Abstract
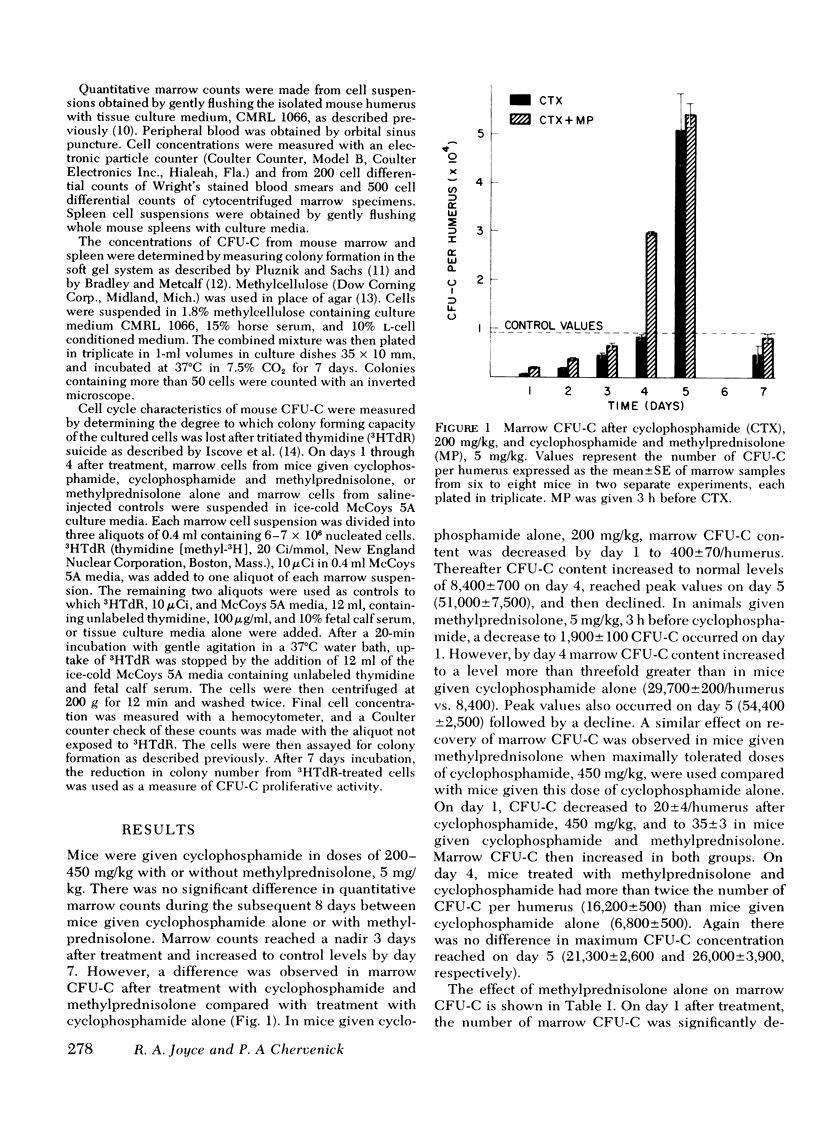
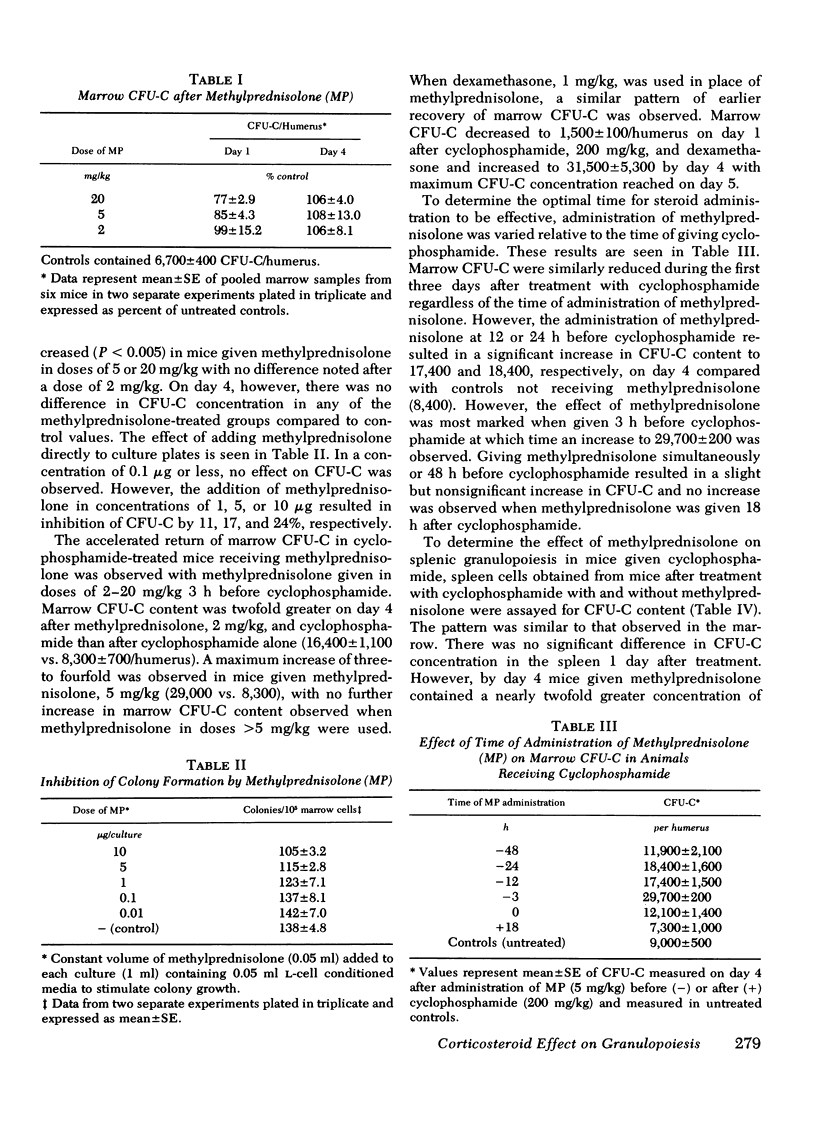
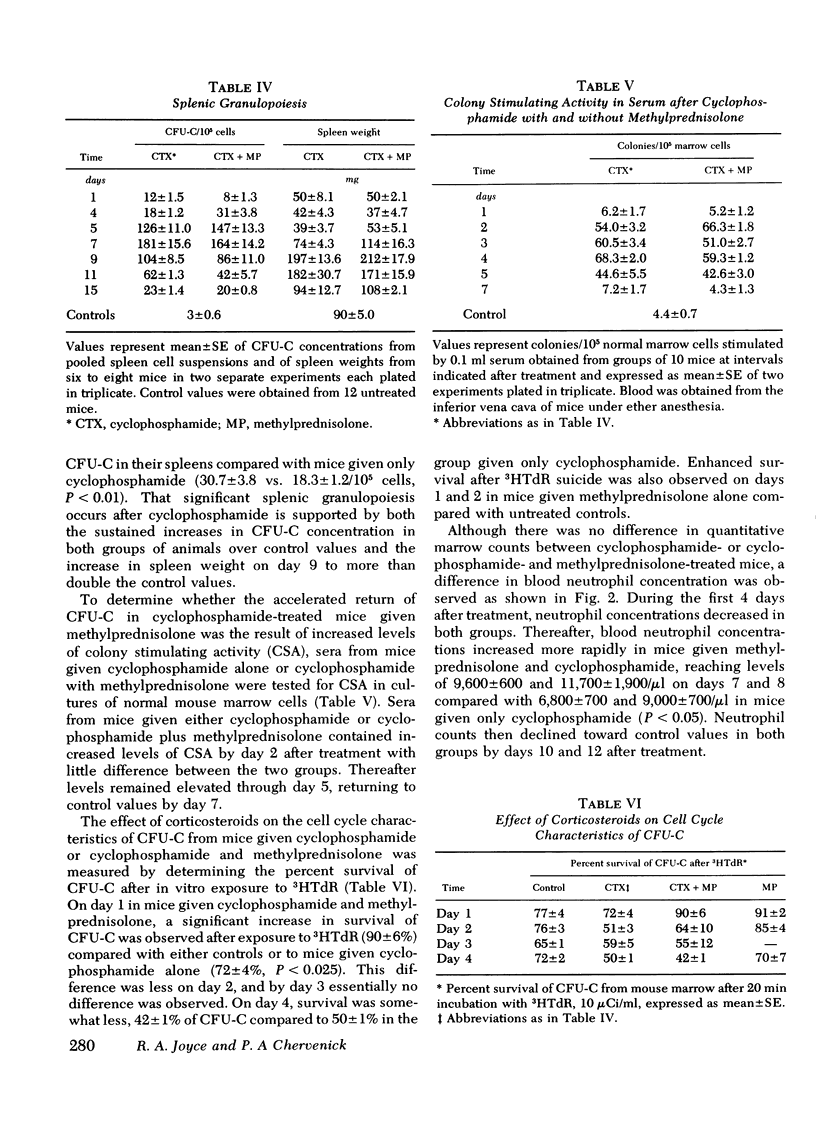
Corticosteroids cause an enhanced return of granulopoiesis as measured by in vitro growth of granulocytic progenitor cells (CFU-C) in mice treated with cyclophosphamide. After methylprednisolone and cyclophosphamide, a greater than threefold increase in marrow CFU-C was measured on day 4 compared to mice given cyclophosphamide alone (29,700±200 vs. 8,400±700/humerus). The accelerated return of marrow CFU-C was observed with cyclophosphamide in doses of 200 and 450 mg/kg and methylprednisolone, 2-20 mg/kg, with no significant differences using >5 mg/kg, and was detected when dexamethasone was used in place of methylprednisolone. This effect was accompanied by similarly enhanced splenic granulopoiesis as measured by CFU-C concentration. Levels of colony stimulating activity did not differ in mice given methylprednisolone and cyclophosphamide or cyclophosphamide alone. Corticosteroids appear to enhance the return of CFU-C by altering the proliferative state of granulocytic progenitor cells. CFU-C survival to in vitro 3HTdR suicide increased from 72±4% on day 1 after cyclophosphamide to 90±6% in animals given both cyclophosphamide and methylprednisolone. Increased survival after 3HTdR suicide was also observed when methylprednisolone alone was given. After treatment with cyclophosphamide and methylprednisolone, blood neutrophils increased more rapidly and improved survival to infection with Candida albicans was observed. These studies demonstrate that corticosteroids have a beneficial effect on marrow regeneration after myelotoxic chemotherapy with cyclophosphamide and suggest that they act by altering cell cycle characteristics of granulocyte progenitor cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop C. R., Athens J. W., Boggs D. R., Warner H. R., Cartwright G. E., Wintrobe M. M. Leukokinetic studies. 13. A non-steady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J Clin Invest. 1968 Feb;47(2):249–260. doi: 10.1172/JCI105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R., Marsh J. C., Chervenick P. A., Cartwright G. E., Wintrobe M. M. Factors influencing hematopoietic spleen colony formation in irradiated mice. VI. The different effects of foreign plasma, endotoxin, and bleeding on colony-forming cell kinetics. Radiat Res. 1968 Jul;35(1):68–77. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Brecher G., Smith W. W., Wilson S., Fred S. Kinetics of colchicine-induced hemopoietic recovery in irradiated mice. Radiat Res. 1967 Mar;30(3):600–610. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Bone marrow colonies: stimulation in vitro by supernatant from incubated human blood cells. Science. 1970 Aug 14;169(3946):691–692. doi: 10.1126/science.169.3946.691. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. In vitro growth of granulocytic and mononuclear cell colonies from blood of normal individuals. Blood. 1971 Feb;37(2):131–135. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- DeWys W. D., Goldin A., Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970 Jun;30(6):1692–1697. [PubMed] [Google Scholar]
- Farhangi M., Osserman E. F. The treatment of multiple myeloma. Semin Hematol. 1973 Apr;10(2):149–161. [PubMed] [Google Scholar]
- Iscove N. N., Till J. E., McCulloch E. A. The proliferative states of mouse granulopoietic progenitor cells. Proc Soc Exp Biol Med. 1970 May;134(1):33–36. doi: 10.3181/00379727-134-34721. [DOI] [PubMed] [Google Scholar]
- Keizer H. J., van Putten L. M. The radioprotective action on bone marrow CFU during immobilization of mice. Radiat Res. 1976 May;66(2):326–336. [PubMed] [Google Scholar]
- MECHANIC R. C., FREI E., 3rd, LANDY M., SMITH W. W. Quantitative studies of human leukocytic and febrile response to single and repeated doses of purified bacterial endotoxin. J Clin Invest. 1962 Jan;41:162–172. doi: 10.1172/JCI104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Cortisone action on serum colony-stimulating factor and bone marrow in vitro colony-forming cells. Proc Soc Exp Biol Med. 1969 Oct;132(1):391–394. doi: 10.3181/00379727-132-34222. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- Mukherji A. K., Mallick K. C. Disseminated candidosis in cyclophosphamide induced leucopenic state: an experimental study. Indian J Med Res. 1972 Nov;60(11):1584–1591. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rosenoff S. H., Bostick F., Young R. C. Recovery of normal hematopoietic tissue and tumor following chemotherapeutic injury from cyclophosphamide (CTX): comparative analysis of biochemical and clinical techniques. Blood. 1975 Apr;45(4):465–475. [PubMed] [Google Scholar]
- Smith W. W., Brecher G., Fred S., Budd R. A. Effect of endotoxin on the kinetics of hemopoietic colony-forming cells in irradiated mice. Radiat Res. 1966 Apr;27(4):710–717. [PubMed] [Google Scholar]
- Tredger J. M., Chakraborty J., Parke D. V. Effect of natural and synthetic glucocorticoids on rat hepatic microsomal drug metabolism. J Steroid Biochem. 1976 May;7(5):351–356. doi: 10.1016/0022-4731(76)90094-7. [DOI] [PubMed] [Google Scholar]
- Udupa K. B., Reissmann K. R. Acceleration of granulopoietic recovery by androgenic steroids in mice made neutropenic by cytotoxic drugs. Cancer Res. 1974 Oct;34(10):2517–2520. [PubMed] [Google Scholar]


