Abstract
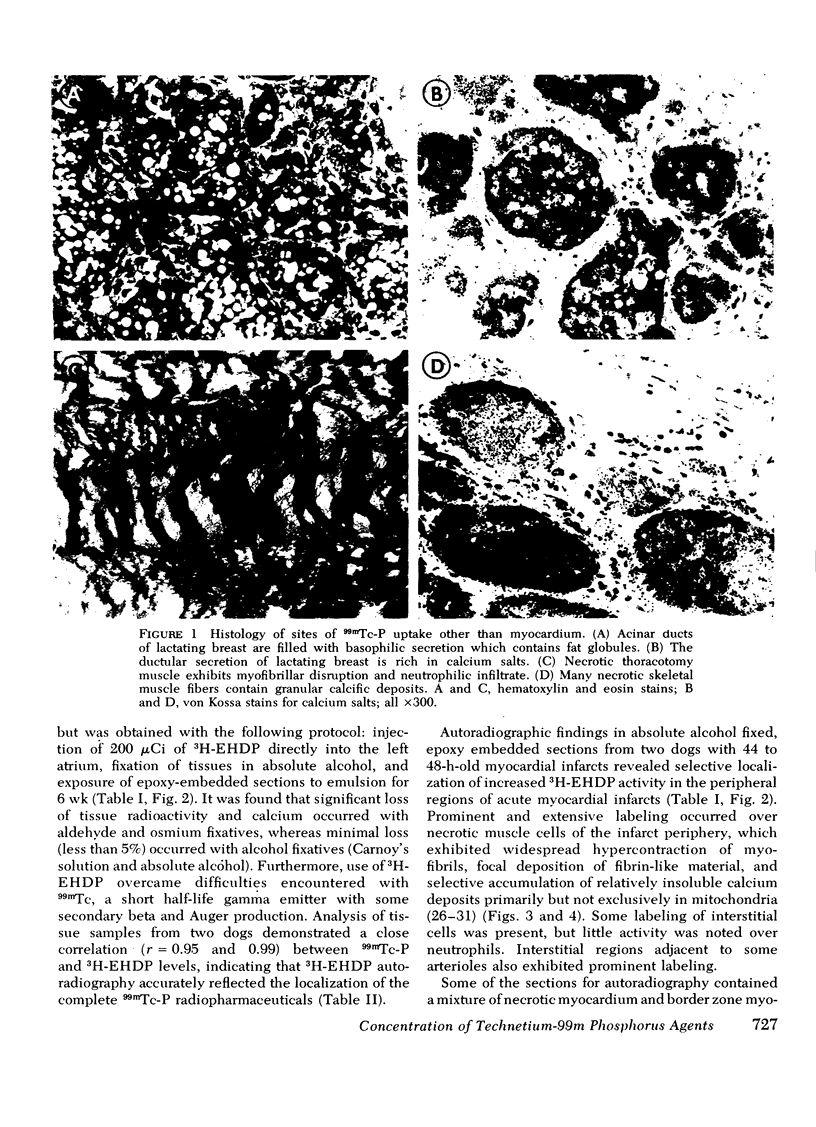
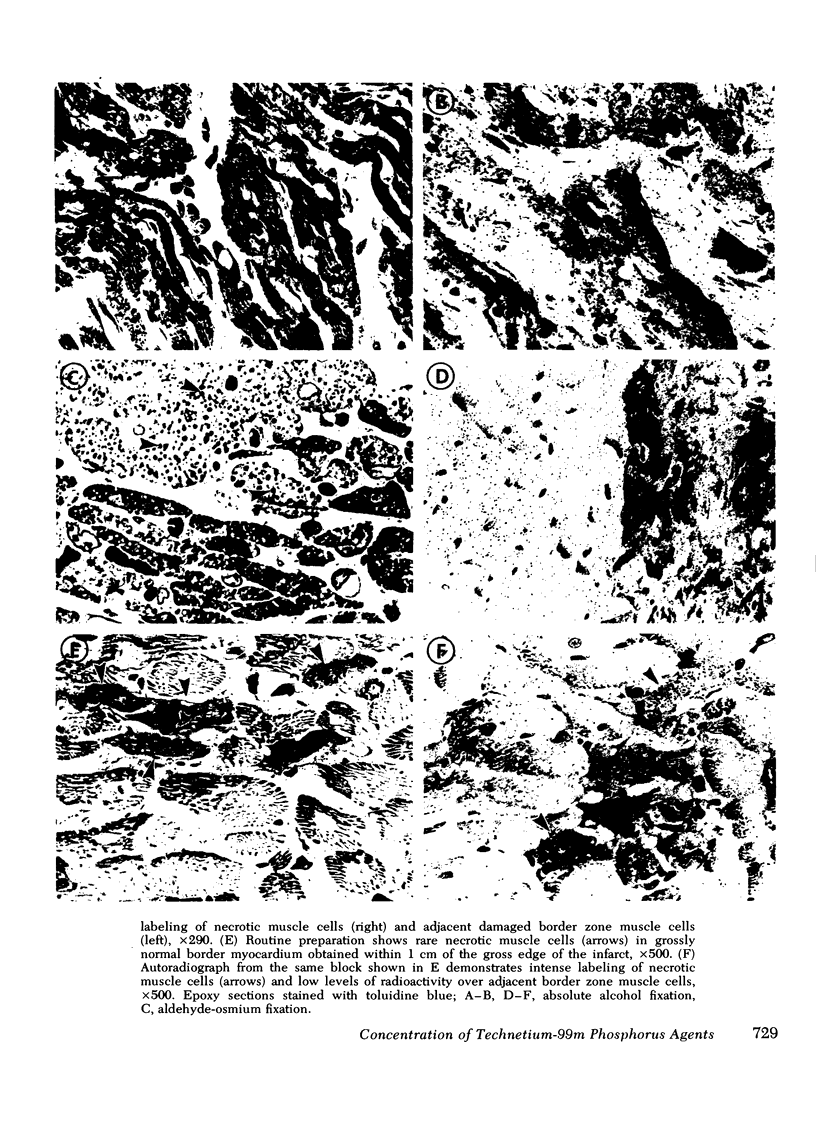
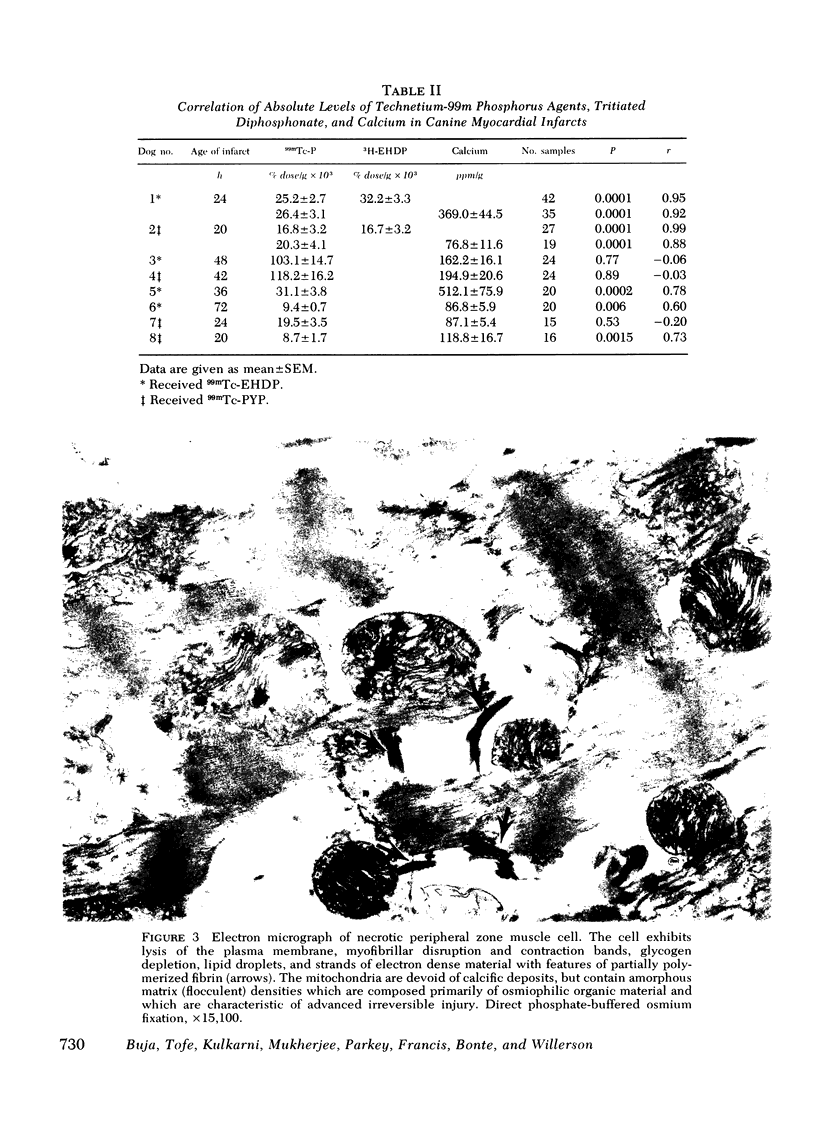
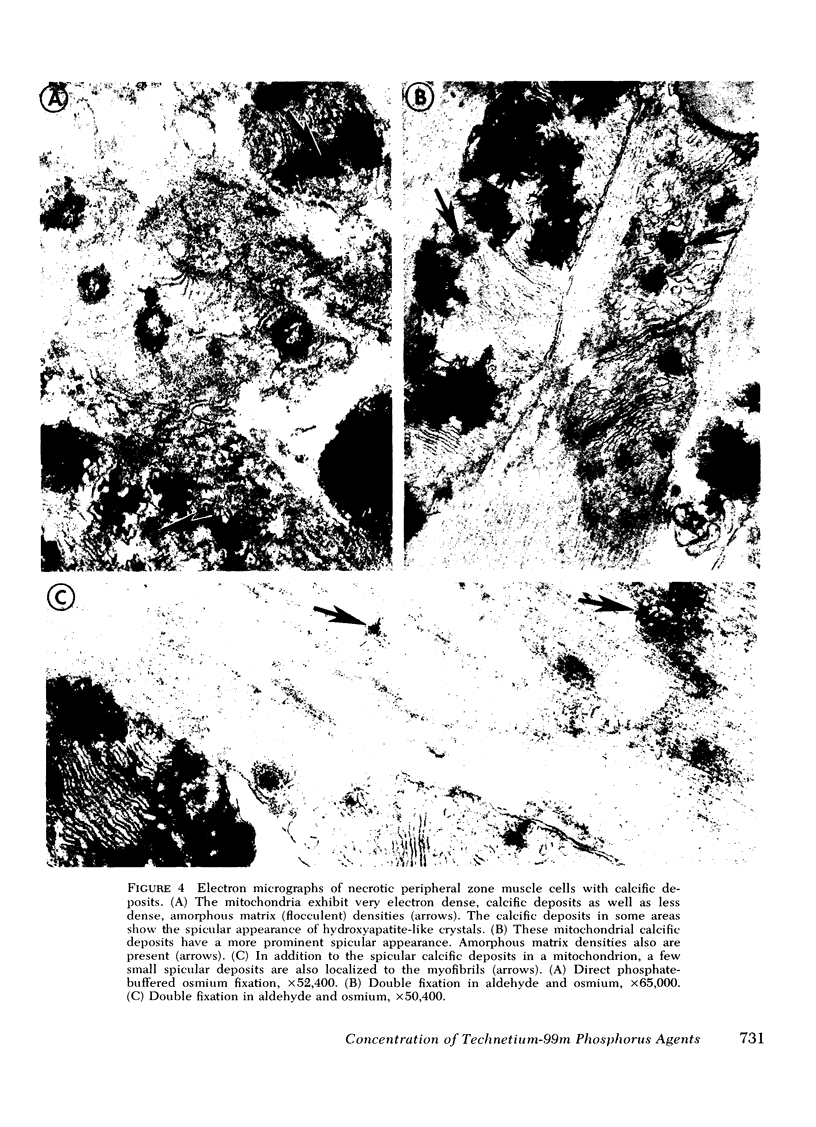
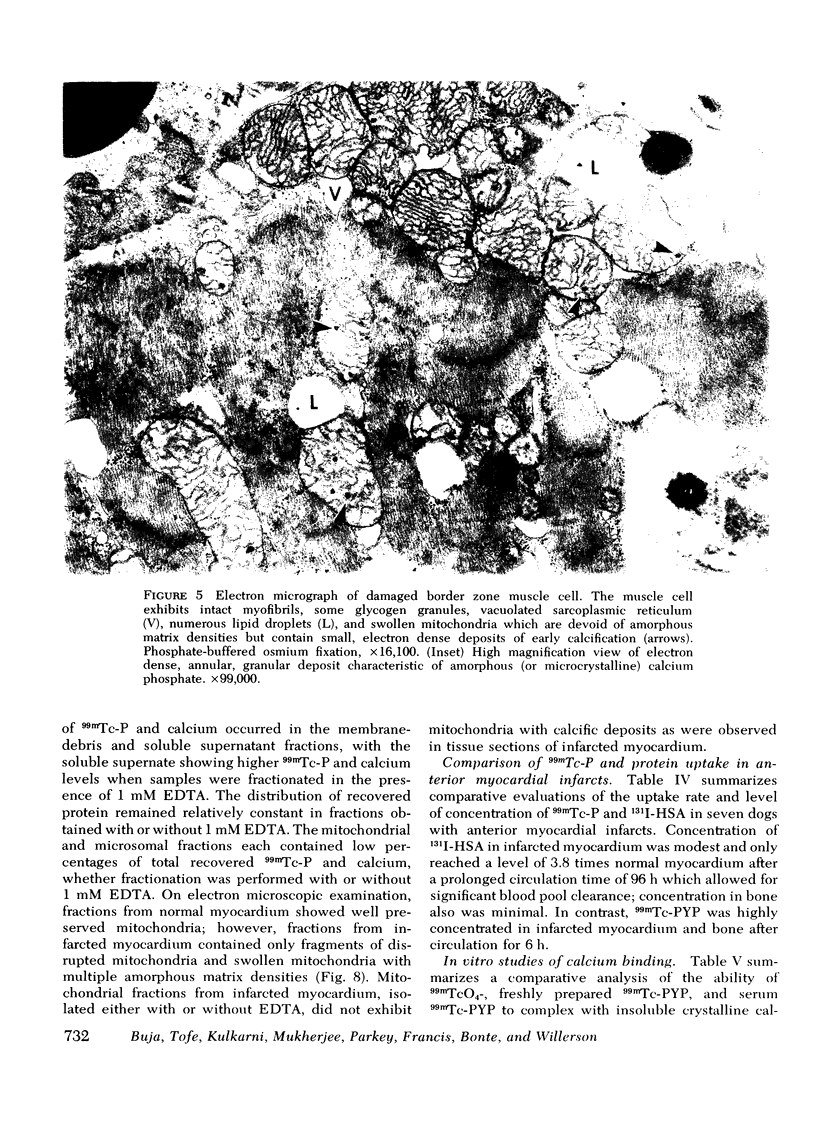
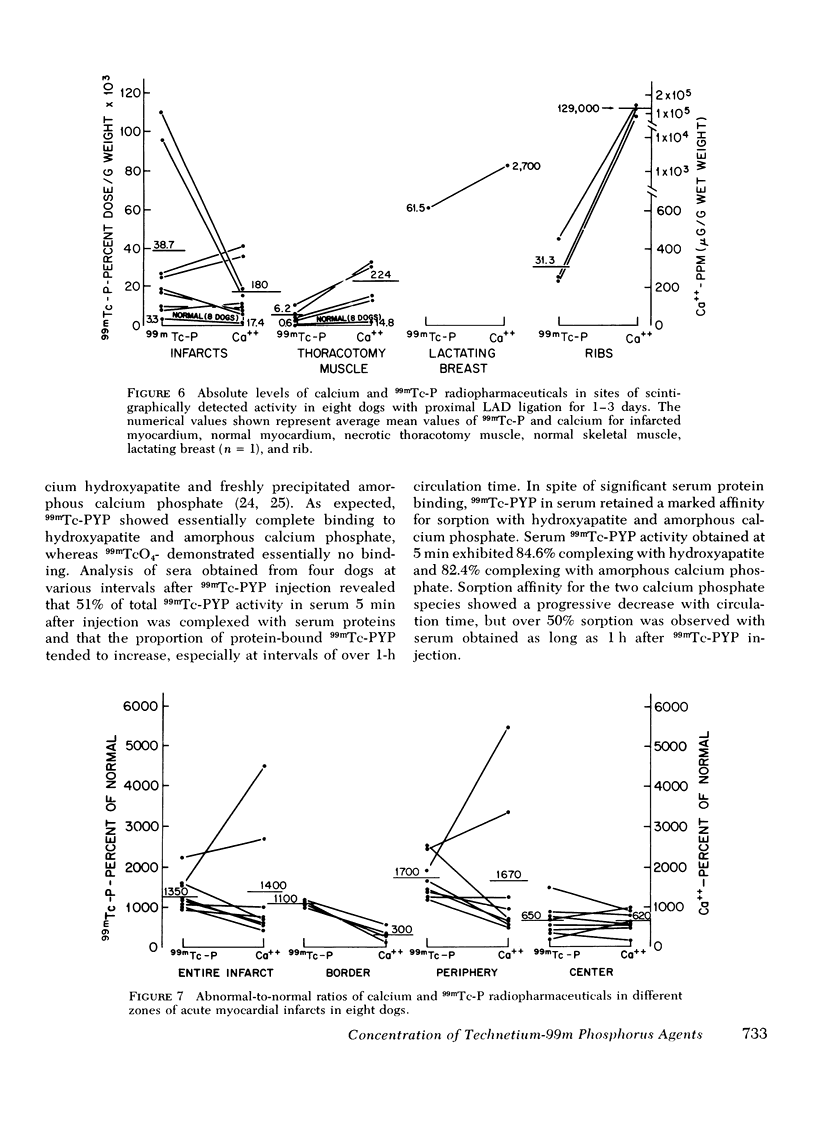
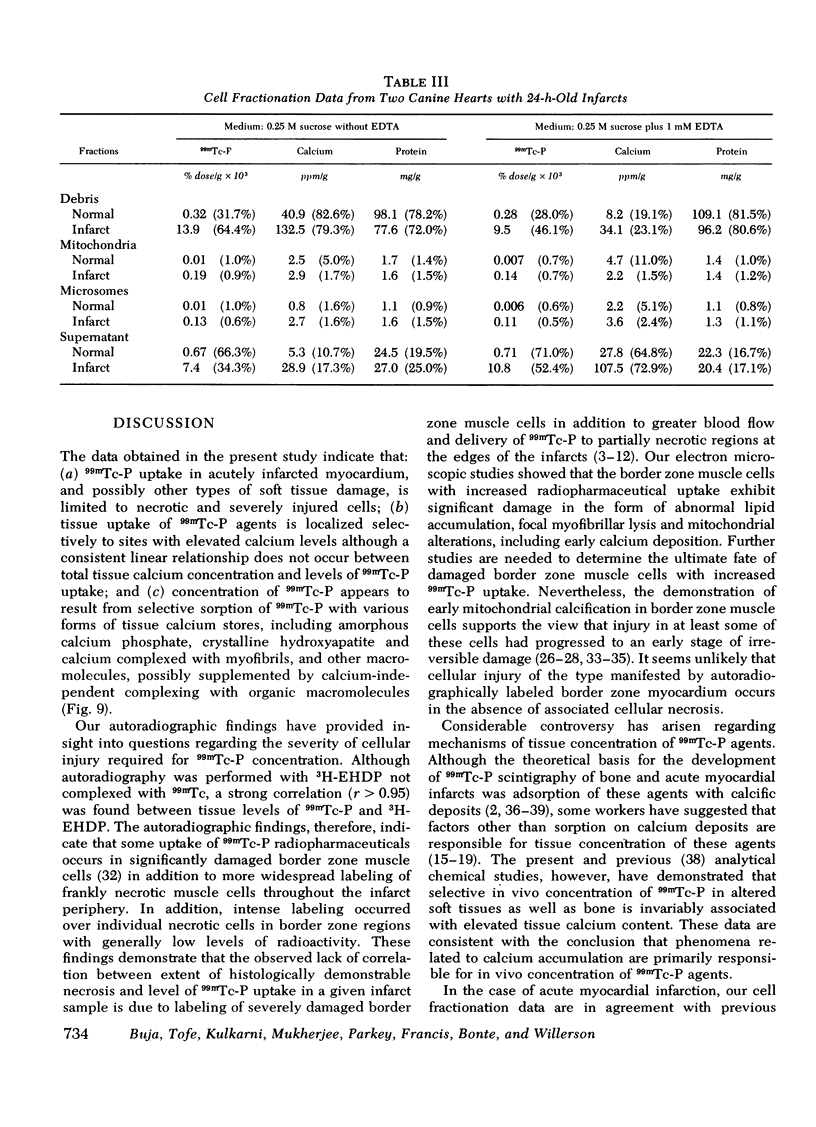
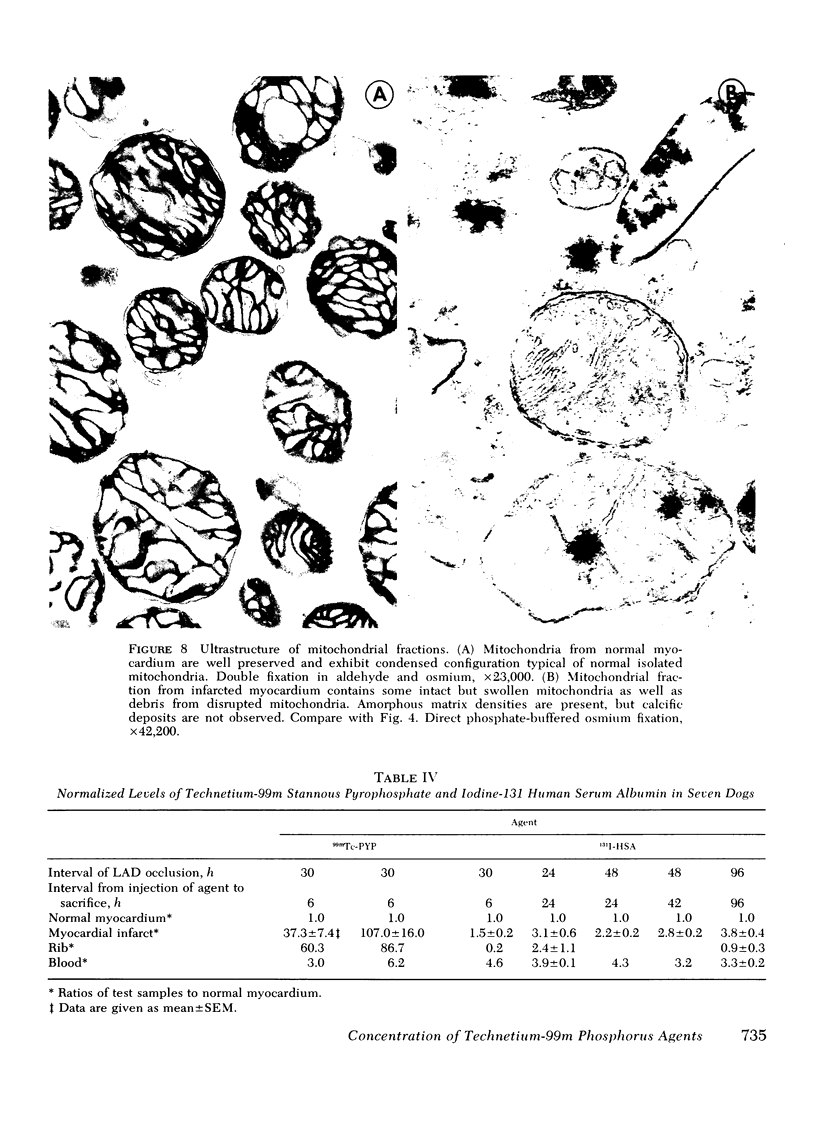
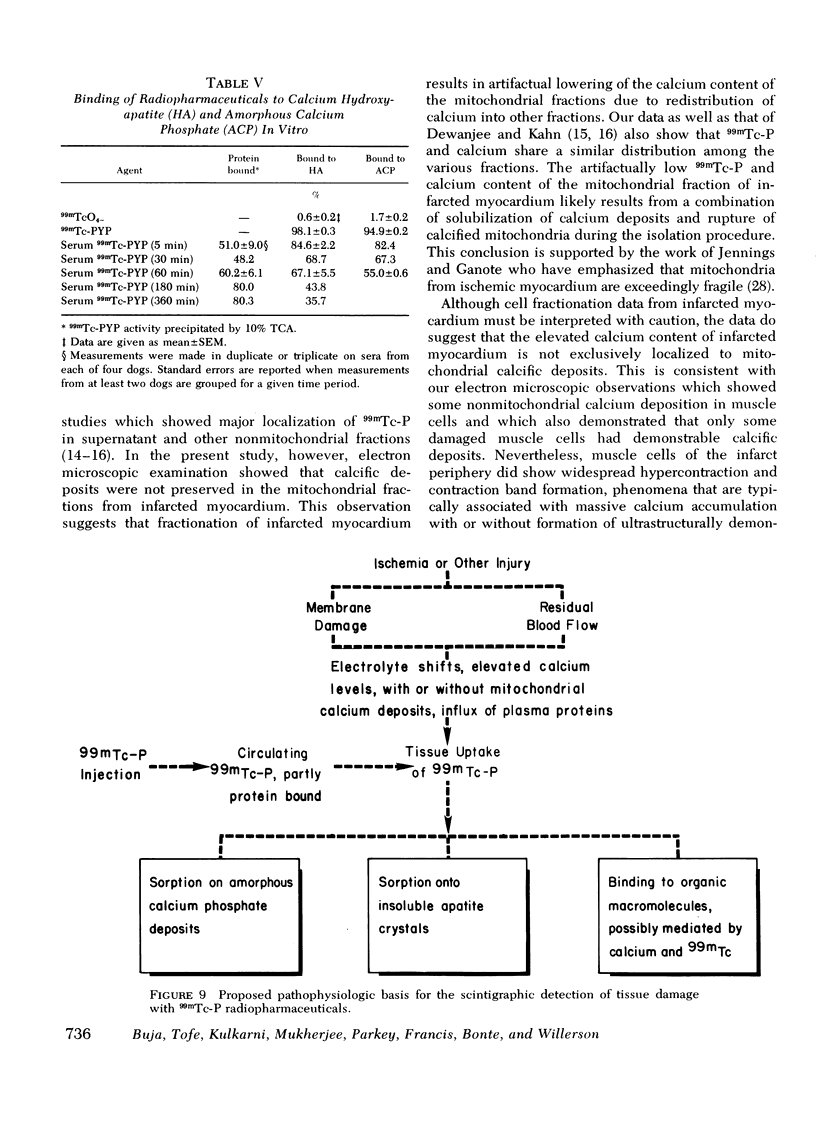
This study was performed to elucidate the localization at the cellular level of technetium-99m phosphorus (99mTc-P) radiopharmaceuticals in acute myocardial infarcts and the mechanisms responsible for 99mTc-P uptake in acute myocardial infarcts and other tissues. In 20 dogs with proximal left anterior descending coronary arterial ligation for 1-3 days, elevated calcium levels were measured at all sites of increased 99mTc-P uptake (acute myocardial infarcts, necrotic thoracotomy muscle, lactating breast, and normal bone); however, a consistent linear relationship between 99mTc-P and calcium levels was not observed. A strong correlation (r = 0.95 and 0.99, n = 2 dogs) was demonstrated between levels of 3H-diphosphonate and 99mTc-P in infarcted myocardium. Autoradiographic studies with 3H-diphosphonate revealed extensive labeling in the infarct periphery which contained necrotic muscle cells with features of severe calcium overloading, including widespread hypercontraction as well as more selective formation of mitochondrial calcific deposits. Autoradiography also demonstrated labeling of a small population of damaged border zone muscle cells which exhibited prominent accumulation of lipid droplets and focal, early mitochondrial calcification. Cell fractionation studies revealed major localization of both 99mTc-P and calcium in the soluble supernate and membrane-debris fractions of infarcted myocardium and less than 2% of total 99mTc-P and calcium in the mitochondrial fractions; however, electron microscopic examination showed that mitochondria with calcific deposits were not preserved in the mitochondrial fractions. In vitro studies evaluating the role of serum protein binding on tissue uptake of 99mTc-P agents demonstrated that, in spite of significant complexing with serum proteins, serum 99mTc-P activity retained the ability to adsorp to calcium hydroxyapatite and amorphous calcium phosphate. In vivo studies showed that concentration of human serum albumin (labeled with iodine-131) in infarcted myocardium reached a maximum of only 3.8 times normal after a circulation time of 96 h, whereas 99mTc-P uptake was at least 10 times normal after a circulation time as short as 1 h. It is concluded that: (a) 99mTc-P uptake in acutely infarcted myocardium, and possibly other types of soft tissue damage, is limited to necrotic and severely injured cells; (b) concentration of 99mTc-P results from selective adsorption of 99mTc-P with various forms of tissue calcium stores, including amorphous calcium phosphate, crystalline hydroxyapatite, and calcium complexed with myofibrils and other macromolecules, possibly supplemented by calcium-independent complexing with organic macromolecules; and (c) lack of a linear relationship between 99mTc-P and tissue calcium levels mainly results from local differences in composition and physicochemical properties of tissue calcium stores and from local variations in levels of blood flow for delivery of 99mTc-P agents.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey A. C., Solomons C. C., Ciricillo J., Miller N. L. Extraosseous calcification. Evidence for abnormal pyrophosphate metabolism in uremia. J Clin Invest. 1976 Mar;57(3):692–699. doi: 10.1172/JCI108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte F. J., Parkey R. W., Graham K. D., Moore J., Stokely E. M. A new method for radionuclide imaging of myocardial infarcts. Radiology. 1974 Feb;110(2):473–474. doi: 10.1148/110.2.473. [DOI] [PubMed] [Google Scholar]
- Botvinick E. H., Shames D., Lappin H., Tyberg J. V., Townsend R., Parmley W. W. Noninvasive quantitation of myocardial infarction with technetium 99m pyrophosphate. Circulation. 1975 Nov;52(5):909–915. doi: 10.1161/01.cir.52.5.909. [DOI] [PubMed] [Google Scholar]
- Bruno F. P., Cobb F. R., Rivas F., Goodrich J. K. Evaluation of 99mtechnetium stannous pyrophosphate as an imaging agent in acute myocardial infarction. Circulation. 1976 Jul;54(1):74–78. doi: 10.1161/01.cir.54.1.74. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Dees J. H., Harling D. F., Willerson J. T. Analytical electron microscopic study of mitochondrial inclusions in canine myocardial infarcts. J Histochem Cytochem. 1976 Mar;24(3):508–516. doi: 10.1177/24.3.57191. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Ferrans V. J., Graw R. G., Jr Cardiac pathologic findings in patients treated with bone marrow transplantation. Hum Pathol. 1976 Jan;7(1):17–45. doi: 10.1016/s0046-8177(76)80004-4. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Parkey R. W., Dees J. H., Stokely E. M., Harris R. A., Jr, Bonte F. J., Willerson J. T. Morphologic correlates of technetium-99m stannous pyrophosphate imaging of acute myocardial infarcts in dogs. Circulation. 1975 Oct;52(4):596–607. doi: 10.1161/01.cir.52.4.596. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Parkey R. W., Stokely E. M., Bonte F. J., Willerson J. T. Pathophysiology of technetium-99m stannous pyrophosphate and thallium-201 scintigraphy of acute anterior myocardial infarcts in dogs. J Clin Invest. 1976 Jun;57(6):1508–1522. doi: 10.1172/JCI108421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. E., Klein M. S., Ahmed S. A., Weiss E. S., Buchholz W. M., Sobel B. E. Mechanisms contributing to myocardial accumulation of technetium-99m stannous pyrophosphate after coronary arterial occlusion. Am J Cardiol. 1977 Jan;39(1):55–59. doi: 10.1016/s0002-9149(77)80011-8. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Holman B. L., Carmel A. N. Evaluation of radiopharmaceuticals sequestered by acutely damaged myocardium. J Nucl Med. 1976 Oct;17(10):911–917. [PubMed] [Google Scholar]
- Dewanjee M. K., Kahn P. C. Mechanism of localization of 99mTc-labeled pyrophosphate and tetracycline in infarcted myocardium. J Nucl Med. 1976 Jul;17(7):639–646. [PubMed] [Google Scholar]
- Dewanjee M. K. Localization of skeletal-imaging 99mTc chelates in dead cells in tissue culture: concise communication. J Nucl Med. 1976 Nov;17(11):993–997. [PubMed] [Google Scholar]
- DiCola V. C., Freedman G. S., Downing S. E., Zaret B. L. Myocsrdial uptake of technetium-99m stannous pyrophosphate following direct current transthoracic countershock. Circulation. 1976 Dec;54(6):980–986. doi: 10.1161/01.cir.54.6.980. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A., Janke J., Döring H. J., Leder O. Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab. 1974;4:563–580. [PubMed] [Google Scholar]
- Francis M. D., Slough C. L., Tofe A. J. Factors affecting uptake and retention of technetium-99m-diphosphonate and 99m-pertechnetate in osseous, connective and soft tissues. Calcif Tissue Res. 1976 Jun 14;20(3):303–311. doi: 10.1007/BF02546417. [DOI] [PubMed] [Google Scholar]
- Francis M. D., Webb N. C. Hydroxyapatite formation from a hydrated calcium monohydrogen phosphate precursor. Calcif Tissue Res. 1971;6(4):335–342. doi: 10.1007/BF02196214. [DOI] [PubMed] [Google Scholar]
- Genant H. K., Bautovich G. J., Singh M., Lathrop K. A., Harper P. V. Bone-seeking radionuclides: an in vivo study of factors affecting skeletal uptake. Radiology. 1974 Nov;113(2):373–382. doi: 10.1148/113.2.373. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Hays M. T., Green F. A. In vitro studies of 99m Tc-pertechnetate binding by human serum and tissues. J Nucl Med. 1973 Mar;14(3):149–158. [PubMed] [Google Scholar]
- Holman B. L., Davis M. A., Hanson R. N. Myocardial infarct imaging with technetium-labeled complexes. Semin Nucl Med. 1977 Jan;7(1):29–35. doi: 10.1016/s0001-2998(77)80005-6. [DOI] [PubMed] [Google Scholar]
- Jones A. G., Francis M. D., Davis M. A. Bone scanning: radionuclidic reaction mechanisms. Semin Nucl Med. 1976 Jan;6(1):3–18. doi: 10.1016/s0001-2998(76)80032-3. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Repke D. I. Calcium-membrane interactions in the myocardium: effects of ouabain, epinephrine and 3',5'-cyclic adenosine monophosphate. Am J Cardiol. 1973 Feb;31(2):193–201. doi: 10.1016/0002-9149(73)91032-1. [DOI] [PubMed] [Google Scholar]
- Kaye M., Silverton S., Rosenthall L. Technetium-99m-pyrophosphate: studies in vivo and in vitro. J Nucl Med. 1975 Jan;16(1):40–45. [PubMed] [Google Scholar]
- Kent S. P. Intracellular plasma protein: a manifestation of cell injury in myocardial ischemia. Nature. 1966 Jun 18;210(5042):1279–1281. doi: 10.1038/2101279b0. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Jennings R. B. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974 Dec;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G. T., Huebotter R. J., Walsh C. F., Taylor J. R., Kehr M. D., Tubis M., Blahd W. H. Kinetics of 99mTc-labeled pyrophosphate and polyphosphate in man. J Nucl Med. 1975 Feb;16(2):109–115. [PubMed] [Google Scholar]
- Krug A. Alterations in myocardial hydrogen ion concentration after temporary coronary occlusion: a sign of irreversible cell damage. Am J Cardiol. 1975 Aug;36(2):214–217. doi: 10.1016/0002-9149(75)90529-9. [DOI] [PubMed] [Google Scholar]
- Krug A. The extent of ischemic damage in the myocardium of the cat after permanent and temporary coronary occlusion. J Thorac Cardiovasc Surg. 1970 Aug;60(2):242–247. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Buja L. M., Saffer S., Mishelevich D., Stokely E., Lewis S., Parkey R., Bonte F., Willerson J. Experimental infarct sizing using computer processing and a three-dimensional model. Science. 1977 Jul 8;197(4299):167–169. doi: 10.1126/science.327541. [DOI] [PubMed] [Google Scholar]
- Lowenthal I. S., Tow D. E., Chang Y. C. Accumulation of 99mTc-polyphosphate in two squamous cell carcinomas of the lung: Case report. J Nucl Med. 1975 Nov;16(11):1021–1023. [PubMed] [Google Scholar]
- Marcus M. L., Tomanek R. J., Ehrhardt J. C., Kerber R. E., Brown D. D., Abboud F. M. Relationships between myocardial perfusion, myocardial necrosis, and technetium--99m pyrophosphate uptake in dogs subjected to sudden coronary occlusion. Circulation. 1976 Oct;54(4):647–653. doi: 10.1161/01.cir.54.4.647. [DOI] [PubMed] [Google Scholar]
- Meloan S. N., Puchtler H., Valentine L. S. Alkaline and acid alizarin red S stains for alkali-soluble and alkali-insoluble calcium deposits. Arch Pathol. 1972 Mar;93(3):190–197. [PubMed] [Google Scholar]
- Merrick M. V. Review article-Bone scanning. Br J Radiol. 1975 May;48(569):327–351. doi: 10.1259/0007-1285-48-569-327. [DOI] [PubMed] [Google Scholar]
- Poe N. D. Rationale and radiopharmaceuticals for myocardial imaging. Semin Nucl Med. 1977 Jan;7(1):7–14. doi: 10.1016/s0001-2998(77)80003-2. [DOI] [PubMed] [Google Scholar]
- Posner A. S. Crystal chemistry of bone mineral. Physiol Rev. 1969 Oct;49(4):760–792. doi: 10.1152/physrev.1969.49.4.760. [DOI] [PubMed] [Google Scholar]
- Pugh B. R., Buja L. M., Parkey R. W., Poliner L. R., Stokely E. M., Bonte F. J., Willerson J. T. Cardioversion and "false positive" technetium-99m stannous pyrophosphate myocardial scintigrams. Circulation. 1976 Sep;54(3):399–403. doi: 10.1161/01.cir.54.3.399. [DOI] [PubMed] [Google Scholar]
- RENAUD S. Superiority of alcoholic over aqueous fixation in the histochemical detection of calcium. Stain Technol. 1959 Sep;34:267–271. doi: 10.3109/10520295909114687. [DOI] [PubMed] [Google Scholar]
- Rona G., Boutet M., Hüttner I., Peters H. Pathogenesis of isoproterenol-induced myocardial alterations: functional and morphological correlates. Recent Adv Stud Cardiac Struct Metab. 1973;3:507–525. [PubMed] [Google Scholar]
- Schelbert H. R., Ingwall J. S., Sybers H. D., Ashburn W. L. Uptake of infarct-imaging agents in reversibly and irreversibly injured myocardium in cultured fetal mouse heart. Circ Res. 1976 Dec;39(6):860–868. doi: 10.1161/01.res.39.6.860. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Eanes E. D. Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif Tissue Res. 1972;10(3):171–197. doi: 10.1007/BF02012548. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Parkey R. W., Bonte F. J., Meyer S. L., Atkins J. M., Stokley E. M. Technetium stannous pyrophosphate myocardial scintigrams in patients with chest pain of varying etiology. Circulation. 1975 Jun;51(6):1046–1052. doi: 10.1161/01.cir.51.6.1046. [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]
- Zaret B., DiCola V. C., Donabedian R. K., Puri S., Wolfson S., Freedman G. S., Cohen L. S. Dual radionuclide study of myocardial infarction. Relationships between myocardial uptake of potassium-43, technetium-99m stannous pyrophosphate, regional myocardial blood flow and creatine phosphokinase depletion. Circulation. 1976 Mar;53(3):422–428. doi: 10.1161/01.cir.53.3.422. [DOI] [PubMed] [Google Scholar]
- Zimmer A. M., Isitman A. T., Holmes R. A. Enzymatic inhibition of diphosphonate: a proposed mechanism of tissue uptake. J Nucl Med. 1975 May;16(5):352–356. [PubMed] [Google Scholar]
- Zweiman G. F., Holman L. B., O'Keefe A., Idoine J. Selective uptake of 99mTc complexes and 67Ga in acutely infarcted myocardium. J Nucl Med. 1975 Nov;16(11):975–979. [PubMed] [Google Scholar]