preparation of eMax
X. axonopodis pv citri was grown on Kings B plates for 48 h at 30°C. The bacteria were harvested and sonicated three times for 2 min (50 W; SONOPULS HD UW2070; Bandelin). Supernatant was dialyzed (molecular weight cut off: 4 to 6 kD; Roth) and separated by anion exchange chromatography (Tris-HCl, pH 8.0; Q-Sepharose; GE Healthcare). Fractions with highest activity as inducer of ethylene biosynthesis in the double mutant fls2 efr were pooled and termed eMax.
PTI
For testing effects on growth of Pseudomonas syringae pv tomato strain DC3000 (Katagiri et al., 2002), Arabidopsis leaves were pretreated for 12 h by injection with 100 μL of buffer (0.4 mM Tris, pH 8.0, containing 5 mM NaCl) or buffer with eMax (0.6 µg protein). Bacteria in 10 mM MgCl2 were applied at a density of 105 colony-forming units mL−1 (OD600 = 0.002) and colony-forming units in extracts of leaves determined 1, 24, and 48 h later.
For assaying the effect on growth of Botrytis cinerea BO5-10 (Mengiste et al., 2003), leaves were sprayed with buffer (0.4 mM Tris, pH 8.0, containing 5 mM NaCl) or eMax in buffer (6 µg protein mL−1). After 12 h of pretreatment, leaves were infected by spotting 5-μL drops of a suspension containing 105 conidia mL−1. Plants were incubated at 100% relative humidity at 22°C under short-day conditions, and appearance of visual symptoms was monitored over 4 d.
Cloning of Receptor Constructs
All PCRs were performed with the Phusion Hot Start DNA Polymerase (Fermentas), and constructs were cloned via SmaI and BamHI sites into vectors derived from pPGT, a derivative of pPZP212 (Hajdukiewicz et al., 1994) that contains the cauliflower mosaic virus 35S promoter, the GFP5(S65T) coding region (Friedrichsen et al., 2000), and the rbcS terminator (Hajdukiewicz et al., 1994). Where indicated, receptor constructs were C-terminally fused to a GFP tag. PCR was based on genomic DNA or cDNA with primers listed in Supplemental Figure 9 online. Chimeric constructs were generated by fusion of two separate PCR products as described (Albert et al., 2010) with the primers indicated in Supplemental Figure 10 online.
Expression in Nicotiana benthamiana Leaves
Agrobacterium tumefaciens carrying plasmids encoding the gene for expression were mixed 1:1 with A. tumefaciens harboring the p19 suppressor of silencing and pressure infiltrated into leaves of 4- to 5 week-old N. benthamiana as described (Voinnet et al., 2003). Leaves were cut and used for bioassays at 24 to 48 h after infiltration or extracted for immunoblot blot analysis at 48 to 72 h after infiltration, respectively.
Expression and Functionality Assay in Arabidopsis Mesophyll Protoplasts
Transient expression in leaf mesophyll protoplasts was performed as described (Yoo et al., 2007). Aliquots of 80,000 protoplasts were cotransformed with 5 µg of plasmid DNA encoding firefly luciferase under the FRK1 promoter (pFRK1:luc) (Asai et al., 2002) and 20 µg of plasmid DNA encoding the receptor construct to be tested. The protoplasts were resuspended in W5 solution supplemented with 200 µM firefly luciferin and distributed to wells in a 96-well plate (10,000 protoplasts/well). After 16 h of preincubation, protoplasts were assayed for response to MAMPs by measuring luminescence of protoplasts using a luminometer (Mithras LB 940; Berthold).
Mapping a Locus Important for eMax Perception
The collection of 114 RILs from Ler Sha (Clerkx et al., 2004) was tested for response to eMax. The 24 RILs with recombinations between the markers NGA59, F21M12, and F3F19 on top of chromosome 1 were analyzed with the two additional AFLP (amplified fragment length polymorphism) markers T7I23 and T1G11 (www.inra.fr/internet/Produits/vast/msat.php). For further analysis, an additional 13 RILs from a cross between Bay-0 and Sha (Loudet et al., 2002; West et al., 2006) with mapped recombinations between T1G11 and F21M12 were used.
Accession Numbers
Sequence information can be found in the GenBank/EMBL or Arabidopsis Genome Initiative databases for RLP1 (At1g07390) and at the Sol Genomics Network for Eix2 (Solyc07g008630.1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Extracellular Alkalinization in fls2 efr Cells.
Supplemental Figure 2. Response to eMax in Leaves of Different Plant Species.
Supplemental Figure 3. Responsiveness to eMax Maps to Top of Chromosome 1.
Supplemental Figure 4. The accession Shakhdara has a 7336 bp deletion on chromosome 1.
Supplemental Figure 5. Primary Sequence of ReMAX and the Xylanase Receptor Eix2.
Supplemental Figure 6. Complementation of the rlp1-3 Mutant and Sha with g-ReMAX.
Supplemental Figure 7. Eix2 Functions as Xylanase Receptor in Arabidopsis Protoplasts.
Supplemental Figure 8. ReMAX and Eix2 After Heterologous Expression in N. benthamiana.
Supplemental Figure 9. Expression of ReMAX, Eix2 and Chimeric Receptor Forms in N. benthamiana Leaves.
Supplemental Figure 10. Primers Used in This Study.
Supplemental Table 1. MAMP Response in 61 Accessions of Arabidopsis.
Acknowledgments
This work was supported by the German Research Foundation as part of the programs SFB766, ERA-Net plant genomics “RLKRLP,” and Grant FE 962/2-1. We thank Ilonka Bock (University of Tuebingen) for technical assistance, Karin Schumacher (University of Heidelberg) for the pPGT clone, and Delphine Chinchilla (University of Basel) and Marisé Borja (PlantResponse, Madrid) for critical reading of the article.
AUTHOR CONTRIBUTIONS
A.K.J., M.L., M.A., V.F.-M., U.F., and K.M. performed research and analyzed data. A.K.J., M.L., and G.F. designed the research and wrote the article.
Glossary
- PRR
pattern recognition receptor
- MAMP
microbe-associated molecular pattern
- LPS
lipopolysaccharide
- RLK
receptor-like kinase
- PTI
pattern-triggered immunity
- Xac
Xanthomonas axonopodis pv citri strain 306
- Col-0
Columbia-0
- Sha
Shakhdara
- RIL
recombinant inbred line
- Ler
Landsberg erecta
- Bay-0
Bayreuth
- SNP
single nucleotide polymorphism
- GFP
green fluorescent protein
- Pen
Penicillium
References
- Akira S., Uematsu S., Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell 124: 783–801 [DOI] [PubMed] [Google Scholar]
- Albert M., Jehle A.K., Mueller K., Eisele C., Lipschis M., Felix G. (2010). Arabidopsis thaliana pattern recognition receptors for bacterial elongation factor Tu and flagellin can be combined to form functional chimeric receptors. J. Biol. Chem. 285: 19035–19042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gómez-Gomez L., Boller T., Ausubel F.M., Sheen J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bleckmann A., Weidtkamp-Peters S., Seidel C.A., Simon R. (2010). Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol. 152: 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bonas U., Stall R.E., Staskawicz B. (1989). Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol. Gen. Genet. 218: 127–136 [DOI] [PubMed] [Google Scholar]
- Cardoso S., Boscariol-Camargo R., Cruz R., Mourão Filho F., Bergamin Filho A. (2010). Reduction in susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis expressing the rice Xa21 gene. Plant Pathol. 59: 68–75 [Google Scholar]
- Clark R.M., et al. (2007). Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317: 338–342 [DOI] [PubMed] [Google Scholar]
- Clerkx E.J., El-Lithy M.E., Vierling E., Ruys G.J., Blankestijn-De Vries H., Groot S.P., Vreugdenhil D., Koornneef M. (2004). Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol. 135: 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A.C., et al. (2002). Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417: 459–463 [DOI] [PubMed] [Google Scholar]
- de Jonge R., van Esse H.P., Maruthachalam K., Bolton M.D., Santhanam P., Saber M.K., Zhang Z., Usami T., Lievens B., Subbarao K.V., Thomma B.P. (2012). Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 109: 5110–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow M., Newman M.A., von Roepenack E. (2000). The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu. Rev. Phytopathol. 38: 241–261 [DOI] [PubMed] [Google Scholar]
- Enkerli J., Felix G., Boller T. (1999). The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 121: 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Fradin E.F., Abd-El-Haliem A., Masini L., van den Berg G.C.M., Joosten M.H.A.J., Thomma B.P.H.J. (2011). Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 156: 2255–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen D.M., Joazeiro C.A., Li J., Hunter T., Chory J. (2000). Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 123: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Laylin L.K., Krishnamurthy N., Tör M., Sjölander K.V., Jones J.D. (2005). Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol. 138: 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman-Matarasso N., Cohen E., Du Q., Chejanovsky N., Hanania U., Avni A. (1999). A point mutation in the ethylene-inducing xylanase elicitor inhibits the beta-1-4-endoxylanase activity but not the elicitation activity. Plant Physiol. 121: 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. (2000). FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L., Felix G., Boller T. (1999). A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 18: 277–284 [DOI] [PubMed] [Google Scholar]
- Gust A.A., Biswas R., Lenz H.D., Rauhut T., Ranf S., Kemmerling B., Götz F., Glawischnig E., Lee J., Felix G., Nürnberger T. (2007). Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem. 282: 32338–32348 [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P., Svab Z., Maliga P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Hoffmann M.H. (2002). Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). J. Biogeogr. 29: 125–134 [Google Scholar]
- Jeong S., Trotochaud A.E., Clark S.E. (1999). The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.A., Thomas C.M., Hammond-Kosack K.E., Balint-Kurti P.J., Jones J.D. (1994). Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266: 789–793 [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Katagiri, F., Thilmony, R., and He, S.Y. (2002), The Arabidopsis thaliana-Pseudomonas syringae interaction. In The Arabidopsis Book 1, e0039. doi/10.1199/tab.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe S., Rougon-Cardoso A., Sherwood E., Peeters N., Dahlbeck D., van Esse H.P., Smoker M., Rallapalli G., Thomma B.P., Staskawicz B., Jones J.D., Zipfel C. (2010). Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 28: 365–369 [DOI] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Loudet, O., Chaillou, S., Camilleri, C., Bouchez, D., and Daniel-Vedele, F. (2002). Bay-0 x Shahdara recombinant inbred line population: A powerful tool for the genetic dissection of complex traits in Arabidopsis Theor. Appl. Genet. 104: 1173–1184. [DOI] [PubMed]
- Mengiste T., Chen X., Salmeron J., Dietrich R. (2003). The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15: 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., Narusaka Y., Kawakami N., Kaku H., Shibuya N. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K., Bittel P., Chinchilla D., Jehle A.K., Albert M., Boller T., Felix G. (2012). Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell 24: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J.A., Sack F.D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Nekrasov V., et al. (2009). Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO Journal 28: 3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M., Avni A. (2004). The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Dunning F.M., Pfund C., Weingarten R., Bent A.F. (2006). Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18: 764–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuerig B., Felix G., Binder A., Boller T., Tamm L. (2005). An extract of Penicillium chrysogenum elicits early defense-related responses and induces resistance in Arabidopsis thaliana independently of known signalling pathways. Physiol. Mol. Plant Pathol. 67: 180–193 [Google Scholar]
- van der Hoorn R.A., Wulff B.B., Rivas S., Durrant M.C., van der Ploeg A., de Wit P.J., Jones J.D. (2005). Structure-function analysis of cf-9, a receptor-like protein with extracytoplasmic leucine-rich repeats. Plant Cell 17: 1000–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter M.M., Kronholm I., He F., Häweker H., Reymond M., Bergelson J., Robatzek S., de Meaux J. (2012). Flagellin perception varies quantitatively in Arabidopsis thaliana and its relatives. Mol. Biol. Evol. 29: 1655–1667 [DOI] [PubMed] [Google Scholar]
- Voinnet O., Rivas S., Mestre P., Baulcombe D. (2003). An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33: 949–956 [DOI] [PubMed] [Google Scholar]
- Wang G., et al. (2008). A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 147: 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Fiers M., Ellendorff U., Wang Z., de Wit P.J.G.M., Angenent G.C., Thomma B.P.H.J. (2010b). The diverse roles of extracellular leucine-rich repeat-containing receptor-like proteins in plants. Crit. Rev. Plant Sci. 29: 285–299 [Google Scholar]
- Wang G., Long Y., Thomma B.P., de Wit P.J., Angenent G.C., Fiers M. (2010a). Functional analyses of the CLAVATA2-like proteins and their domains that contribute to CLAVATA2 specificity. Plant Physiol. 152: 320–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt S.A., Tellström V., Patschkowski T., Niehaus K. (2006). Identification of the bacterial superoxide dismutase (SodM) as plant-inducible elicitor of an oxidative burst reaction in tobacco cell suspension cultures. J. Biotechnol. 126: 78–86 [DOI] [PubMed] [Google Scholar]
- West M.A., van Leeuwen H., Kozik A., Kliebenstein D.J., Doerge R.W., St Clair D.A., Michelmore R.W. (2006). High-density haplotyping with microarray-based expression and single feature polymorphism markers in Arabidopsis. Genome Res. 16: 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R., et al. (2011). Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA 108: 19824–19829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff B.B., Heese A., Tomlinson-Buhot L., Jones D.A., de la Peña M., Jones J.D. (2009). The major specificity-determining amino acids of the tomato Cf-9 disease resistance protein are at hypervariable solvent-exposed positions in the central leucine-rich repeats. Mol. Plant Microbe Interact. 22: 1203–1213 [DOI] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C., Robatzek S., Navarro L., Oakeley E.J., Jones J.D., Felix G., Boller T. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]