Summary
The adipocyte is central to organismal metabolism and exhibits significant functional and morphological plasticity during its formation and lifespan. Remarkable transformations of this organ occur during obesity and lactation, two metabolic processes where a better understanding of adipocyte function is essential. Considering the critical importance of the cellular organelle endoplasmic reticulum (ER) in adapting to fluctuations in synthetic processes, we explored the role of XBP1, a central regulator of ER adaptive responses, in adipocyte formation and function. Unexpectedly, deletion of adipocyte-XBP1 in vivo in mice (XBP1ΔAd) had no effect on adipocyte formation or systemic homeostatic metabolism on regular or high fat diet. However, during lactation XBP1ΔAd dams displayed increased adiposity, decreased milk production, and decreased litter growth compared to control dams. Moreover, we demonstrate that XBP1 is regulated during lactation, where it responds to prolactin to alter lipogenic gene expression. These results demonstrate a previously unrecognized role for adipocyte-XBP1 in regulation of lactational metabolism.
Introduction
The fat cell, or adipocyte, is a central regulator of metabolism that is conserved in organisms from flies to humans. At the core of adipocyte function is its ability to store and release lipid in flux with the energy demands of the organism. As such, the life of the adipocyte encompasses many extreme fluctuations in lipid storage capacity, beginning with the development of a pre-adipocyte into a mature adipocyte, and then continuing to respond to metabolic signals. For example, the adipocyte must deplete its lipid supply under nutrient-scarce or high-energy demand states such as starvation or lactation, or increase its lipid stores under nutrient-rich conditions, including obesity (Attie and Scherer, 2009).
We have previously shown that under obese conditions, adipose tissue function is compromised, and possible mechanisms behind the dysfunction include the upregulation of inflammatory and/or endoplasmic reticulum (ER) stress signaling due to disruption of the functional integrity of this organelle and activation of its unfolded protein response (UPR). Indeed, in both mice and humans the UPR was activated in adipose tissue in obesity (Hotamisligil, 2010). One specific molecule identified in these studies was X-box binding protein 1 (XBP1), a transcription factor involved in the most ancient arm of the UPR. The UPR transmembrane sensor inositol-requiring enzyme 1α (IRE1α) functions upon activation as an endoribonuclease to cleave 26 base pairs from the mRNA of XBP1. This spliced form of XBP1 (sXBP1) encodes an active transcription factor that upregulates target genes involved in a myriad of ER-related processes including protein synthesis, protein folding, ER associated degradation, and ER expansion (Ron and Walter, 2007). In addition to its role in ER stress, XBP1 is known to be essential for the in vivo differentiation of various secretory cell types such as plasma cells, and pancreatic and salivary exocrine cells (Glimcher, 2010). Recently, experimental suppression of XBP1 in preadipocyte cell lines was reported to result in incomplete adipogenesis in vitro (Basseri et al., 2009; Sha et al., 2009). However, the role of XBP1 in adipocytes in vivo remains unknown. Since adipose tissue XBP1 expression is increased in mouse and human obesity characterized by robust expansion of adipose tissue, we set out to interrogate the biology of this molecule under conditions that represent extremes of adipose tissue fluctuation, namely obesity and lactation, in vivo.
We found that genetic deletion of adipocyte XBP1 did not, in contrast to expectations, affect adipose tissue formation and function under homeostatic metabolic conditions. However, we report the unanticipated role of XBP1 in regulation of adipose tissue function during the homeorhetic, or directional, metabolism of lactation.
Results
In vivo deletion of adipocyte XBP1
We began our study by suppressing Xbp1 expression in cultured adipocytes and observed an inhibition of differentiation as reported previously (Fig.1A-C)(Sha et al., 2009). However, this function of XBP1 was limited in vitro and this inhibitory effect on adipocyte differentiation could be overcome by stronger stimuli such as thiazolidinediones that promote adipogenesis (Fig.1B,C). Therefore, we utilized adipocyte-specific deletion models of XBP1 to investigate adipocyte formation and function in its physiological context in vivo in mice. The loxP-Cre system utilizing Cre under the aP2 promoter efficiently deleted XBP1 in adipose tissue, both at the mRNA and protein levels (Fig.S1A & Fig.1D). We next performed metabolic characterization of the mice harboring adipocyte deletion of XBP1 (XBP1ΔAd). Compared to wild-type (WT) mice, deletion of adipocyte XBP1 did not affect body weight, adipose tissue mass, serum insulin, or glucose homeostasis when fed a regular chow diet (Fig.S1B-G). Interestingly, when animals were challenged with high fat diet, representing one extreme of adipocyte plasticity and function, body weight and adiposity were still unaltered (Fig.1E-G). In addition, no differences were found in adipose tissue morphology, serum insulin, serum adiponectin, or systemic glucose tolerance between WT and XBP1ΔAd mice (Fig.1H-K). These results indicate that in mice, XBP1 is not a contributing factor to the formation or expansion of adipose tissue and, moreover, it does not regulate adipocyte function or glucose homeostasis under the stress of obesity.
Figure 1. Targeted deletion of XBP1 in the adipocyte does not affect tissue formation or function in homeostatic metabolism.
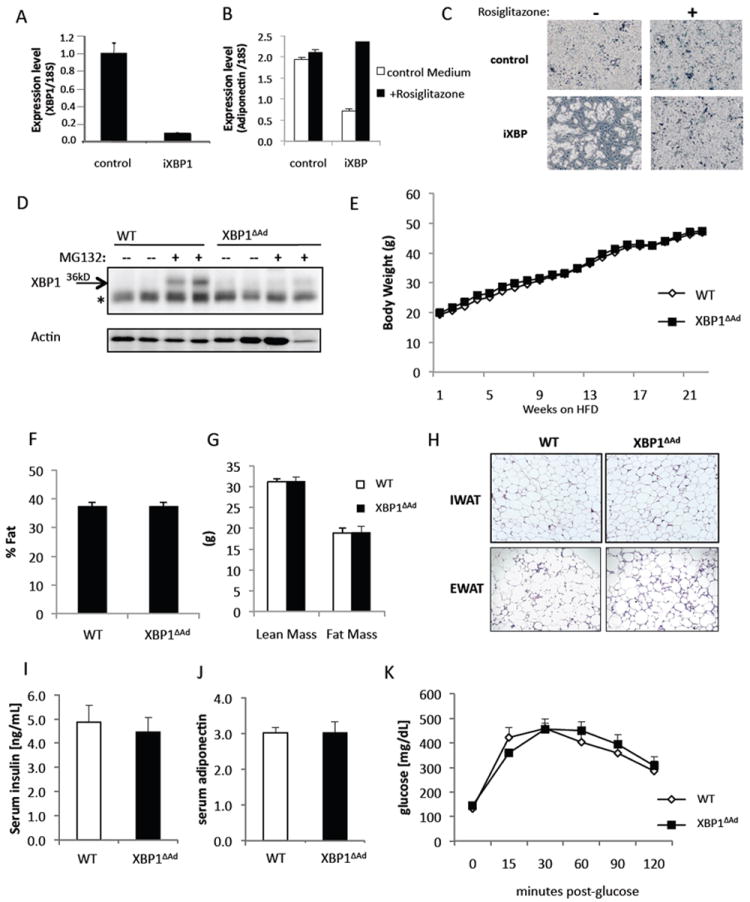
(A-C) Lentiviral suppression of XBP1 mRNA in 3T3L1 preadipocytes. (A) Xbp1 mRNA levels were measured by real-time quantitative RT-PCR (QPCR). Preadipocytes carrying control or XBP1 shRNA (iXBP1) were induced to differentiate with or without rosiglitazone (10uM). (B) On day 8 of differentiation, mRNA levels of the Adiponectin gene were measured by QPCR and (C) bright phase microscopy images were taken. (D) XBP1 protein levels in fat explants from WT and XBP1ΔAd mice after treatment with or without protease inhibitor MG132 (25μM) for 20 hours to stabilize XBP1 protein. Protein extracts were probed using XBP1 or Actin antibody (Santa Cruz). *denotes a non-specific band. (E-K) were performed with male mice (n=7-12) on high fat diet (HFD). (E) Body weight of WT and XBP1ΔAd mice over time on HFD. (F) Percent fat, (G) Lean Mass, and Fat Mass of WT and XBP1ΔAd mice (n=5-11) as measured by DEXA analysis. (H) Hematoxylin and Eosin staining (H&E) of adipose tissue sections from WT and XBP1ΔAd mice (Magnification 100x). Inguinal or epididymal white adipose tissue (IWAT or EWAT). (I) serum insulin and (J) adiponectin levels in WT and XBP1ΔAd mice (n=5,6). (K) Glucose tolerance test performed after 16 weeks on HFD with (1.0 g/kg glucose injection, n=6). All error bars indicate +/- SEM. See also Figure S1.
XBP1 is regulated during lactation in adipocytes
Next, we tested another extreme condition of adipocyte transformation: lactation. Throughout pregnancy maternal body weight increases, and after parturition, lactation commences and adipose tissue lipid stores are utilized as components of or energy for the production of milk. In order to achieve this partitioning of nutrients, adipocytes undergo a dramatic transformation involving severe lipid depletion and suppression of glucose and lipid uptake. Although this phenomenon has been observed morphologically (Elias et al., 1973), little is known about the functional role of the adipocyte during lactation or any molecular mediators involved in this process.
To determine the potential relevance of XBP1 to adipocytes during lactation, we examined the regulation of Xbp1 expression in vivo in adipose tissue during pregnancy and throughout lactation. In gonadal adipose tissue, a depot that is devoid of mammary gland, we observed significant upregulation of total and spliced forms of Xbp1 mRNA during lactation but not pregnancy, corresponding to the time of substantial adipocyte transformation (Fig.2A). We also observed increased total but not spliced Xbp1 mRNA in the inguinal adipose depot, which develops into mammary gland during lactation (Fig.S2A). In order to explore XBP1 regulation further, we treated cultured adipocytes with prolactin, the primary lactogenic hormone orchestrating milk production (Fig. 2B-C). Previous studies have identified the prolactin receptor on mouse and human adipocytes and have shown that its expression increases in adipose tissue during lactation (Flint et al., 2003; Ling et al., 2000). We found that exposure of adipocytes to prolactin in vitro resulted in increased total and spliced Xbp1 expression (Fig.2B,C), similar to the patterns seen in lactating tissues in vivo. The effect of prolactin in adipocytes also revealed no changes or mild increases in other UPR markers. These results warrant further investigation into the role of the UPR in adipocytes during lactation and may suggest a potential distinctive role for adipocyte XBP1.
Figure 2. Adipocyte XBP1 deletion disturbs metabolic flux during lactation.
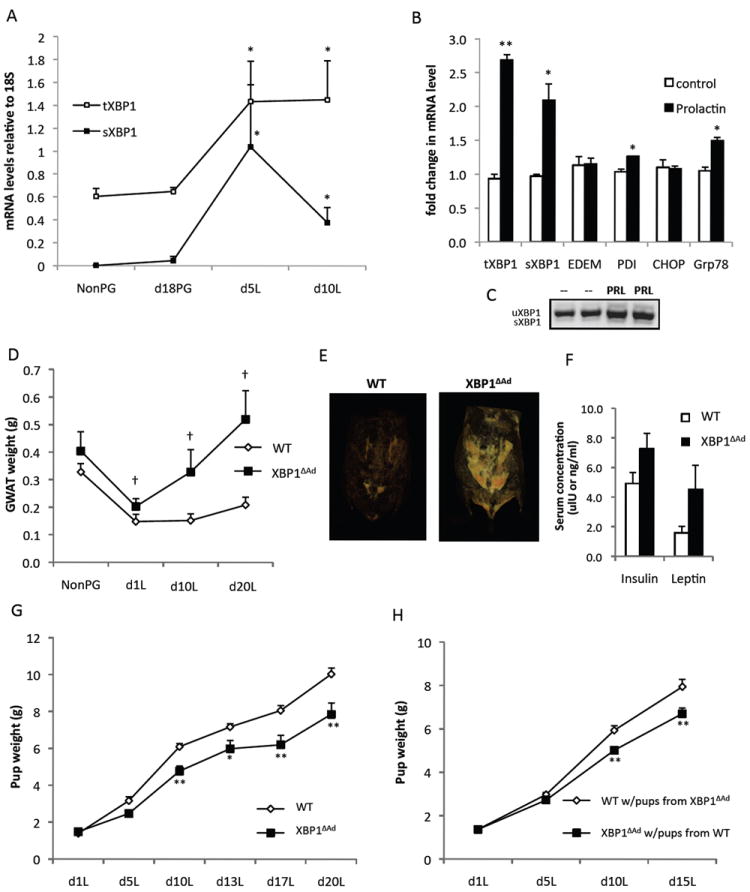
(A) mRNA levels of Xbp1 in gonadal adipose tissue from WT mice that were non-pregnant (NonPG), in late pregnancy (d18PG), or in day 5 or 10 of lactation(L). (B) Fully differentiated adipocytes were treated with or without prolactin for 6 hours and mRNA levels of UPR genes were measured by QPCR. (C) Both spliced and unspliced forms of XBP1 are shown. (D) Gonadal white adipose tissue (GWAT) wet weights of WT and XBP1ΔAd dams from non-pregnant (NonPG) and day 1, 10 and 20 lactation (L) timepoints (n=4-9). (E) Representative images from CT scans of WT and XBP1ΔAd dams during peak lactation (day 12). Adipose tissue density is displayed. (F) Serum insulin and leptin levels of WT and XBP1ΔAd dams at day 20 lactation (n=3). (G) Pup weights of litters nursed by WT or XBP1ΔAd dams during lactation. All litters are mixtures of pup genotypes (WT and XBP1ΔAd) (n=6-10 litters per genotype). (H) Cross-foster of XBP1ΔAd and WT litters. Average pup weight during lactation from cross-fostered litters switched on day 1 lactation (n=8-10, litters were pared to 6 pups). All error bars indicate +/- SEM. *p<0.05, **p<0.01 using standard t-test. † denotes p<0.007 as measured by regular 2-way ANOVA. See also Figure S2.
Loss of adipocyte XBP1 disturbs lactational metabolism
Interestingly, we observed that when XBP1ΔAd dams underwent lactation, they displayed an increase in adiposity compared to WT controls (Fig.2D,E). Under normal conditions, metabolic characteristics of female XBP1ΔAd mice such as body weight, adipose tissue weight, blood glucose, serum insulin and lipid levels were similar to WT controls (Table S1). In addition, during pregnancy, no significant differences were observed between WT and XBP1ΔAd dams in terms of body weight, blood glucose or insulin levels, litter weights upon delivery, and number of pups per litter (Fig.S2B-F). Food intake during pregnancy and lactation was also similar between WT and XBP1ΔAd dams (Fig.S2G,H).
However, during lactation adipose tissue mass was significantly increased in the XBP1ΔAd dams, while it remained low in the WT dams compared to non-pregnant mice (Fig. 2D,E). We also observed increases in serum insulin and leptin levels, and a trend toward increased total body weight in XBP1ΔAd dams, again indications of increased adiposity (Fig.2F, S2I). Strikingly, litters from XBP1ΔAd dams gained less weight during lactation than those of WT dams (Fig.2G). It was then critical to examine whether maternal or fetal genotype was the driver of this phenotype and whether WT dams could rescue this effect. For this, we performed cross-fostering experiments. Nursing of XBP1ΔAd pups by WT dams completely rescued the pup phenotype and, conversely, WT pups nursed by XBP1ΔAd dams showed a significant decrease in body weight during lactation (Fig.2H). These experiments demonstrated that the effect on pup weight resulted from the mother’s genotype during lactation and was not the result of an in utero effect. Taken together, these results indicate that lactating XBP1ΔAd dams harbor increased adipose tissue lipid stores, and insufficient nutrients are reaching the pups during lactation.
XBP1 contribution to lactation performance is adipocyte-specific
As participation of adipocytes in the metabolic transition of lactation is an unexpected finding, it was critical to ensure that the observed effects arose from adipocyte XBP1-deficiency, per se. Inguinal adipocytes are in close proximity to the mammary gland during lactation as they share the same depot. In contrast, visceral and gonadal adipose tissues do not contain any mammary gland. To verify the specificity of the adipocyte deletion in the loxP-Cre model we first performed fractionation of mammary tissue from day 1 and day 10 of lactation. Figure 3A reveals that Xbp1 is dramatically deleted in the floating adipocytes of the XBP1ΔAd tissues compared to the stromal-vascular fractions. Second, we performed laser capture microdissection of mammary alveoli from day 1 lactation tissues and demonstrated that no deletion of Xbp1 occurred in the captured cells (Fig.3B), indicating again the adipocyte-specificity of the model employed in our study. Since regulation of lactation through adipocyte XBP1 is a central tenant of our observations, we also performed additional genetic experiments to validate the adipocyte-specificity of the effects of XBP1. First, we examined a potential contribution from macrophages since the aP2 promoter can drive gene expression in these cells (Makowski et al., 2001). For this, we tested the effects of a macrophage-specific deletion of XBP1 in our model using the LysM-driven Cre to delete XBP1 in the myeloid lineage. Loss of macrophage XBP1 did not affect pup growth during lactation, ruling out a contribution of these cells to the phenotype (Fig.3C). Next, we utilized a second independent adipocyte-specific XBP1-deletion model, with Cre expression driven by the Adiponectin promoter (Eguchi et al., 2011). Importantly, litters from these XBP1ΔAd dams also displayed significantly decreased growth during lactation compared to litters of WT dams (Fig. 3D).
Figure 3. XBP1 is deleted specifically in the adipocytes of the mammary gland.
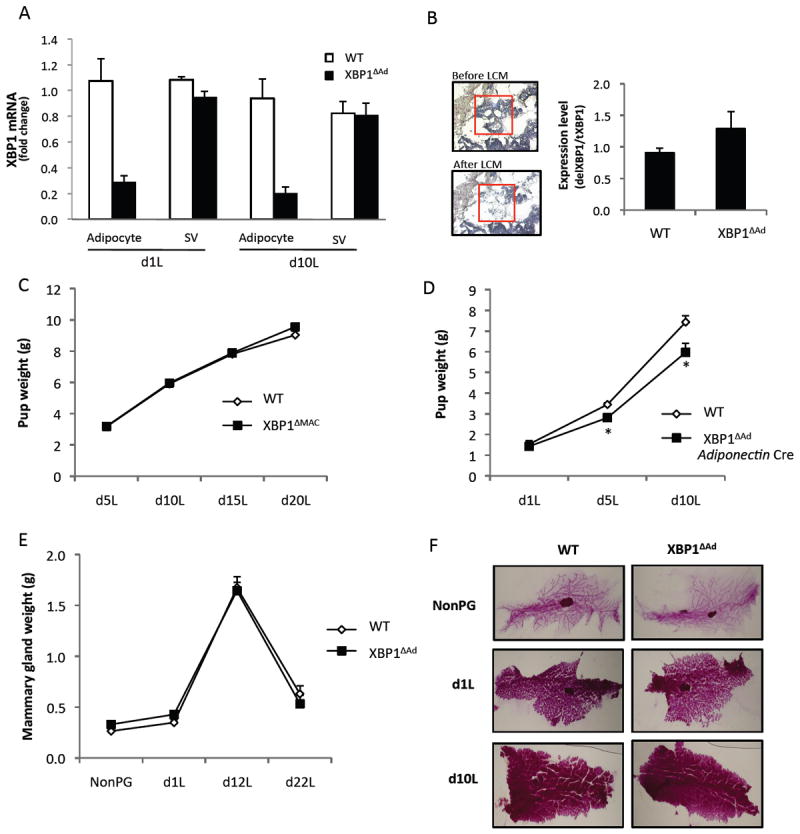
(A) Xbp1 mRNA levels in fractionated mammary tissue from day 1 and day 10 lactation. Tissues from WT and XBP1ΔAd mice were separated into adipocyte and stromal-vascular (SV) fractions (n=4-5). (B) Laser Capture Microdissection (LCM) of mammary epithelial cells from mammary glands on day 1 lactation. Total RNA was extracted from the microdissected cells to determine levels of Xbp1 deletion. Pictures of histology illustrate the area of capture before and after dissection. (C) Average pup weights of litters nursed by WT and XBP1ΔMAC(LysM Cre) dams throughout lactation (n=5-6). (D) Average pup weights of litters nursed by WT and Adiponectin Cre XBP1ΔAd dams throughout lactation (n=5-6). (E) Mammary gland wet weights of WT and XBP1ΔAd dams (n=4-9). (F) Whole mount stains of mammary glands from WT and XBP1ΔAd mice at different stages of mammary development (non-pregnant (NonPG), day 1 or day 10 lactation). All error bars indicate +/- SEM. *denotes p<0.05 using standard t-test. See also Figure S3.
We next examined WT and XBP1ΔAd mammary glands for morphological or developmental differences. In virgin mice we observed slightly reduced mammary gland branching post-puberty in XBP1ΔAd glands compared to WT. However, when mammary epithelia from XBP1ΔAd glands were isolated and transplanted into a WT recipient fat pad, mammary gland branching was indistinguishable between genotypes (Fig.S3A,B), indicating again that it is the XBP1-deleted adipose tissue in our model that influences the mammary gland environment.
Next, we analyzed tissue weights and also utilized whole mount staining technique to visualize the development of the mammary epithelia and alveoli during lactation. In these experiments, we did not observe any differences in tissue weight, morphology or extent of development in the XBP1ΔAd glands compared to WT controls (Fig.3E,F). Cross-sections of the tissues stained by H&E also showed similar mammary development between WT and XBP1ΔAd tissues (Fig.S3C). We also did not observe any large cytoplasmic lipid droplets apparent in the alveolar epithelium, which would indicate a secretory defect. Finally, as a marker of prolactin action, we measured Stat5 signaling in lactating mammary gland tissues from WT and XBP1ΔAd mice and observed no significant differences between genotypes (Fig.S3D,E). Hence, the data obtained thus far suggests normal mammary gland function in XBP1ΔAd dams.
In sum, the results obtained in examination of tissues, ex vivo investigations, and in multiple independent tissue-specific deletion models in vivo demonstrate that XBP1 deletion in the adipocyte is the causative manipulation that produces a disturbed lactational metabolism.
Analysis of milk composition and quantity in XBP1ΔAd dams
Since adipose tissue lipids are partitioned to the mammary gland during lactation, it was possible that the gonadal accumulation of lipid in XBP1ΔAd dams would affect milk composition. We performed lipidomic analysis of the major lipid classes and fatty acid composition of those classes in milk produced from each genotype. No significant differences were found among the studied lipid classes in XBP1ΔAd milk compared to WT (Fig.4A,B & Fig.S4A). Additionally, fatty acid composition was not altered in XBP1ΔAd milk (Fig.4C) and cluster analysis did not indicate inherent differences even when the whole data set was incorporated into the comparisons (Fig.S4B). We also measured lactose and total protein levels in XBP1ΔAd milk and found these constituents were similar to WT milk (Fig.4D,E). Silver staining of milk proteins also did not reveal any gross differences (Fig.S4C). Therefore, all of the major components tested show no effect on milk composition in XBP1ΔAd dams that would support a content-related defect. Hence, we moved on to test if the amount of milk produced was affected by adipocyte deletion of XBP1. Indeed, when milk quantity was measured in pups before and after controlled feeding, the XBP1ΔAd dams produced significantly decreased amounts of milk compared to WT controls (Fig.4F). This reduction in milk production was unlikely to result from a lack of sufficient prolactin release upon suckling since serum prolactin levels in the lactating dams were not different between genotypes (Fig.4G). Decreased milk volume can be associated with a reduction in mammary gland lipogenesis (Boxer et al., 2006). Therefore we examined lipogenic enzymes in lactating mammary glands of WT and XBP1ΔAd dams. Our analysis revealed no significant differences in the mRNA levels of lipogenic genes Fas, Scd1, Dgat2 and also similar protein levels of FAS, SCD1, ACC, and ACLY in WT and XBP1ΔAd dams (Fig.S4D,E). In addition, we measured phosphorylation of lipogenic enzymes ACC and ACL to assess pathway activity and observed no significant differences between genotypes (Fig. S4E,F). Hence, with the measures employed in this study, XBP1ΔAd mammary gland lipogenesis appears normal. In sum, although the mechanism underlying less milk volume in the XBP1ΔAd dams is unclear at present, we conclude that adipocyte deletion of XBP1 does not impact milk composition but results in decreased milk production during lactation.
Figure 4. Adipocyte XBP1 affects milk quantity and lipogenic gene expression.
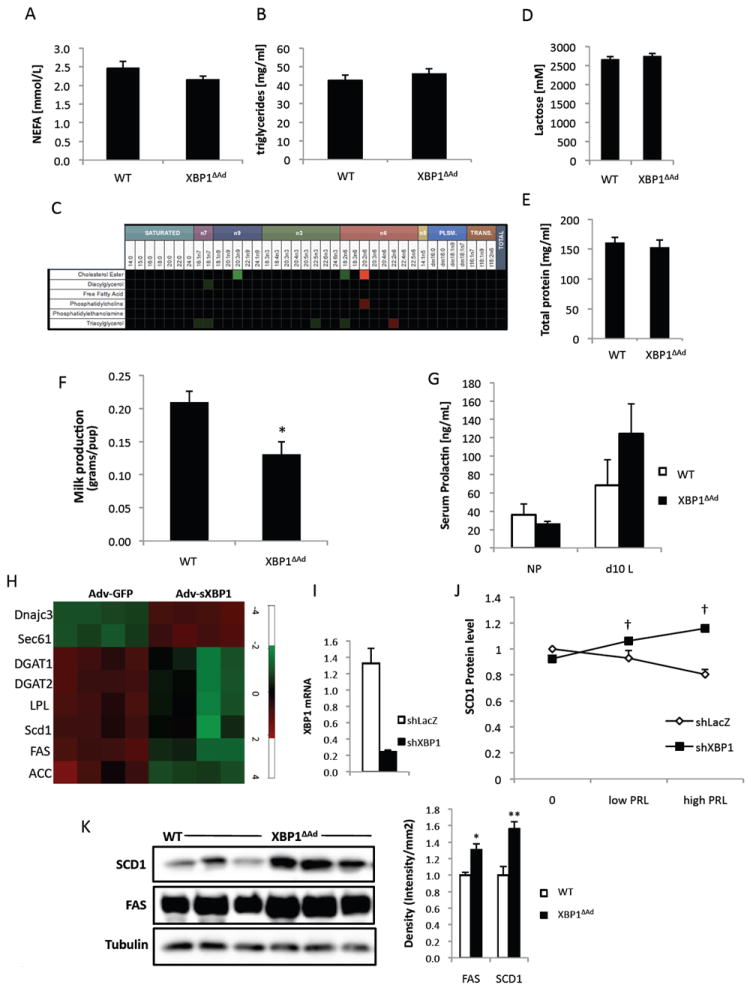
Milk was collected from WT and XBP1ΔAd dams at day 12 lactation. (A) non-esterified fatty acid (NEFA) and (B) triglyceride levels in milk. (C) Heatmap of lipidomics analysis in XBP1ΔAd and WT milk. (D) Lactose and (E) total protein concentrations in WT and XBP1ΔAd milk. (F) Milk quantitation assay. Amount of milk produced from each dam was measured as difference in pup weight before and after a controlled 1.5 hour feeding. Litters were pared to 6 pups. (G) Serum prolactin in NP and d10L WT and XBP1ΔAd mice. *p<0.02 using standard t-test. (H) Heatmap of gene expression from F442A adipocytes overexpressing sXBP1 or GFP control. (I) QPCR demonstrating suppression of Xbp1 mRNA by adenoviral shRNA. (J) Graph of densitometry data for protein levels of SCD1 in cultured adipocytes during low or high (50-500ng/ml) prolactin treatments. SCD1 protein levels were normalized to adiponectin. Basal WT SCD1 protein level was set at 1. (K) Western blot analysis of FAS, SCD1, and Tubulin proteins in gonadal adipose tissue from d10 lactation. Densitometry measures are provided in the graph to the right. All error bars indicate +/- SEM. *p<0.05, **p<0.004 using standard t-test. † denotes p<0.004 as measured by regular 2-way ANOVA. See also Figure S4.
XBP1 regulates the lipogenic program in adipocytes
While the particular role of the adipocyte during lactation has not yet been defined, it has long been appreciated that adipose tissue must moderate lipogenesis and lipid storage activities in order to direct dietary nutrients to the mammary gland to support milk production. The adipocytes themselves show decreased lipid uptake and lipogenic activity for the sake of re-directing nutrients to the milk-producing secretory cells (Bell and Bauman, 1997; Vernon and Pond, 1997). In more specific studies investigating the effects of prolactin on adipocytes, the results have been inconsistent and often species-dependent (Ben-Jonathan et al., 2008). For instance, in mice prolactin is thought to not affect lipolysis. Indeed, when we treated adipose tissue explants with prolactin, we also did not observe a lipolytic effect of prolactin, or any differences between WT and XBP1ΔAd lipolytic activity (Fig.S4G,H). Prolactin has also been reported to decrease glucose uptake in rodent adipocytes and reduce expression of the lipogenic gene fatty acid synthase (FAS) (Flint et al., 2003; Hogan and Stephens, 2005), hypothetically leading to less lipogenesis and nutrient redirection to the mammary gland. We reasoned that since XBP1 is regulated by prolactin in adipocytes and is also involved in lipid metabolism in liver (Deng et al., 2013; Lee et al., 2008; Wang et al., 2012), it may be involved in the regulation of the adipocyte lipogenic program during lactation. To investigate the function of XBP1 in adipocytes, we first overexpressed sXBP1 which resulted in the expected upregulation of XBP1 target genes such as Sec61 and Dnajc3, validating our experimental system (Fig.4H). Of note, sXBP1 expression in adipocytes resulted in a broad down-regulation of lipogenic/lipid storing genes such as Scd1, Fas, Acc, Dgat1/2 and Llp (Fig.4H). Next, we suppressed Xbp1 expression in adipocytes through a viral shRNA and examined the effect of prolactin on a key lipogenic enzyme, stearyl CoA desaturase 1 (SCD1). Interestingly, prolactin treatment caused an increase in SCD1 protein expression in shXBP1 cells (Fig.4I,J). We also tested these observations in vivo. Importantly, gonadal adipose tissue from XBP1ΔAd dams during lactation showed higher levels of FAS and SCD1 proteins (Fig.4K), consistent with the data from prolactin-treated adipocytes in vitro. These data indicate that XBP1 may be a critical factor in prolactin-mediated effects on adipocytes given its regulation and requirement for suppressing lipogenic genes in a prolactin-rich environment.
Discussion
The adipocyte is an extraordinary cell type poised to act at the intersection of energy supply and demand. Capable of extreme lipid storage under conditions of energy surplus such as obesity, the adipocyte can also liberate its lipid stores dramatically when called upon by the demand of the organism, such as during lactation. We were surprised to find that although the UPR transcription factor XBP1 can influence in vitro differentiation, adipocyte formation and function were not altered when XBP1 was deleted in adipocytes in vivo, either under normal or high fat diet conditions. Although obesity induces UPR activation in adipose tissue, it may be that under metabolic stress other UPR branches compensate for the loss of XBP1 and this results in little to no phenotype (Hotamisligil, 2010). Indeed, it is possible that XBP1 plays a smaller role in the UPR response of the obese adipocyte but has a distinct role in other processes that demand extreme fluctuations of adipose depots and their function. Supporting this claim, deletion of adipocyte XBP1 resulted in increased maternal adipose tissue, reduced milk production, and reduced weight gain in XBP1ΔAd litters during lactation. Furthermore, we showed that prolactin induces XBP1 expression and suggest that in response to prolactin XBP1 functions in adipocytes to maintain a low-level lipogenic activity. We postulate that without XBP1, adipose tissue accumulates lipid during lactation, milk production is deprived of this energy, and this results in less milk and therefore less weight gain for the pups. Interestingly, poor lactation performance is associated with increased adiposity or obesity in women and also cows, referred to as ‘fat cow syndrome’ (Rasmussen, 2007). However, the biological mechanisms underlying these observations have remained enigmatic.
Decreased pup weight from a lactation defect has been linked to low triglyceride levels or increased viscosity of the milk making it difficult to release (Schwertfeger et al., 2003; Zhu et al., 2005). Others have reported similar composition but decreased volume of milk attributable to decreased mammary gland lipogenic activity (Boxer et al., 2006; Rudolph et al., 2010). We have not seen any alterations in milk composition or evidence of decreased lipogenic activity in the mammary gland, but further investigation of these aspects may be fruitful. Therefore, we suggest that mammary gland function may be unchanged in the XBP1ΔAd mice and that signals from adipose tissue are needed to maintain not the developmental or functional integrity but the level of production of milk. Interestingly, two hormonal signals, insulin and leptin, are elevated in the serum of the XBP1ΔAd dams during lactation and these hormones are also increased in obese women. Therefore, it will be worth investigating the effects of the mother’s hormonal milieu on milk production. It is also possible that other actions of XBP1, such as synthesis and secretion of an unknown mediator to the mammary gland or an immunological response, could be involved in the effect of adipocyte function during lactation.
Thus, we suggest that this work introduces a new context in which to study adipocyte function and biology, as well as UPR, given that the majority of studies involving adipocytes have focused on metabolic homeostasis. Here we reveal the adipocyte’s influence on the directional or homeorhetic metabolism of lactation, and anticipate future studies will uncover further essential adipocyte functions in this vital process of early mammalian growth and survival.
Experimental Procedures
Generation and breeding of XBP1ΔAd mice
C57BL/6 mice harboring loxP sites around exon 2 of XBP1 (Lee et al., 2008) were crossed to mice carrying the Cre recombinase gene under the aP2, adiponectin, or LysM promoters, all three of the C57BL/6 genetic background. Breeding strategy was followed so that control (XBP1 flox/flox-no Cre) and experimental mice (XBP1flox/flox-Cre) were always littermates. Female mice used for the pregnancy and lactation studies came from crosses of flox/flox-no Cre females to flox/flox Cre males and were fed standard breeding chow (PicoLabs, Mouse Diet 20). In all experiments pup numbers from WT and XBP1ΔAd litters were similar. The Institutional Animal Care and Use Committee of the Harvard School of Public Health approved all studies.
Laser capture microdissection
Frozen mammary tissue sections of 5μm thickness from day 1 lactation were H&E stained 20 minutes prior to laser capture microdissection (LCM). LCM was performed at the Specialized Histopathology Core in the Dana-Farber/Harvard Cancer Center in Boston, MA, using the Arcturus PixCell II instrument along with Macro CapSure Caps (Molecular Devices). For each animal, one slide of two serial sections was captured for 50-60 minutes at laser beam size of 7.5μm with a power of 50mW. RNA extraction of the isolated cells was performed using the PicoPure RNA Isolation kit (Molecular Devices) and cDNA was synthesized from the total RNA extracted (Fermentas). Levels of deleted (primers specific to exon 2) and total (primers not in exon 2) Xbp1 mRNA were assessed by quantitative RT-PCR.
Preparation of mammary gland whole mounts
Whole mount preparations of mammary glands were performed according to the protocol found on the NIH Biology of the Mammary Gland website (http://mammary.nih.gov/tools/histological/Histology/index.html). Briefly, inguinal #4 mammary glands were dissected, spread on glass slides, and fixed overnight in Carnoy’s fixative. The next day, tissues were hydrated and then stained in Carmine-Aluminum stain overnight. Tissues were dehydrated, cleared in xylene, and mounted between two glass slides using Permount mounting media.
Adipocyte cell culture
3T3L1 and F442A preadipocytes were maintained in DMEM supplemented with 10% Bovine Calf serum. To induce differentiation, 3T3L1 cells were grown to confluence and fed induction medium (DMEM, 10% Cosmic Calf serum, 5 μg/ml insulin, 0.5mM IBMX, 1μM dexamethasone, with or without 10μM Rosiglitazone). After two days, the medium was changed to DMEM, 10% CCS and 5μg/ml insulin. F442A cells were differentiated in only the DMEM-CCS-Insulin medium. Adipocytes were considered fully differentiated at day 8.
CT scan imaging
Mice at day 12 lactation were anesthetized and scanned using the GE explore CT 120 Micro-CT scanner. Data capture and reconstructions of the adipose tissue were done using Osirix software.
Milk collection and analysis
Following overnight pup removal to facilitate milk accumulation, mice were anesthetized at day 12 lactation. Milk was collected by gentle manual nipple stimulation. Lactose was measured per kit instructions (Abcam) and total protein was measured via the DC Protein Assay (Bio-Rad). Lipid extraction was done on a 5μl sample of milk and triglyceride and free fatty acid content was determined using Sigma and WAKO diagnostics assays, respectively. In depth lipidomics analysis was performed as described (Cao et al., 2008) by Lipomics Inc.
Milk quantitation
Quantitation of milk production was performed as previously described (Jara-Almonte and White, 1972). Briefly, 10-day-old pups were removed from dams and fasted for 6 hours. Pups were then returned to dams for 1.5 hours and litter weights were taken before (post-fast) and after milking. The difference in litter weight represents the amount of milk consumed.
Supplementary Material
Acknowledgments
This study is supported in part by a grant from NIH to GSH (DK052539). We thank Evan Rosen for the generous gift of Adiponectin Cre mice ahead of publication. We also thank A. White, S. Widenmaier, and S. Watkins for help with animal experiments, lactose measurements and lipidomic analyses. MFG is supported by the NIH Environmental Health Training grant T32 ES007155-24 and the Donald and Sue Pritzker Scholar award.
Footnotes
Author contributions: MFG designed, performed, and analyzed experiments and wrote the manuscript. ESM, LY, KI, SH and BB performed and analyzed experiments. AHL provided the XBP1-flox mice. GSH designed and analyzed experiments and co-wrote the manuscript.
References
- Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res. 2009;50(Suppl):S395–399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseri S, Lhotak S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50:2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 1997;2:265–278. doi: 10.1023/a:1026336505343. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, Belka GK, Cho H, Rathmell JC, Thompson CB, Birnbaum MJ, Chodosh LA. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006;4:475–490. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wang ZV, Tao C, Gao N, Holland WL, Ferdous A, Repa JJ, Liang G, Ye J, Lehrman MA, Hill JA, Horton JD, Scherer PE. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J Clin Invest. 2013;123:455–468. doi: 10.1172/JCI62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JJ, Pitelka DR, Armstrong RC. Changes in fat cell morphology during lactation in the mouse. Anat Rec. 1973;177:533–547. doi: 10.1002/ar.1091770407. [DOI] [PubMed] [Google Scholar]
- Flint DJ, Binart N, Kopchick J, Kelly P. Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary. 2003;6:97–102. doi: 10.1023/b:pitu.0000004800.57449.67. [DOI] [PubMed] [Google Scholar]
- Glimcher LH. XBP1: the last two decades. Ann Rheum Dis. 2010;691(Suppl):i67–71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- Hogan JC, Stephens JM. The regulation of fatty acid synthase by STAT5A. Diabetes. 2005;54:1968–1975. doi: 10.2337/diabetes.54.7.1968. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-Almonte M, White JM. Milk production in laboratory mice. J Dairy Sci. 1972;55:1502–1505. doi: 10.3168/jds.S0022-0302(72)85703-5. [DOI] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Hellgren G, Gebre-Medhin M, Dillner K, Wennbo H, Carlsson B, Billig H. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology. 2000;141:3564–3572. doi: 10.1210/endo.141.10.7691. [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–121. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC. Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab. 2010;299:E918–927. doi: 10.1152/ajpendo.00376.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res. 2003;44:1100–1112. doi: 10.1194/jlr.M300045-JLR200. [DOI] [PubMed] [Google Scholar]
- Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RG, Pond CM. Adaptations of maternal adipose tissue to lactation. J Mammary Gland Biol Neoplasia. 1997;2:231–241. doi: 10.1023/a:1026380220364. [DOI] [PubMed] [Google Scholar]
- Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, Davidson NO, Kaufman RJ. IRE1alpha-XBP1s Induces PDI Expression to Increase MTP Activity for Hepatic VLDL Assembly and Lipid Homeostasis. Cell Metab. 2012;16:473–486. doi: 10.1016/j.cmet.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Anderson GW, Mucha GT, Parks EJ, Metkowski JK, Mariash CN. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology. 2005;146:3343–3350. doi: 10.1210/en.2005-0204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


