Abstract
Objective
To describe the association between isoniazid preventive therapy (IPT) and mortality among individuals starting antiretroviral therapy (ART) in a workplace programme in South Africa where tuberculosis incidence is very high.
Methods
ART-naïve individuals starting ART from January 2004 to December 2007 were followed for up to 12 months. Deaths were ascertained from clinic and human resources data. The association between IPT and mortality was assessed using Cox regression.
Results
3,270 individuals were included (median age 45; 93% male; median baseline CD4 155 cells/mm3 (IQR:87–221); 45% WHO stage 3/4). 922(28%) individuals started IPT either prior to, or within three months of, starting ART. Individuals who started IPT tended to have less advanced HIV disease at ART initiation. 259 (7.9%) deaths were observed, overall mortality rate 8.9/100 person years [pyrs] (95%CI:7.9–10.6). The unadjusted mortality rate was lower among those who received IPT vs. not (3.7/100 pyrs versus 11.1/100 pyrs respectively, hazard ratio (HR) 0.34 [95%CI:0.24–0.49]); this association remained after adjustment for age, baseline CD4, baseline WHO stage, year of ART start and individual company (HR 0.51, 95%CI 0.32–0.80). In sensitivity analyses restricted to those with no previous history of TB (n=3036) or with no TB symptoms at ART initiation (n=2251), IPT remained associated with reduced mortality (adjusted HRs 0.51 (95%CI: 0.32 – 0.81) and 0.48 (95%CI: 0.24–0.96) respectively).
Conclusions
Mortality was lower among individuals receiving IPT with or prior to ART start. These results support routine use of IPT in conjunction with ART.
Keywords: isoniazid, mortality, antiretroviral therapy, South Africa, workplace
INTRODUCTION
Antiretroviral therapy (ART) is increasingly available in developing countries, delivered through a diverse range of programmes. Although reported outcomes of such programmes are encouraging, early mortality is high despite effective antiretroviral regimens[1] especially in the first 12 months of treatment [2]. Tuberculosis (TB) was the most important cause of morbidity and mortality among HIV-infected adults in developing countries in the pre-ART era[3, 4]. Although ART reduces the risk of TB substantially [5],[6],[7], tuberculosis remains the most common cause of morbidity and mortality in patients on ART[8],[9],[10, 11].
Isoniazid preventive therapy (IPT) reduces the risk of active tuberculosis by approximately 33% overall and by approximately 64% among those with a positive tuberculin skin test (TST)[12],[13]. IPT was also effective in preventing tuberculosis when implemented routinely in an HIV care programme for gold miners in South Africa prior to ART availability[14]. Despite World Health Organization guidelines recommending IPT as part of routine HIV care[15], there has been little IPT implementation at country level[16]. South African HIV treatment guidelines have previously not recommended IPT for individuals taking ART.
In a large workplace HIV programme in South Africa, where both the prevalence of latent tuberculosis and the incidence of active tuberculosis are very high (exceeding 4000 per 100,000 in 2002)[17, 18], IPT has been part of HIV care since its inception in 1999 and is recommended whether or not individuals are also taking ART. In this setting, we evaluated the effect of IPT, given either prior to or concurrent with the start of ART, on early mortality following ART initiation.
METHODS
Study design and population
We carried out an observational cohort analysis using prospectively collected data among individuals enrolled in a workplace ART programme in a group of industrial companies with operations primarily in South Africa. The design of the ART programme has been described elsewhere [19]. HIV disease management is based on guidelines developed in accordance with local and international best practice. During the time period of this analysis, employees were eligible for ART if they had a CD4 count below 250 cells/μl; were in World Health Organization (WHO) clinical stage 4 regardless of CD4 count; or were in WHO stage 3 with a CD4 count below 350 cells/μl. The preferred first line regimen was zidovudine and lamivudine (combined as Combivir) and efavirenz (with stavudine substituted for zidovudine for individuals with anaemia) until December 2007, replaced by tenofovir and emtricitabine (combined as Truvada) and efavirenz from January 2008.
According to programme guidelines, IPT (isoniazid 300mg daily for 6 months) is recommended for individuals with no evidence of active tuberculosis, and no prior history of tuberculosis. Screening for active tuberculosis is done using symptom screening and chest radiography: sputum is sent for microscopy and culture from tuberculosis suspects. TST is not part of the eligibility criteria for IPT in this programme. Cotrimoxazole (960mg daily) is recommended for individuals with a CD4 count below 250 cells/μl. All employees (regardless of HIV status) receive annual evaluation for TB disease through a workplace TB control programme based on radiological screening. All employees diagnosed with TB receive short-course directly observed treatment using a rifampicin-based four drug regimen.
In this analysis, we included all ART-naïve adults starting ART in this HIV care programme from January 2004 to December 2007. Subjects were followed until the earliest of: death, leaving employment or the end of the period of this analysis (12 months after ART start). We excluded individuals who were presumed to be on TB treatment at the start of ART (started TB treatment <6 months prior to ART start). In settings of high tuberculosis prevalence, the effect of IPT is thought to wane after 12 months [20], thus we excluded from the analysis individuals who started IPT greater than 12 months before starting ART. Since the risk of death changes rapidly after ART start, in order to have a more homogeneous IPT group, we excluded from the analysis individuals who started IPT more than three months after starting ART.
Data collection
Information on ART and all preventive therapies given was recorded routinely as part of the HIV disease management programme. Details of past medical history were collected at the first clinic visit. HIV clinical disease stage was derived from history and physical examination done by the physician at the first visit to the clinic. The baseline CD4 count and viral load values were those closest to the ART treatment start date, within a window of 6 months before or within 5 days after ART initiation. Virological response on treatment was determined using a viral load done closest to the 6 week time point (between 30 –120 days) after starting ART. For the purpose of this analysis, a satisfactory response was defined as a one log drop in viral load at 6 weeks. TB symptoms are collected routinely at every clinic visit on standardised clinic forms that required yes/no answers for the following symptoms: cough, night sweats, sputum production, fever and weight loss.
Ascertainment of outcome
All deaths among individuals enrolled in the ART programme are reported using standardised forms to the monitoring team by the clinic physicians. Human resources data were used as an additional source of information concerning deaths in employment, and reasons for leaving employment. To allow for the possibility of deaths among individuals who left employment due to ill-health, a secondary analysis was conducted using a combined mortality outcome defined as “death or termination from work on medical grounds”.
Laboratory methods
CD4 lymphocyte counts were measured by flow cytometry (FACSCount, BD Diagnostics, Franklin Lakes, New Jersey, US). HIV RNA quantification was carried out using the branched-DNA HIV-1 RNA 3.0 assay (Bayer Versant, New York, USA) according to the manufacturers’ instructions. For a small portion of patients (25) HIV RNA Quantitation was run on Roche COBAS Ampliprep/COBAS Taqman HIV-1 test version 2.0, Roche, NJ, USA.
Statistical methods
Baseline characteristics of individuals who started and did not start IPT were compared using the chi-square test or Wilcoxon rank sum test, as appropriate. Individuals were followed from date of ART initiation until the earliest of death, leaving employment, or the end of the analysis period (12 months from ART initiation). Kaplan Meier methods were used to illustrate the association between IPT and time to death. Cox proportional hazards regression was used to investigate the effect of IPT on the risk of death following initiation of ART. Factors that showed a strong confounding effect (based on an important change in the hazard ratio for association of IPT with mortality) were considered for the full adjusted analysis. In the multivariable analysis we started by adjusting for the strongest confounder based on the previous analysis, and included other variables one at a time. These were retained in the model if the hazard ratio for IPT changed. We used a rule-of thumb of including a variable if the hazard ratio changed by more than 10%. Age was included in the model a priori. Unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CIs) are reported and the likelihood ratio test used to assess whether use of IPT was associated with mortality. In addition, as an indicator of early response to ART, change in HIV viral load at 6 weeks was included in the model.
As this was not a comparison of randomised groups, a number of sensitivity analyses were conducted to explore issues which might cause bias and to take into account informative censoring which may have arisen due individuals leaving the workforce due to medical reasons. These sensitivity analyses were conducted as follows: a) restricted to individuals with no previous history of tuberculosis, as IPT was only indicated for these individuals in the programme guidelines, b) restricted to individuals without any symptoms suggesting tuberculosis (cough, night sweats, weight loss, fever, sputum production, regardless of duration, thus a more sensitive definition than is generally used to define a TB suspect) within the period 30 days before to 15 days after ART initiation, to allow for the possibility that individuals that were symptomatic were less likely to start IPT and may have had a higher mortality rate c) classifying individuals who left employment on medical grounds as deaths and d) combination of two above, restricted to individuals with no previous history of TB and no TB symptoms at initiation. To assess whether the effect of IPT on mortality was modified by baseline CD4 count, we modelled an interaction between IPT and baseline CD4 strata. We also assessed for interaction between IPT and time period following ART (<3 months, >3 months).
Ethical considerations
The analysis was approved by the Research Ethics Committees of University of KwaZulu Natal, South Africa, and the London School of Hygiene and Tropical Medicine.
RESULTS
In total, 3752 ART-naïve individuals started ART between 1 January 2004 and 31 December 2007. 213 individuals were excluded due to starting IPT later than 3 months after ART start, and 14 who started IPT greater than 12 months prior to ART. 255 individuals were on TB treatment at the start of ART and were excluded. Of the 3270 included individuals, 93% were male, mean age was 44.9 years, median baseline CD4 155 cells/μl (interquartile range [IQR] 87 – 221), and median viral load 48 817 copies/ml (IQR 17 860 – 128 629). Total follow-up time was 2910 person-years (339 (9.03%) censored before 12 months). WHO stage at ART initiation (available for 3 270 individuals), was WHO stage 2 (1803, 55.1%), stage 3 (1123, 34.3%) or stage 4 (344, 10.5%).
922 (28%) individuals started IPT either before or during the first three months of starting ART; the vast majority (n=882, 96%) within 0–7 days of starting ART. 234 individuals (7.2%) had previously been treated for tuberculosis prior to starting ART. 1338 (40.9%) individuals started cotrimoxazole either before starting ART (94% within a month of starting ART) or during the follow up time on ART. Baseline and 6 week viral load results were available for 1986 individuals (60.6%), of whom 1648 (83%) achieved a greater than 1 log10 reduction in HIV RNA by 6 weeks.
Baseline characteristics among those who started versus did not start IPT are compared in Table 1. Individuals who were started on IPT tended to have less advanced HIV disease as they had higher CD4 counts (CD4 <100cells/μl, 26.2% vs. 32.9%), lower viral load (viral load >100 000copies/ml, 24.3% vs. 33.1%), and higher haemoglobin levels (haemoglobin >10g/dl, 94.1% vs. 87%) at ART initiation and fewer were in WHO stage 3 or 4 (29.8% vs. 50.3%). The groups also differed with regards to whether they had had an episode of TB (8.9% in those never started on IPT and 2.5% in those who had started on IPT) and markedly by whether they were also on cotrimoxazole (84.8% in those who had started on IPT and 29.9% in those never started on IPT).
Table 1.
Baseline characteristics of individuals starting antiretroviral therapy, comparing those who did vs. did not receive isoniazid preventive therapy (n=3270).
| Baseline characteristic | No IPT N (%) |
IPT N (%) |
P value |
|---|---|---|---|
| All | 2348 | 922 | |
|
| |||
| Age (years) | |||
| Median (IQR) | 45 (38 – 51) | 46 (37 – 52) | P2=0.25 |
| 18 – 29 | 220 (9.4) | 93 (10.1) | P1=0.005 |
| 30 – 39 | 740 (31.5) | 269 (29.2) | |
| 40 – 49 | 965 (41.1) | 346 (37.5) | |
| ≥ 50 | 423 (18.0) | 214 (23.2) | |
|
| |||
| Gender: number (%) male | 2182 (92.9) | 857 (94.0) | P1=0.26 |
|
| |||
| Baseline CD4 count* (cells/μl) | |||
| Median (IQR) | 152 (81 – 224) | 158 (98 – 215) | P2=0.56 |
| <50 | 323 (14.6) | 95 (10.7) | P1=0.002 |
| 50 – 100 | 383 (17.3) | 138 (15.5) | |
| 101 – 150 | 385 (17.5) | 182 (20.5) | |
| 151 – 200 | 395 (17.9) | 194 (21.8) | |
| >200 | 718 (32.6) | 281 (31.6) | |
|
| |||
| Baseline WHO stage | |||
| 1 or 2 | 1167 (49.7) | 648 (70.3) | P1<0.001 |
| 3 | 873 (37.2) | 233 (25.3) | |
| 4 | 308 (13.1) | 41 (4.5) | |
|
| |||
| Baseline viral load** (copies/ml) | |||
| Median (IQR) | 52,071 (19,134–141,461) | 42,418 (15,420–97,844) | P2<0.001 |
| VL>100 000 | 654 (33.1) | 202 (24.3) | P1<0.001 |
|
| |||
| Baseline haemoglobin*** (g/dl) | |||
| Median (IQR) | 12.9 (11.2–14.1) | 13.4 (12.2 – 14.6) | P2<0.001 |
| Haemoglobin<10 | 250 (13.0) | 50 (5.9) | P1<0.001 |
|
| |||
| Previous tuberculosis | 211 (8.9) | 23 (2.5) | P1<0.001 |
|
| |||
| Cotrimoxazole started | 603 (25.7) | 735 (79.7) | P1<0.001 |
|
| |||
| Year started ART | |||
| 2004 | 633 (27.0) | 91 (9.9) | P1<0.001 |
| 2005 | 587 (25.0) | 181 (19.6) | |
| 2006 | 692 (29.5) | 284 (30.8) | |
| 2007 | 436 (18.6) | 366 (39.7) | |
|
| |||
| Company | |||
| Company A | 2165 (92.2) | 484 (52.5) | P1<0.001 |
| Company B | 78 (3.3) | 347 (37.6) | |
| Company C | 105 (5.5) | 91 (10.0) | |
|
| |||
| Viral response at 6wks**** | 1101 (82.7) | 547 (83.6) | P1=0.58 |
IPT: isoniazid preventive therapy; IQR: interquartile range; ART: antiretroviral therapy
available in 3094 individuals
available in 2806 individuals
available for 2771 individuals
>1 log reduction in viral load at 6 weeks after ART initiation, available for 1986 individuals
P1= Pearson chi-squared test
P2=Wilcoxon rank-sum (Mann-Whitney) test
In the first 12 months following initiation of ART, 259 (7.9%) individuals died giving an overall mortality rate of 8.90/100 person years (py) (95% confidence interval (CI) 7.88 – 10.05). The mortality rate of those never started on IPT was 11.08/100py (227/2047, 95% CI 9.74 – 12.63) and those who were started IPT was 3.71/100py (32/863, 95% CI 2.62 – 5.24). The unadjusted hazard ratio (HR) for mortality from the Cox model for those started on IPT compared to those not started on IPT was 0.34 (95% CI 0.24 – 0.49).
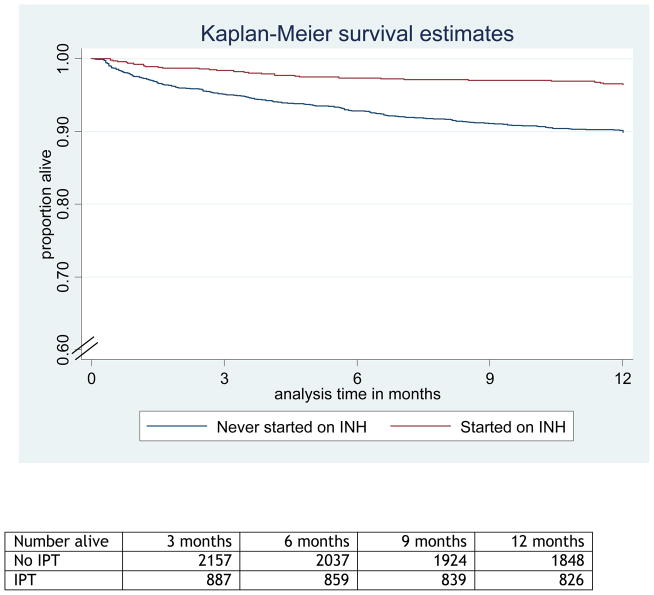
Table 2 shows associations of various risk factors with mortality. The final adjusted model included age group, baseline CD4 group, WHO stage at ART initiation, year of starting ART, individual company and receipt of IPT Table 2). In the adjusted model, IPT use remained strongly associated with decreased mortality (adjusted HR: 0.50, 95% CI 0.32 – 0.80). Adding cotrimoxazole use, baseline haemoglobin and baseline viral load to the model did not change the hazard ratio substantially. As an indicator of early response to ART, change in viral load at 6 weeks was included in a model restricted to individuals with available data, who therefore survived at least this long (Table 2); however there was no evidence that this caused confounding in the association between IPT and mortality. All factors met the proportional hazards assumption (P<0.01). Other risk factors for mortality that remained in the adjusted model were older age group (Ptrend<0.001), lower baseline CD4 count (Ptrend<0.001), earlier year of ART start (Ptrend=0.001) and company (P=0.30). We graphically illustrate the association between IPT and mortality using a Kaplan-Meier survival curve (Figure).
Table 2.
Factors associated with mortality, unadjusted and adjusted analyses
| Unadjusted analysis (N=3270) | Adjusted analysis* (N=3094) | ||||
|---|---|---|---|---|---|
|
| |||||
| Rate/100py | Hazard Ratio (HR) | 95% CI (P value) | Hazard Ratio (HR) | 95% CI (P value) | |
| IPT | |||||
| No | 11.10 | 1 | P<0.001 | 1 | P=0.002 |
| Yes | 3.71 | 0.34 | 0.24 – 0.49 | 0.51 | 0.32 – 0.80 |
|
| |||||
| Gender | |||||
| Male | 9.29 | 1 | P=0.005 | ||
| Female | 3.82 | 0.42 | 0.21 – 0.84 | ||
|
| |||||
| Age (years) | |||||
| 18–29 | 4.86 | 1 | PT <0.001 | 1.00 | PT <0.001 |
| 30 – 39 | 6.74 | 1.38 | 0.77 – 2.47 | 0.99 | 0.55 – 1.78 |
| 40–49 | 11.53 | 2.24 | 1.29 – 3.89 | 1.58 | 0.90 – 2.77 |
| ≥ 50 | 10.20 | 2.06 | 1.15 – 3.71 | 1.80 | 0.99 – 3.27 |
|
| |||||
| Baseline WHO stage | |||||
| 1 or 2 | 6.36 | 1 | PT<0.001 | 1 | PT=0.005 |
| 3 | 9.66 | 1.49 | 1.13 – 1.98 | 1.06 | 0.86 – 1.57 |
| 4 | 21.68 | 3.27 | 2.38 – 4.50 | 1.75 | 1.21 – 2.54 |
|
| |||||
| Baseline CD4 (cells/μl) | |||||
| <50 | 19.34 | 4.11 | 2.79 – 6.06 | 3.58 | 2.39 – 5.36 |
| 50–100 | 12.91 | 2.78 | 1.87 – 4.14 | 2.57 | 1.72 – 3.83 |
| 101 – 150 | 9.08 | 1.97 | 1.30 – 2.99 | 1.93 | 1.27 – 2.94 |
| 151 – 200 | 3.81 | 0.84 | 0.50 – 1.42 | 0.86 | 0.51 – 1.46 |
| >200 | 4.58 | 1 | PT <0.001 | 1 | PT<0.001 |
|
| |||||
| Baseline viral load (copies/ml) | |||||
| ≤ 100,000 | 6.85 | 1 | P<0.001 | ||
| >100,000 | 11.77 | 1.70 | 1.29 – 2.24 | ||
|
| |||||
| Year started ART | |||||
| 2004 | 15.10 | 1 | PT<0.001 | 1 | PT=0.001 |
| 2005 | 9.98 | 0.67 | 0.49 – 0.92 | 0.78 | 0.55 – 1.11 |
| 2006 | 6.75 | 0.46 | 0.33 – 0.64 | 0.58 | 0.1 – 0.83 |
| 2007 | 5.62 | 0.38 | 0.26 – 0.55 | 0.60 | 0.41 – 0.89 |
|
| |||||
| Cotrimoxazole started** | |||||
| No | 9.85 | 1 | P=0.001 | ||
| Yes | 7.57 | 0.78 | 0.60 – 1.00 | ||
|
| |||||
| Previous TB | |||||
| No | 8.88 | 1 | P=0.97 | ||
| Yes | 9.19 | 1.01 | 0.62 – 1.63 | ||
|
| |||||
| Baseline Haemoglobin (g/dl) | |||||
| <10 | 26.28 | 1 | P<0.001 | ||
| ≥ 10 | 5.51 | 4.58 | 3.37 – 6.24 | ||
|
| |||||
| Viral response at 6 wks (>1 log decrease) | |||||
| No | 8.32 | 1 | P<0.001 | ||
| Yes | 3.01 | 0.36 | 0.22 – 0.59 | ||
|
| |||||
| Company (clinic site) | |||||
| Company A | 9.70 | 1 | P=0.002 | 1 | P=0.29 |
| Company B | 4.40 | 0.45 | 0.27 – 0.73 | 0.74 | 0.43 – 1.31 |
| Company C | 8.83 | 0.95 | 0.57 – 1.57 | 1.37 | 0.80 – 2.35 |
PT = P value for linear trend
Adjusted for age group, baseline WHO stage, baseline CD4 count, year started on ART, and company.
Cotrimoxazole refers to those put on either cotrimoxazole or dapsone (used in cases of cotrimoxazole hypersensitivity)
IPT: isoniazid preventive therapy; ART: antiretroviral therapy
Figure 1.
Figure Kaplan Meier curve comparing survival among those who started or did not start isoniazid preventive therapy (IPT)
To assess whether the association between IPT and mortality differed by time period after starting ART we assessed for an interaction between IPT use and time period. The data were consistent with no interaction (unadjusted analysis p=0.89, adjusted analysis p=0.94). In the first three months following ART initiation, overall mortality rate was 16.28/100py, higher in those not on IPT (20.15/100py : 113/561py) compared to those started on IPT (6.65/100pyrs :15/226py), HR 0.33 (95% CI 0.19 – 0.57). In the period after three months on ART, overall mortality rate was 6.17/100py, higher in those not on IPT (7.67/100py : 114/1487py) compared to those started on IPT (2.67/100pyrs :17/638py), HR 0.35 (95% CI 0.21 – 0.58). In the multivariable model, the adjusted hazard ratio IPT in the time period less than 3 months was 0.50 (95% CI 0.27 – 0.93) and in the time period greater than 3 months was 0.51 (95% CI 0.29 – 0.91) (Table 4). Similarly we tested for effect modification with cotrimoxazole. When stratifying by whether patients were also on cotrimoxazole, those on IPT continued to have lower mortality rates than those not on IPT, both among individuals taking cotrimoxazole (HR 0.27 (0.11 – 0.56)) and not taking cotrimoxazole (HR 0.33 (0.21 – 0.51); P-value for interaction P=0.68)
Table 4.
Interaction between CD4 count and isoniazid preventive therapy use on mortality (n=2540)
| IPT use | #deaths/pys | Rate per 100 pys | Unadjusted HR (95% CI) | Adjusted HR2 (95% CI) | |
|---|---|---|---|---|---|
| Baseline CD4 | |||||
|
| |||||
| < 50 | No | 58/259 | 22.41 | 11 | 13 |
| Yes | 8/82 | 9.70 | 0.44 (0.21 – 0.93) | 0.68 (0.30 – 1.51) | |
|
| |||||
| 50–100 | No | 50/324 | 15.42 | 1 | 1 |
| Yes | 8/125 | 6.40 | 0.42 (0.20 – 0.89) | 0.58 (0.26 – 1.28) | |
|
| |||||
| 101 – 200 | No | 56/699 | 8.01 | 1 | 1 |
| Yes | 11/359 | 3.07 | 0.39 (0.20 – 0.74) | 0.48 (0.24 – 0.96) | |
|
| |||||
| >200 | No | 38/650 | 5.85 | 1 | 1 |
| Yes | 4/268 | 1.49 | 0.26 (0.09 – 0.73) | 0.33 (0.12 – 0.95) | |
|
| |||||
| Time period following ART initiation | |||||
|
| |||||
| <3 months | No | 113/561 | 20.15 | 1 | 1 |
| Yes | 15/226 | 6.65 | 0.33 (0.19 – 0.57) | 0.50 (0.27 – 0.93) | |
|
| |||||
| >3 months | No | 114/1487 | 7.67 | 1 | 1 |
| Yes | 17/638 | 2.67 | 0.35 (0.21 – 0.58) | 0.51 (0.29 – 0.91) | |
P-value for interaction=0.84;
Adjusted for age group, baseline WHO stage, year started on ART and individual company (and CD4 baseline for time period);
P-value for interaction=0.79 pys=person-years
Table 3 shows the association between IPT and mortality in sensitivity analyses. When analysis was restricted to only those who had not had previous tuberculosis (n= 3036) the adjusted HR was 0.52 (95%CI 0.32 – 0.82). When restricted to those who had data available regarding symptoms at baseline, and had no symptoms suggesting tuberculosis (n=1923), the adjusted HR for the association of IPT and mortality was 0.48 (95%CI 0.24 – 0.96). Using a combined mortality end-point which classified individuals who left employment on medical grounds (n=99) as deaths, the adjusted HR for the association of IPT and mortality was 0.72 (95%CI 0.47 – 1.10). We also looked at the association of IPT with mortality, restricted to those who had no previous TB and also no symptoms suggesting tuberculosis with the period of ART initiation (N=1795); the adjusted HR for the association of IPT and mortality was 0.44 (95% CI 0.22 – 0.89).
Table 3.
Sensitivity analyses for associations between isoniazid preventive therapy and mortality using various scenarios
| # death/pyrs | Rate per 100 pyrs | Unadjusted HR | Adjusted HR* (95% CI) | ||
|---|---|---|---|---|---|
| Restricted to those with no previous tuberculosis (N=3036) | No IPT | 209/1872 | 11.16 | 1 | 1 (P=0.003) |
| IPT | 32/843 | 3.80 | 0.35 | 0.51(0.32 – 0.81) | |
| Restricted to those with no tuberculosis symptoms at ART initiation (N=1923) | No IPT | 66/1011 | 5.99 | 1 | 1 (P=0.08) |
| IPT | 15/683 | 2.19 | 0.37 | 0.48 (0.24 – 0.96) | |
| Including all those medically boarded as deaths (N=3270) | No IPT | 310/2047 | 15.14 | 1 | 1 (P=0.11) |
| IPT | 48/863 | 5.56 | 0.37 | 0.66 (0.45 – 0.97) | |
| Restricted to no previous TB and no TB symptoms at initiation (n=1795) | No IPT | 61/1007 | 6.06 | 1 | 1 (P=0.05) |
| IPT | 15/699 | 2.24 | 0.37 | 0.44 (0.22 – 0.89) |
IPT: isoniazid preventive therapy; ART: antiretroviral therapy
Adjusted for age group, baseline WHO stage baseline CD4 count, year started on ART and individual company
To assess whether the association between IPT and mortality differed by CD4 count strata we assessed for an interaction between IPT use and CD4 count. The data were consistent with no interaction (unadjusted analysis p=0.84, adjusted analysis p=0.81). The effect of IPT use on mortality, stratified by CD4 group at ART initiation was as follows: CD4 <50 cells/mm3, HR 0.80 (95% CI 0.36–1.78); CD4 50–100 cells/mm3, HR 0.48 (95% CI 0.19 – 1.19); CD4 101 – 200cells/mm3, HR 0.48, (95% CI: 0.22 – 1.07), and >200 cells/mm3, HR 0.45, (95% CI: 0.15–1.33) (Table 4).
Discussion
In this observational cohort study, we found approximately a halving of the risk of mortality among people starting ART who were also given IPT. This association persisted after controlling for confounders and in sensitivity analyses exploring possible sources of bias. This is a major reduction in mortality among individuals whose risk of death is very high, with potential for a substantial public health impact. Clinical trials of IPT from the pre-ART era found a smaller reduction in mortality among people with HIV given IPT (RR 0.74, 0.55 –1.00)[13]. However, an effect of this magnitude is plausible among individuals starting ART in a setting of high tuberculosis incidence, given that tuberculosis is the most important cause of death among these individuals, and the incidence of tuberculosis during the first few months of ART is very high [21] [22].
Although there is strong evidence from the pre-ART era that IPT reduces tuberculosis incidence in HIV-infected individuals,[12] [13, 23] there are far fewer data concerning the use of IPT in combination with ART. Observational cohort studies in Brazil and South Africa showed an effect of IPT in addition to ART in reducing TB incidence [24–26], although in the South African study most patients were given IPT a median of one year prior to ART[25]. Neither study assessed the effect of IPT on mortality.
In ART care programmes, IPT is delivered as part of a package of care, and screening to identify active tuberculosis both prior to and during IPT is an essential component. This screening may contribute to a reduction in tuberculosis case-fatality, an issue which is discussed in more detail in the accompanying Opinion piece.
Very few data are available concerning outcomes when IPT and ART are used concurrently, probably because the implementation of IPT has been so limited[27]. We restricted this analysis to individuals who started IPT relatively close to the ART start date in order that the exposure of interest (IPT at the time of ART start) was more homogeneous. Given that our HIV care programme guidelines promote early use of IPT as part of pre-ART care, it was a little surprising to us that the great majority of individuals started IPT within one week of starting ART (and thus this restriction resulted in few [227/3752] exclusions). There are concerns that IPT should not be given to individuals starting ART on the basis that it is difficult to reliably exclude active tuberculosis when the prevalence of undiagnosed tuberculosis is so high[7]. Our data suggest that such theoretical concerns should be weighed against a potentially large reduction in mortality if IPT is initiated with ART. Randomised trials currently in progress investigating the effect of adding IPT to ART may help resolve this issue.
Although INH was recommended by the programme for all patients with no previous TB, INH was not always prescribed in all eligible patients. We believe that the most important reason for inconsistent implementation is variability in clinician willingness to use IPT. Low uptake of IPT is acknowledged in many settings, mainly thought to be due to operational problems relating to feasibility of tuberculin testing, as well as concerns about the development of isoniazid drug resistance[28]. We recently conducted a study investigating barriers to IPT use, presented in this same supplement, showing that barriers to use of IPT were predominantly derived from clinic staff, in particular prescribing physicians who often lacked sufficient knowledge and familiarity with the use of INH[29]. It seems to be that certain physicians are more likely than others to prescribe INH mainly based on their own experience. We believe that this is the most likely explanation for why some patients received IPT and others did not.
Strengths of our analysis include the large number of individuals included, and the relatively complete ascertainment of deaths, via human resources records and follow-up of defaulters. Limitations include lack of data on the effect of IPT on tuberculosis incidence due to absence of electronic tuberculosis clinic records and no information on adherence to IPT, although we believe that the adherence in this programme may have been better than previously experienced due to the strong emphasis on adherence as this was offered as a “package” with ART.
An important limitation of this study is that it was not a randomised clinical trial and so we cannot exclude systematic differences between individuals who received IPT and those who did not. The analysis of baseline factors does indicate that the patients not given IPT may have been more unwell. Our analyses controlled for all well-recognised potential confounding factors, and the sensitivity analyses explored potential issues which could result in healthier individuals receiving IPT and hence experiencing lower mortality, yet in all analyses the association between IPT and lower mortality was of similar magnitude. However we cannot exclude that there may have been confounders that we did not control for. In addition, there were missing data for some variables such as viral load and haemoglobin at baseline, again making it harder to exclude residual confounding. Another limitation was that we were not able to report cause of death for these patients; however TB is an important cause of death in patients on ART, although it is often underreported[11], and is well documented to be an important cause of death in miners who have HIV infection[30]. In addition, we have documented high TB incidence among employees with HIV in the largest of the sites in this study[31].
Data from large cohorts of patients in treatment programmes can be complementary to those from randomised trials; specifically, this analysis may give insights into the effect of the “package” of IPT along with more intensive tuberculosis case finding, which was not measured by clinical trials of IPT. In ART care programmes, IPT is delivered as part of a “package” of care, and screening to identify active tuberculosis both prior to and during IPT is an essential component. However, in randomised controlled trials of IPT, the intensive screening component was implemented in both arms[32–34], and thus only the IPT component was tested. We hypothesise that individually randomised controlled trials may have underestimated the impact of an IPT programme on TB case-fatality (see Opinion piece).
The risk of death after starting ART remains high until the CD4 cell count increases[22]. Additional interventions are therefore urgently needed to reduce the risk of death among ART initiators. These data from our treatment cohort suggest that individuals starting ART may have a substantial reduction in mortality if IPT is given concurrently, and add to accumulating evidence concerning the additional value of IPT along with ART[25] [26]. Although the results of randomised trials of IPT with ART are awaited[35, 36], our data support the routine use of IPT in HIV care programmes in line with WHO recommendations.
Acknowledgments
Sources of support: The study was funded by Anglo American plc. Alison Grant was supported by a UK Department of Health Public Health Career Scientist award. Katherine Fielding and Gavin Churchyard are part supported by the Consortium to Respond Effectively to the AIDS and TB Epidemic (CREATE).
We would like to acknowledge other staff in the Aurum ART team, including the clinical support and data management department, for their contributions to this programme. We would specifically like to thank Michael Eisenstein, Piotr Hippner, Pule Seatlanyane, Othia Letlape who were involved in the day-to-day running of the project. We would also like to take this opportunity to acknowledge the contribution of all the company leaders and collaborators for supporting this work, specifically Professor Richard Chaisson, Dr Brian Brink, Dr Lettie La Grange, Dr Jan Pienaar and Dr Lesego Rametsi.
The programme was funded through the Anglo American PLC companies. C J Hoffmann was supported by NIH DK074348, RE Chaisson by NIH AI5535901 and AI016137, and AD Grant by a UK Department of Health Public Health Career Scientist Award.
Footnotes
This work was presented in part at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, California, February 17-21, 2010.
Conflicts of interest: SC: none, ADG: none, CI: none, CJH: none, RD: none, JP: none, KLF: none, GJC: none
References
- 1.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 3.Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N’Gbichi JM, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Grant AD, Djomand G, De Cock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11 (Suppl B):S43–54. [PubMed] [Google Scholar]
- 5.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis J, Churchyard G. Complementary roles of antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in resource-limited settings. Lancet Infect Dis. doi: 10.1016/S1473-3099(10)70078-5. (in press) [DOI] [PubMed] [Google Scholar]
- 8.Saraceni V, King BS, Cavalcante SC, Golub JE, Lauria LM, Moulton LH, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–772. [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 10.Innes C, Charalambous S, Pemba LF, Bogoshi M, Steele J, Senoge S, et al. Causes of Mortality in Patients on Antiretroviral Therapy in South Africa. XVI International AIDS Conference; Toronto, Canada. 2006. [published abstract CDC0001] [Google Scholar]
- 11.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004:CD000171. doi: 10.1002/14651858.CD000171.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. :CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant AD, Charalambous S, Fielding KL, Day JH, Corbett EL, Chaisson RE, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA. 2005;293:2719–2725. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- 15.Organization WH. WHO 3 I’s Meeting, Report of a Joint HIV/AIDs and TB department Meeting. World Health Organization; 2008. [Accessed on 5th September 2010]. at http://www.who.int/hiv/pub/tb/3is_mreport/en/index.html. [Google Scholar]
- 16.Churchyard GJ, Scano F, Grant AD, Chaisson RE. Tuberculosis preventive therapy in the era of HIV infection: overview and research priorities. J Infect Dis. 2007;196 (Suppl 1):S52–62. doi: 10.1086/518662. [DOI] [PubMed] [Google Scholar]
- 17.Corbett EL, Churchyard GJ, Charalambos S, Samb B, Moloi V, Clayton TC, et al. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis. 2002;34:1251–1258. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 18.Hanifa Y, Mngadi K, Lewis J, Fielding K, Churchyard G, Grant AD. Evaluation of the Arkansas method of urine testing for isoniazid in South Africa. Int J Tuberc Lung Dis. 2007;11:1232–1236. [PubMed] [Google Scholar]
- 19.Charalambous S, Grant AD, Day JH, Pemba L, Chaisson RE, Kruger P, et al. Establishing a workplace antiretroviral therapy programme in South Africa. AIDS Care. 2007;19:34–41. doi: 10.1080/09500340600677872. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JL, Okwera A, Hom DL, Mayanja H, Mutuluuza Kityo C, Nsubuga P, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15:2137–2147. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 23.Bucher H. Isoniazid Prophylaxis for Tuberculosis in HIV Infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 24.Golub JE, Chaisson RE, Martinson NA. Additive effects of isoniazid preventive therapy and HAART. AIDS. 2009;23:1446–1447. doi: 10.1097/QAD.0b013e32832d53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Epidemiology, strategy, financing. World Health Organization; Geneva: 2009. [Accessed on 5th September 2010]. Global tuberculosis control 2009. WHO/HTM/TB/2009.411. from http://www.who.int/tb/publications/global_report/2009/en/index.html WHO/HTM/TB/2009.411. [Google Scholar]
- 28.Hiransuthikul N, Hiransuthikul P, Nelson KE, Jirawisit M, Paewplot R, Kasak S. Physician adherence to isoniazid preventive therapy guidelines for HIV-infected patients in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:1208–1215. [PubMed] [Google Scholar]
- 29.Lester R, Hamilton R, Charalambous S, Chandler C, Churchyard GJ, Grant AD. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study in South Africa. 2nd SA TB conference; Durban, South Africa. 2010. [Abstract 208, oral presentation] [DOI] [PubMed] [Google Scholar]
- 30.Murray J, Sonnenberg P, Nelson G, Bester A, Shearer S, Glynn JR. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS. 2007;21 (Suppl 6):S97–S104. doi: 10.1097/01.aids.0000299416.61808.24. [DOI] [PubMed] [Google Scholar]
- 31.Charalambous S, Morris C, Innes C, Shisana M, Churchyard GJ, Grant AD, et al. TB incidence in HIV-infected patients starting antiretroviral therapy in a South African mining population. 39th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease; Paris, France. 2008. [TS-82295–19] [Google Scholar]
- 32.Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala BN, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–2457. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 34.Hawken MP, Meme HK, Elliott LC, Chakaya JM, Morris JS, Githui WA, et al. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS. 1997;11:875–882. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Gideon HP, du Toit E, Maartens G. Evaluation of IGRA for detection of prevalent tuberculosis (TB) amongst asymptomatic HIV-1 infected adults on combined antiretroviral treatment (ART) being screened for a TB prevention study in Khayelitsha, South Africa. 5th International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009. [TUPEB154] [Google Scholar]
- 36.ClinicalTrials.gov. [Accessed on 28.01.10];Early antiretroviral treatment and/or early isoniazid prophylaxis against tuberculosis in HIV-infected adults (ANRS 12136 TEMPRANO) at: http://clinicaltrials.gov/ct2/show/NCT00495651. In.