Abstract
Incubation of isolated rat epididymal fat cells is associated with the accumulation of adenosine in the incubation medium. To more clearly define the effect of adenosine on lipolysis, isolated rat epididymal adipocytes were studied with the perifusion system.
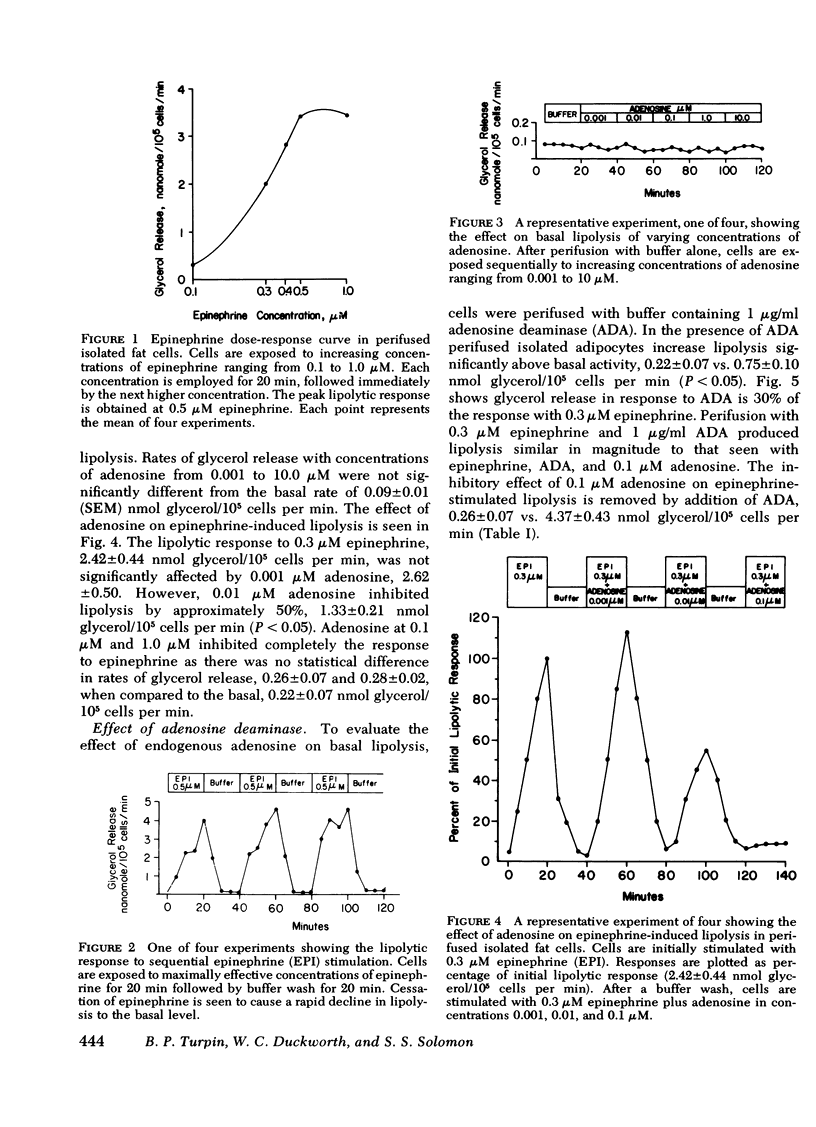
Various combinations of epinephrine, adenosine, and adenosine deaminase were perifused through the adipocytes. Exogenous adenosine, 0.001-10.0 μM, had no discernible influence upon unstimulated lipolysis; but exogenous adenosine inhibited epinephrine-sensitive lipolysis in a concentration-dependent manner. Cells perifused with 0.3 μM epinephrine plus 0.001 μM adenosine did not show any impairment of the lipolytic response to 0.3 μM epinephrine alone. Adenosine, 0.01 μM, inhibited the response to epinephrine by 50%; response to 0.3 μM epinephrine plus 0.1 μM adenosine was similar to the basal rate.
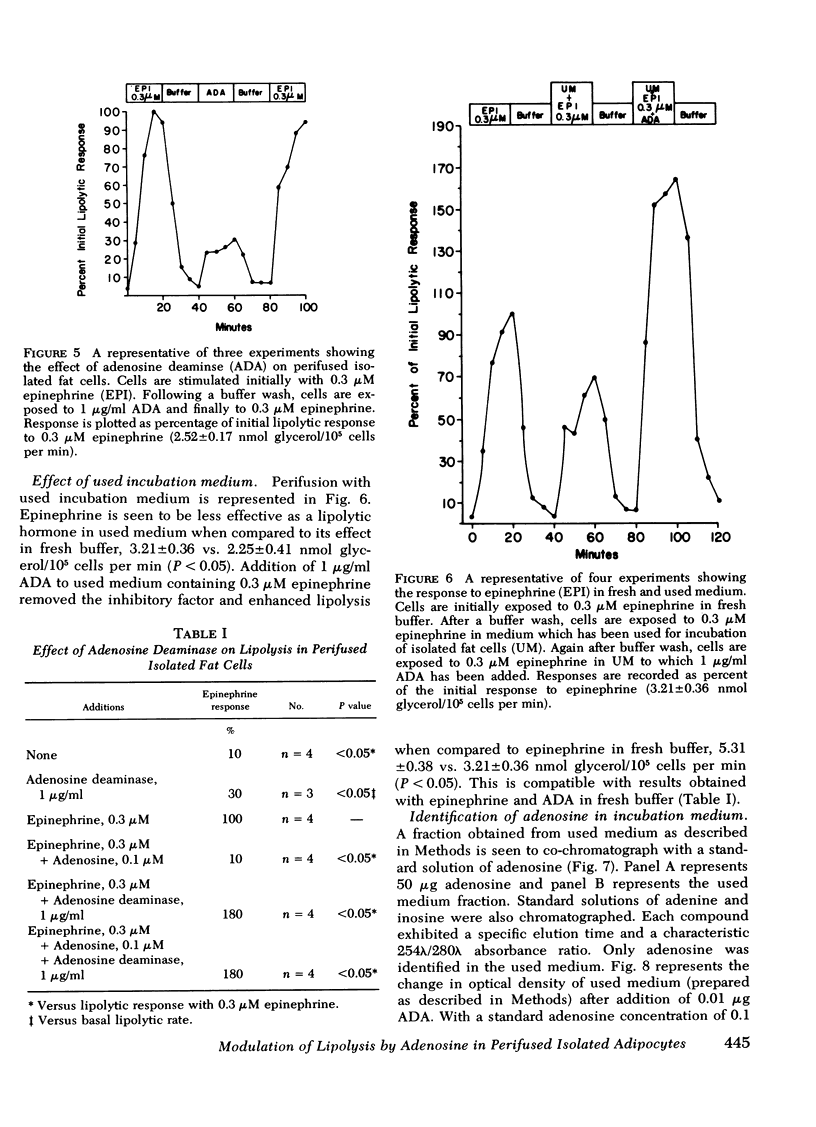
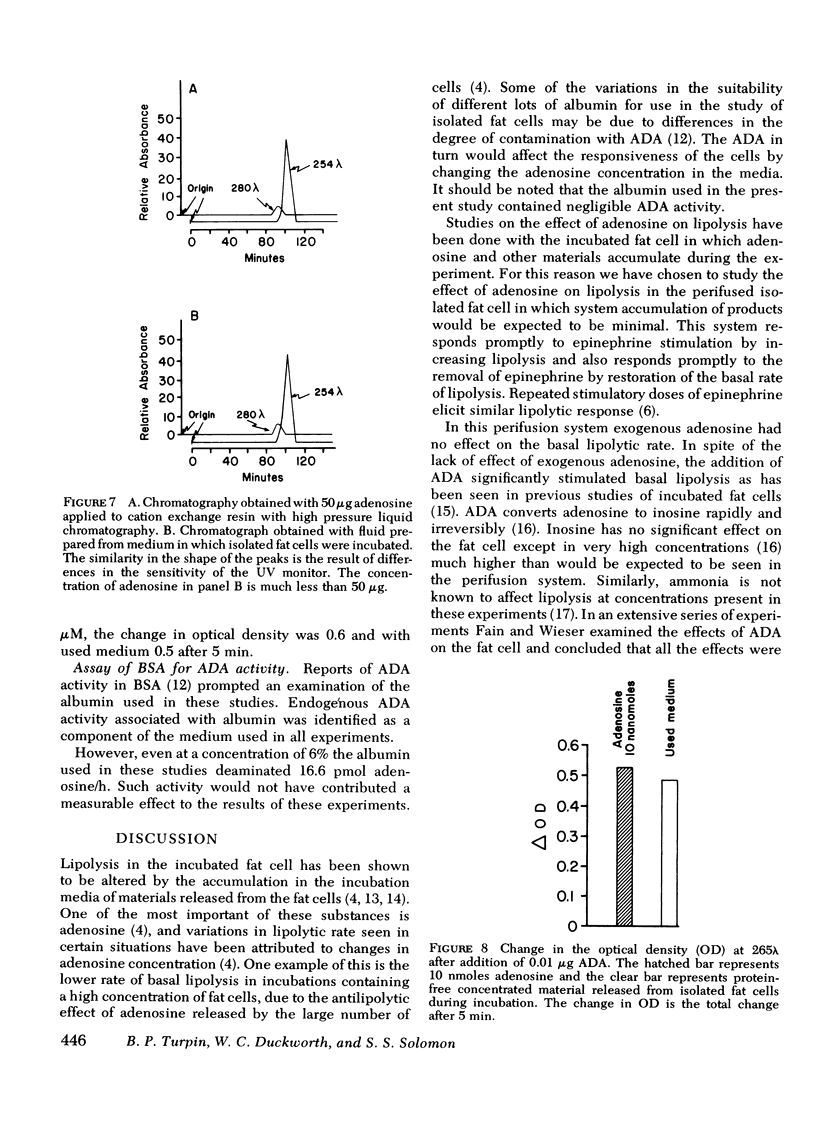
Perifusion with adenosine deaminase significantly increased basal lipolysis to 30% of the epinephrine response. Adenosine deaminase and epinephrine were synergistic in stimulating lipolysis to 180% of the response to epinephrine alone. Isolated fat cells were incubated for 30 min, and the cell-free used medium was perifused through fresh fat cells. Epinephrine in used medium was less effective in promoting lipolysis than epinephrine in fresh buffer. High-pressure liquid chromatography identified adenosine in the used medium. Bovine serum albumin possessed adenosine deaminase activity but accounted for negligible conversion of adenosine to inosine.
Adenosine is shown to have a modulating effect upon basal and hormone-stimulated lipolysis in the perifusion system. Sufficient endogenous adenosine (<0.01 μM) is present to maximally affect basal lipolysis. Hormone-stimulated lipolysis, although inhibited somewhat by endogenous adenosine, requires the addition of exogenous adenosine for complete inhibition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebert R., Schwabe U. Studies on the antilipolytic effect of adenosine and related compounds in isolated fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):247–259. doi: 10.1007/BF00500286. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Inhibition of adenosine cyclic 3', 5'-monophosphate accumulation in fat cells by adenosine, N6-(phenylisopropyl) adenosine, and related compounds. Mol Pharmacol. 1973 Sep;9(5):595–604. [PubMed] [Google Scholar]
- Fain J. N., Pointer R. H., Ward W. F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972 Nov 10;247(21):6866–6872. [PubMed] [Google Scholar]
- Fain J. N., Shephard R. E. Inhibition of adenosine 3':k'-monophosphate accumulation white fat acids, lactate, and beta-hydroxybutyrate. J Lipid Res. 1976 Jul;17(4):377–385. [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Free fatty acids as feedback regulators of adenylate cyclase and cyclic 3':5'-AMP accumulation in rat fat cells. J Biol Chem. 1975 Aug 25;250(16):6586–6592. [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- Gliemann J. Insulin-like activity of dilute human serum assayed by an isolated adipose cell method. Diabetes. 1965 Oct;14(10):643–649. doi: 10.2337/diab.14.10.643. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Fredholm B. B. Cyclic AMP-dependent and independent inhibition of lipolysis by adenosine and decreased pH. Acta Physiol Scand. 1976 Feb;96(2):170–179. doi: 10.1111/j.1748-1716.1976.tb10186.x. [DOI] [PubMed] [Google Scholar]
- Ho R., Sutherland E. W. cAMP-mediated feedback regulation in target cells. Adv Cyclic Nucleotide Res. 1975;5:533–548. [PubMed] [Google Scholar]
- Katocs A. S., Jr, Largis E. E., Allen D. O., Ashmore J. Perifused fat cells. Effect of lipolytic agents. J Biol Chem. 1973 Jul 25;248(14):5089–5094. [PubMed] [Google Scholar]
- Mosinger B., Vaughan M. Effects of electrolytes on epinephrine stimulated lipolysis in adipose tissue in vitro. Biochim Biophys Acta. 1967 Dec 5;144(3):556–568. doi: 10.1016/0005-2760(67)90045-8. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Jones C. E., Smith E. E. Determination of acid-soluble nucleosides and bases in myocardium by thin-layer methods. J Chromatogr. 1973 May 16;79:360–363. doi: 10.1016/s0021-9673(01)85316-4. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rubio R., Berne R. M., Bockman E. L., CURNISH R. R. Relationship between adenosine concentration and oxygen supply in rat brain. Am J Physiol. 1975 Jun;228(6):1896–1902. doi: 10.1152/ajplegacy.1975.228.6.1896. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R. Stimulation of cyclic adenosine 3',5'-monophosphate accumulation and lipolysis in fat cells by adenosine deaminase. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):33–44. doi: 10.1007/BF00647401. [DOI] [PubMed] [Google Scholar]
- Solomon S. S., Duckworth W. C. Effect of antecedent hormone administration on lipolysis in the perifused isolated fat cell. J Lab Clin Med. 1976 Dec;88(6):984–994. [PubMed] [Google Scholar]
- Solomon S. S., King L. E., Jr, Hashimoto K. Studies of the biological activity of insulin, cyclic nucleotides and concanavalin A in the isolated fat cell. Horm Metab Res. 1975 Jul;7(4):297–304. doi: 10.1055/s-0028-1093718. [DOI] [PubMed] [Google Scholar]
- Weissel M., Raberger G., Kraupp O. The effects of intra-arterial adenosine infusion on substrate levels and blood flow in skeletal muscle of the dog. Naunyn Schmiedebergs Arch Pharmacol. 1973;277(3):239–252. doi: 10.1007/BF00505663. [DOI] [PubMed] [Google Scholar]


