Abstract
MSCs are hypothesized to potentially give rise to sarcomas after transformation and therefore serve as a good model to study sarcomagenesis. Both spontaneous and induced transformation of MSCs have been reported, however, spontaneous transformation has only been convincingly shown in mouse MSCs while induced transformation has been demonstrated in both mouse and human MSCs. Transformed MSCs of both species can give rise to pleomorphic sarcomas after transplantation into mice, indicating the potential MSC origin of so-called non-translocation induced sarcomas. Comparison of expression profiles and differentiation capacities between MSCs and sarcoma cells further supports this. Deregulation of P53- Retinoblastoma-, PI3K-AKT-and MAPK pathways has been implicated in transformation of MSCs. MSCs have also been indicated as cell of origin in several types of chromosomal translocation associated sarcomas. In mouse models the generated sarcoma type depends on amongst others the tissue origin of the MSCs, the targeted pathways and genes and the differentiation commitment status of MSCs. While some insights are glowing, it is clear that more studies are needed to thoroughly understand the molecular mechanism of sarcomagenesis from MSCs and mechanisms determining the sarcoma type, which will potentially give directions for targeted therapies.
Keywords: MSC, Sarcoma, Bone tumour, Soft tissue tumour, Osteosarcoma, Ewing sarcoma
Introduction
MSCs have been under intensive research and application efforts since their first establishment by Friedenstein and his colleagues in 1968 [1]. Standard criteria developed by the International Society for Cellular Therapy define MSCs by three characteristics: 1) plastic adherence under standard culture conditions, 2) expression of CD105, CD73 and CD90 and no expression of CD45, CD34, CD14, CD11b, CD79b, CD19 and HLA-DR and 3) capacity to differentiate into osteoblasts, chondroblasts and adipocytes in vitro, termed trilineage differentiation potential (Figure 1) [2].
Figure 1.
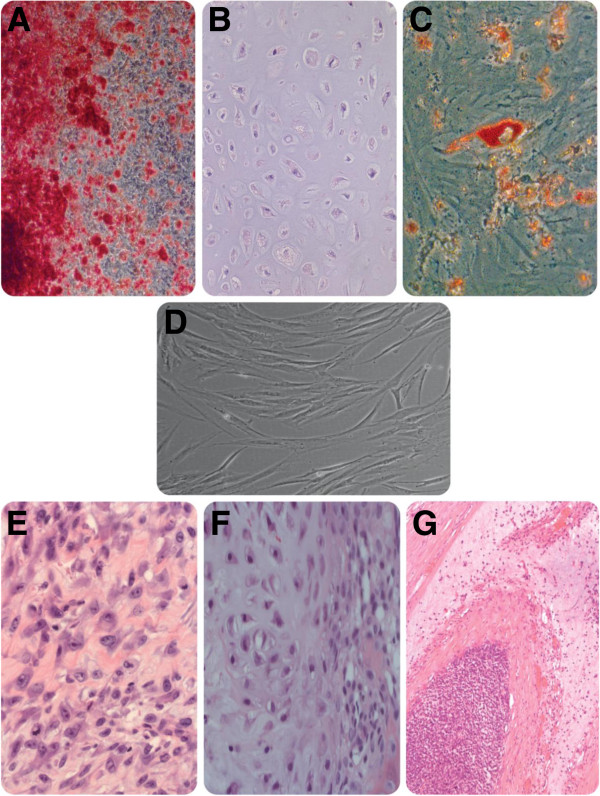
Trilineage differentiation capacity of human MSCs and representative types of sarcomas. Human MSCs are capable of differentiating into osteoblasts, chondrocytes and adipocytes under proper inductions. This differentiation spectrum corresponds with the histological spectrum of different types of sarcomas, represented here by osteosarcoma, chondrosarcoma and liposarcoma. This correlation supports the hypothesis that MSCs are the cell of origin of sarcomas. A: alizarin red staining for osteoblast differentiation assay, B: toluidine blue staining for chondrocyte differentiation assay of MSC pellets, C: oil red staining for adipocyte differentiation. D: human MSC cell culture. E: osteosarcoma, F: chondrosarcoma, G: liposarcoma.
Owing to the ease of isolation, expansion, the multi-lineage differentiation potential and a variety of physiological functions, MSCs are applied in a wide range of experimental and medical applications. Among them are the enhancement of hematopoietic stem cell engraftment, the amelioration of acute graft-versus-host disease, cardiac diseases and regenerative medicine approaches for especially bone and cartilage [2].
Cell transformation is a process during which genetic changes occur, resulting in cells with the ability to grow indefinitely and anchorage-independently and with tumorigenic properties upon transplantation [3-7]. Senescence has been overcome in these transformed cells [4,8-10]. On one hand, the potentials of MSCs to transform, to initiate sarcomas and under some conditions to facilitate tumour progression are calling for caution for MSC-based applications [5,11]. On the other hand, the transforming property of MSCs and their possible role as sarcoma progenitors make these cells useful for studying sarcomagenesis and progression. In this review we present an overview of the roles of MSCs in sarcomas, with a specific focus on tumorigenic transformation and sarcomagenesis.
MSC transformation
Spontaneous mouse MSC transformation
Mouse MSCs have been consistently demonstrated to spontaneously undergo tumorigenic transformation after long term ex vivo culture [4,6]. This transformation process can also be induced by certain manipulations, including both gene targeting and drug or chemical treatment to affect crucial pathways (Table 1) [11-14]. In contrast, human MSCs do not spontaneously transform in vitro, even after long term culturing, which will be discussed later in more detail [9,13,15].
Table 1.
A summary of spontaneous transformation studies with mouse BMMSCs
| Transformation after | Type of sarcoma | Associated genetic event(s) | Reference |
|---|---|---|---|
| Long term culture |
Osteosarcoma |
Aneuploidy + CDKN2A/p16 loss |
[4] |
| Abnormal karyotype |
[6] |
||
| Fibrosarcoma |
p53 mutations |
[24] |
|
| Chromosomal instability + TERT and c-myc expression |
[16] |
||
| Undiff. soft tissue sarcomas |
Aneuploidy + chromosomal translocations |
[17] |
|
| Short term culture | Soft tissue sarcomas | Aneuploidy | [18] |
Mouse MCSs are reported to spontaneously undergo changes in morphology, proliferation rate, migration ability, cell surface marker profile, genomic constitution and most importantly tumorigenicity after long term in vitro culture [4,9,20,21]. Meanwhile, one study has also revealed that mMSCs could transform even after short term in vitro culture. Injection of passage 3 mMSCs into mice resulted in formation of tumours comparable with soft tissue sarcomas [19]. Transformed mouse MSCs always show a higher proliferation rate than the native cells [3,4]. These transformed cells exhibit tumorigenicity, as shown by anchorage-independent growth assay and xeno-transplantation in mice and zebrafish, while this is not observed with low passage mouse MSCs before their transformation [3,4,6,22]. Interestingly, the readiness of in vitro tumorigenic transformation seems to be a unique property of mouse MSCs since it is absent in most other mouse stem cells, including hematopoietic stem cells and embryonic stem cells [23]. This readiness can be probably ascribed to the genetic instability already shown in mouse MSCs very shortly after isolation from bone marrow, although the cytogenetic abnormalities in low passage mouse MSCs are considerably less in number than in transformed mouse MSCs [23]. Interestingly, MSC spontaneous transformation happens much less frequently in vivo, as shown by the low incidence of spontaneous sarcomagenesis in mice. This can be possibly explained by the different microenvironment of in vitro and in vivo conditions for MSCs. Solid research on the role of the in vivo niche of BMMSCs in guarding its genomic stability is needed to answer this question more exactly.
Induced mouse MSC transformation
Transformation of mouse MSCs has been induced by an array of manipulations, including knockout of tumour suppressor genes, overexpression of oncogenes and drug administration to affect signaling pathways. The pathways targeted by these manipulations are mostly involved in cell cycle checkpoint control, cell survival, proliferation and apoptosis (Table 2) [14]. In one study, loss of tumour suppressor P21 and Tp53 in mouse adipose derived MSCs (AMSC) induced in vitro transformation and in vivo so-called fibrosarcoma formation after transplantation [24]. In another study, both Tp53−/− Rb−/− and Tp53−/− mouse AMSCs were generated through Cre mediated excision of loxP flanked loci. Leiomyosarcoma-like tumours were developed in the in vivo tumorigenicity assays of these 2 types of mouse AMSCs [8]. The combination of Cdkn2a loss and C-myc overexpression in mouse BMMSCs gave rise to osteosarcomas accompanied by the loss of adipogenic differentiation capacity in transformed mouse BMMSCs [16]. Besides directly targeting in vitro cultured MSCs, several genetically engineered mouse models have been developed to investigate the effects of genes on transformation process. A conditional mouse model with Tp53 homozygous deletion has been created by crossing Prx1-Cre transgenic mice to mice bearing alleles of Tp53 flanked by loxP. Prx1 is specifically expressed in the early mesenchymal tissues of embryonic limb buds [17]. In these P53-deficient mice many types of sarcomas occurred in the mesenchymal cells of limb buds and osteosarcoma was the most common type. A mouse model with loss of RB generated also through Cre-loxP system was not found to display tumorigenesis. However, loss of RB accelerated tumorigenesis in P53-deficient mice [17]. These induced transformation studies established the importance of the P53 pathway in preventing mouse MSC transformation. Besides, in spontaneous transformation studies of mouse MSCs, defects in Tp53 or Cdkn2a genes were frequently found [18]. P53 and P14, proteins encoded by these two genes, are both important members of P53 pathway, further corroborating the crucial role of P53 pathway in mouse MSC transformation [4,25]. Upregulated oncogenic pathways have also been shown to induce or potentiate mouse MSC transformation. Fos is an oncogene encoding a transcription factor downstream of many growth factor pathways. The Fos overexpression transgenic mice resulted in the development of bone tumours, with chondrosarcomas as the main type [26]. This is puzzling as the driver mutation in human central chondrosarcoma is IDH1 or IDH2 [27], while in peripheral chondrosarcomas this is not known [27,28], but no indication for involvement of Fos is found [28,29]. The PI3K-AKT pathway is crucially involved in apoptosis and proliferation. In a study a mouse model with homozygous loss of Pten, a negative regulator of the PI3K-AKT pathway in smooth muscle lineage cells developed leiomyosarcomas [30,31]. The MAPK pathway is principally responsible for mitosis. Overexpression of K-ras, a component of the MARK pathway in addition to P53 loss induced sarcoma formation in mice more efficiently than in mice with P53 loss alone [32,33]. These studies underscore the role of different oncogenic pathways to promote mouse MSC transformation.
Table 2.
A summary of induced transformation studies with mouse cells
|
Sarcomas without specific chromosomal translocation | ||||
|---|---|---|---|---|
| Cell type | Inactivated gene(s) | Expressed gene(s)/treatment | Type of sarcoma | Reference |
| mASCs |
p21 + p53 |
- |
“Fibrosarcoma” |
[20] |
| p53 or p53 + Rb |
- |
Leiomyosarcoma |
[7] |
|
| BM-mMSCs |
INK4A/ARF |
c-myc |
Osteosarcoma |
[24] |
| Osteoblastic lineage |
p53 or p53 + Rb |
- |
Osteosarcoma |
[32,33] |
| Mesenchymal cells of limb buds |
p53 |
- |
Osteosarcoma |
[23] |
| p53 + Rb |
- |
Undifferentiated sarcoma |
[23] |
|
| Muscle, uterus |
p53 orINK4A/ARF |
K-RAS |
High-grade sarcoma with myofibroblastic differentiation |
[25] |
| Muscle |
p53 |
K-RAS |
Pleomorphic rhabdomyosarcoma |
[18] |
| Smooth muscle lineage |
PTEN |
- |
Leiomyosarcoma |
[17] |
| MSC progenitors |
APC |
- |
Aggressive fibromatosis |
[67] |
|
Sarcomas with specific chromosomal translocation | ||||
|
Cell type |
Expressed fusion gene |
Type of sarcoma |
Reference |
|
| BM-mMSCs |
EWS-FLI-1 |
Ewing sarcoma |
[63] |
|
| EWS-FLI-1 |
Ewing sarcoma |
[65] |
||
| FUS-CHOP |
Myxoid liposarcoma |
[11] |
||
| PAX3/7-FKHR |
Alveolar rhabdomyosarcoma |
[71] |
||
| mASCs |
FUS- CHOP |
Liposarcoma |
[11] |
|
| Mesenchymal cells of limb buds |
EWS-FLI-1 |
Ewing sarcoma |
[65] |
|
| Differentiated muscle cells (MYF6-expressing cells) | PAX3-FKHR | Liposarcoma | [70] | |
Human MSC transformation
Human MSCs have not been shown to undergo spontaneous transformation in vitro[9,15,43]. There have been few reports on spontaneous human MSC in vitro transformation, of which two turned out to be caused by contamination by tumour cell lines and were retracted afterwards [34,35,44]. Meanwhile, there are several studies demonstrating that human MSCs did not go through transformation in spite of long term in vitro culturing [12,15]. For the possibility of in vivo spontaneous transformation, there have been few cases of osteosarcoma genesis in patients infused with bone marrow MSCs for other diseases [45-47]. The majority studies of human MSC transformation are based on genetic approaches to knock out important tumour suppressor genes and overexpress certain oncogenes Table 3) [14]. In contrast to mouse MSC studies, four of the induced human MSC transformation studies consist of the exogenous expression of hTERT in human cells [38,48-50]. This may be attributed to the much shorter telomeres in human MSCs than their mouse counterparts, the much shorter life span of mice than human and the difference in telomere damage signaling pathways between mouse and human [41,50,51]. Consistent with mouse MSC studies, the disruption of cell cycle control machineries, exemplified by P53 and RB pathways are also important for human MSC transformation. For instance, the introduction of SV40-LT, which perturbs both P53 and RB proteins potently promoted human MSC transformation [38]. Furthermore, the overexpression of some oncogenes has also been shown to contribute to the transformation, such as H-RAS[5-7]. Although the definite spontaneous transformation capacity of mouse MSCs is not a mimicry of human MSCs, the signaling pathways underlying their tumorigenic transformation show high consistency, including the P53 pathway, RB pathway, PI3K-AKT pathway and MAPK pathway and so on.
Table 3.
A summary of human BMMSCs transformation without specific chromosomal translocation
| Type of sarcomas | Expressed gene(s)/treatment | Reference |
|---|---|---|
| Undifferentiated spindle cell sarcoma |
hTERT + HPV16 E6/E7 + SV40-ST + H-RAS |
[44] |
| hTERT + SV40-LT + H-RAS |
[13] |
|
| hTERT + H-RAS + BMI-1 |
[43] |
|
| Tumors with smooth muscle and bone properties |
hTERT4 |
[33] |
| Undifferentiated pleomorphic sarcomas | DKK1 + SV40-LT | [14] |
MSCs as the origin of sarcomas and tumour type specificity
There is substantial evidence supporting a MSC origin of a spectrum of sarcomas, both pleomorphic as well as translocation driven subtypes. In the non-translocation-driven sarcoma types, the correspondence between the differentiation capacity of MSCs and the histological spectrum of different types of sarcomas is reflected (Figure 1). Approaches and methods have also been used to investigate this hypothesis, including differentiation assays, expression profiling and Immunohistochemistry [52-54]. Based on the site of presentation, sarcomas can be categorized into bone tumours and soft tissue tumours. Based on genetic profiles, sarcomas can be categorized into two groups, one with relatively simple genetic alterations, either being associated with point mutations or reciprocal translocations, and the other with extensive genetic changes. Examples of the cytogenetically relatively simple group are alveolar rhabdomyosarcoma, myxoid liposarcoma, Ewing sarcoma and synovial sarcoma. Examples of the other group are leiomyosarcoma, undifferentiated pleomorphic sarcoma and osteosarcoma [55].
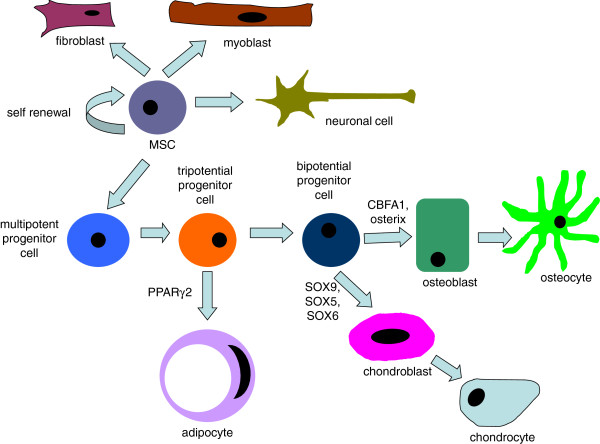
MSC differentiation towards a defined and differentiated cell type is a process with a lot of different signaling pathways and differentiation stages involved (Figure 2). The sarcoma type arising from in vitro transformed MSCs after inoculation into mice seems to be dependent on many factors, including the originating tissue of the MSCs, the differentiation commitment status of the targeted cell and also the targeted molecular pathways. In most cases with bone marrow derived mouse MSCs (BMMSC) or osteochondro progenitors osteosarcoma-like tumours were formed. With AMSCs or smooth muscle cell progenitors leiomyosarcomas were mostly formed (Table 4) [8,16,24]. BMMSCs from aged mice tend to spontaneously give rise to so-called fibrosarcomas instead of osteosarcomas as in most spontaneous transformation studies [25]. It must be added that according to the present view fibrosarcomas is a poorly defined histological entity. It is necessary to perform large scale studies to specifically address the relationship between tissue origin, targeted pathways and the sarcoma type generated, which is currently lacking.
Figure 2.
MSC differentiation scheme. Under different signaling regulations, MSCs can differentiate into different types of cells. The differentiation process involves sequential signaling regulation and many different stages.
Table 4.
A summary of sarcoma types from different transformation studies in mice
| Sarcoma type | Cell of origin | Targeted genes | Reference |
|---|---|---|---|
| Osteosarcoma |
Mouse BMMSCs |
- |
[4] |
| - |
[6] |
||
|
C-myc overexpression and Ink4a/Arf knockout |
[16] |
||
| Mouse osteoblast precursors |
Tp53, Rb double knockout |
[56] |
|
| Mouse osteoblasts |
Tp53knockout |
[57] |
|
|
Tp53 and Rb double knockout [57] | |||
|
Tp53 knockout |
[17] |
||
| Leiomyosarcomas |
Mouse AMSCs |
Tp53 knockout |
[14] |
|
Tp53 knockout |
[24] |
||
|
Tp53 and Rb double knockout [8] | |||
| Mouse smooth muscle lineage cells |
Pten knockout |
[30] |
|
| “Fibrosarcoma” |
Mouse BMMSCs |
- |
[58] |
| Aged mouse BMMSCs |
- |
[25] |
|
| Mouse AMSCs |
P21 knockout, Tp53 heterozygous knockout |
[24] |
|
| Pleomorphic rhabdomyosarcoma | Mouse skeletal muscle cells |
K-ras overexpression and Tp53 knockout |
[32] |
| K- ras overexpression and Tp53 heterozygous knockout [32] |
Bone sarcomas
Ewing sarcoma
Ewing sarcoma arises predominantly in bone but in soft tissues as well. It is a type of a poorly differentiated tumour known to be associated with a the expression of EWSR1–ETS fusions or rarely other chimeras [59-62]. The exogenous expression of the fusion gene EWS-FLI1 alone in mouse MSCs has been shown to transform these cells, demonstrated by in vitro immortalization and in vivo sarcomatous tumour formation after inoculation in immunocompetent mice [63]. In another study a secondary genetic alteration was needed for the induced transformation of mouse MSCs [64]. Similar manipulations have been also applied on human MSCs. Human MSCs with exogenous EWS-FLI1 expression transformed and these transformed cells expressed neuroectodermal markers [65]. Moreover, the knockdown of EWS-FLI1 expression in Ewing sarcoma cell lines restored the in vitro trilineage differentiation ability of the cells [52]. In a transgenic mouse model, by expressing EWS-FLI1 gene specifically in the mesoderm-originated tissues in limbs and simultaneous Tp53 knockout, sarcomas with similar characteristics as Ewing sarcoma occurred while with only Tp53 knockout the primary sarcoma type was osteosarcoma [66]. In brief, Ewing sarcoma, originally considered as tumours arising from the neuroectodermal lineage and not considered of mesenchymal origin could be experimentally derived directly from MSCs, but only upon introducing the typical translocation. This strongly supports an MSC origin of Ewing sarcoma [67].
Osteosarcoma
Osteosarcoma is the most common primary malignant bone tumour among children. It is characterized by the production of osteoid and extensive cytogenetic instability [36]. Different studies have supported the MSC origin of osteosarcoma [4,16]. Both spontaneous and induced MSC models for osteosarcoma have been discussed above. Osteosarcomas mainly arise in the metaphyses of long bones and the peak incidence is in the second decade of human life, correlating with the rapid bone growth during puberty, a process in which MSCs are crucially involved [37]. In both human osteosarcoma cells and transformed MSCs, frequent aberrations in genes encoding components of P53 pathway have been identified [4,39]. In Tp53 knockout mice many types of sarcomas developed and osteosarcoma was the main type [17].
Chondrosarcoma
A study compared the gene expression profiles of chondrosarcomas of different differentiation degree [53]. Less differentiated chondrosarcomas were shown to have more similarity with MSCs of pre-chondrogenic stages and more differentiated chondrosarcomas share more similarity with fully differentiated chondrocytes. This suggests that chondrosarcoma progression probably parallels deregulated chondrocytes differentiation process of MSCs [40,53].
Soft tissue sarcomas
Synovial sarcoma
In synovial sarcoma, exogenous expression of SYT-SSX2 fusion gene in the skeletal-muscle-specific Myf5 expressing lineage induced the formation of synovial sarcomas in vivo. Remarkably, when this fusion gene was introduced into cells more differentiated than myoblasts synovial sarcoma did not occur [68]. This fact emphasizes the important role of cell status in the genesis of specific type of sarcomas. On the other hand, fusion gene silencing in primary synovial sarcoma cells restored both the trilineage differentiation capacity and the MSC marker expression, strongly suggesting cells of MSC lineage as the origin of synovial sarcoma [69]. This may be explained by the fact that although considered as muscle specific Myf5 can also be expressed in some MSCs during development.
Other soft tissue sarcomas
Similar results as described above were seen in a mouse model of liposarcoma, where FUS -CHOP was able to induce liposarcoma genesis in MSCs, whereas no liposarcoma was formed when FUS-CHOP gene was manipulated to be only expressed in differentiated, aP2-expressing adipocytes. This study again underscores the exact cell status as a crucial factor in sarcomagenesis [42,70]. However other studies show that there is considerate plasticity in the different lineages since rhadomyosarcoma, an aggressive skeletal muscle tumour can be generated from adipocytes by activation of Sonic Hedgehog signaling [71]. A third soft tissue sarcoma model is that of clear cell sarcoma, characterized by melanoma-like features and an EWSR1/ATF1 translocation. Conditional expression of the human EWSR1/ATF1 fusion gene in mouse gives rise to tumorigenesis with extreme brief latency. The most stem-like MSCs give rise to fully melanoma-like lesions, whereas more differentiated cells result in a less clear cell sarcoma phenotype [72].
Discussion
Until now there have not been many studies addressing the effect of MSCs of different tissues and different ways of preparation on the role of MSCs as a model for sarcoma genesis. The conspicuous difference between mouse and human MSCs in spontaneous transformation can be possibly explained by many factors. In human cells, the telomeric DNA is often 5–10 kb long and mouse cells have a telomeric DNA length of 30–40 kb [41,51]. The longer telomeres in mouse MSCs allow cells to proliferate many generations before reaching reaching the telomere length limit, giving higher chance for cell to acquire aberrations [41,51]. Since mice have a shorter life span than humans, the genome maintenance in mouse cells is also less stringent than in human cells [73].
Niche is one of the most important factors in the determination of stem cell characteristics. The function of niche in stem cell differentiation and pluripotency maintenance is well known. There has also been research showing that the low oxygen tension is important for multipotency maintenance of MSCs, while normal oxygen level will induce differentiation [74]. Besides, niche has also been indicated to be involved in tumorigenesis [75]. This suggests the important role of niche in genomic instability and therefore tumourigenetic ability. One special feature of the bone marrow niche is the partnership of MSCs and haematopoietic stem cells, which deserves further exploration [76,77].
Future considerations
The numerous but well documented studies on MSCs giving rise to sarcomas in experimental set-up provide excellent models to study this devastating malignancy in a systematic and controlled way. This offers opportunities for preclinical testing of experimental therapies, thereby providing convincing data that may facilitate application in actual clinical trials despite small patient cohorts.
Conclusions
Although mouse MSCs have exhibited definite readiness to transform in vitro, human MSCs do not go through transformation in ex vivo expansion and need additional manipulation before progression into sarcomas. Therefore, although there are few cases of osteosarcoma genesis in patients infused with bone marrow MSCs [45-47], it is considered generally safe to use human MSCs in clinic.
Abbreviations
AKT: v-akt murine thymoma viral oncogene homolog; AMSC: Adipose tissue derived MSC; APC: Adenomatous polyposis coli; BMI-1: B lymphoma Mo-MLV insertion region 1 homolog; BMMSC: Bone marrow derived MSC; C-myc: v-myc myelocytomatosis viral oncogene; Cdkn2a: Cyclin-dependent kinase inhibitor A; CHOP: C/EBP homologous protein; DKK1: dickkopf 1 homolog; EWS: Ewing Sarcoma; ETS: E-twenty six; FKHR: Forkhead in rhabdomyosarcoma; FLI1: Friend leukemia integration 1; Fos: FBJ murine osteosarcoma viral oncogene; FUS: Fused in sarcoma; H-RAS: v-Ha-ras Harvey rat sarcoma viral oncogene homolog; HLA-DR: Human leukocyte antigen-DR chain; HPV: Human papillomavirus; hTERT: Human telomerase reverse transcriptase; IDH: Isocitrate dehydrogenase; INK4A/ARF: Inhibitor of CDK4 A/Alternative Reading Frame; K-ras: v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; MAPK: Mitogen-activated protein kinase; MSC: Mesenchymal Stem Cell; MYF6: Myogenic factor 6; PAX3/7: Paired box 3/7; PI3K: Phosphatidylinositol-3-kinase; Prx1: Peroxiredoxin 1; Pten: Phosphatase and tensin homolog; Rb: Retinoblastoma; SSX2: Synovial sarcoma translocation 2; SV40-LT: Simian virus 40-large antigen; SYT: Synaptotagmin; TERT: Telomerase reverse transcriptase.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Wei Xiao drafted the manuscript. Alexander B. Mohseny, Anne-Marie Cleton-Jansen and Pancras C. W. Hogendoorn provided comments and suggestions. All authors read and approved the final manuscript.
Contributor Information
Wei Xiao, Email: w.xiao@lumc.nl.
Alexander B Mohseny, Email: A.B.Mohseny@lumc.nl.
Pancras C W Hogendoorn, Email: P.C.W.Hogendoorn@lumc.nl.
Anne-Marie Cleton-Jansen, Email: A.M.Cleton-Jansen@lumc.nl.
References
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS. et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Mohseny AB, Xiao W, Carvalho R, Spaink HP, Hogendoorn PC, Cleton-Jansen AM. An osteosarcoma zebrafish model implicates Mmp-19 and Ets-1 as well as reduced host immune response in angiogenesis and migration. J Pathol. 2012;227:245–253. doi: 10.1002/path.3998. [DOI] [PubMed] [Google Scholar]
- Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-De BI, De JD. et al. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- Mohseny AB, Hogendoorn PCW. Concise review: mesenchymal tumors: when stem cells go mad. Stem Cells. 2011;29:397–403. doi: 10.1002/stem.596. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis MA, McElmurry RT, Bell S. et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111:249–257. doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R, Garcia-Castro J, Gutierrez-Aranda I, Paramio J, Santos M, Catalina P. et al. Deficiency in p53 but not retinoblastoma induces the transformation of mesenchymal stem cells in vitro and initiates leiomyosarcoma in vivo. Cancer Res. 2010;70:4185–4194. doi: 10.1158/0008-5472.CAN-09-4640. [DOI] [PubMed] [Google Scholar]
- Choumerianou DM, Dimitriou H, Perdikogianni C, Martimianaki G, Riminucci M, Kalmanti M. Study of oncogenic transformation in ex vivo expanded mesenchymal cells, from paediatric bone marrow. Cell Prolif. 2008;41:909–922. doi: 10.1111/j.1365-2184.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, He L, Wan Y, Song J. H3K9me-enhanced DNA hypermethylation of the p16(INK4a) gene: an epigenetic signature for spontaneous transformation of rat mesenchymal stem cells. Stem Cells Dev. 2013;22:256–267. doi: 10.1089/scd.2012.0172. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Rubio R, Gutierrez-Aranda I, Melen GJ, Elosua C, Garcia-Castro J. et al. FUS-CHOP fusion protein expression coupled to p53 deficiency induces liposarcoma in mouse but not in human adipose-derived mesenchymal stem/stromal cells. Stem Cells. 2011;29:179–192. doi: 10.1002/stem.571. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N. et al. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H. et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Rubio R, Menendez P. Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res. 2012;22:62–77. doi: 10.1038/cr.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A. et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Ishikawa T, Sugihara E, Kuninaka S, Miyamoto T, Mabuchi Y. et al. c-MYC overexpression with loss of Ink4a/Arf transforms bone marrow stromal cells into osteosarcoma accompanied by loss of adipogenesis. Oncogene. 2010;29:5687–5699. doi: 10.1038/onc.2010.312. [DOI] [PubMed] [Google Scholar]
- Lin PP, Pandey MK, Jin FH, Raymond AK, Akiyama H, Lozano G. Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis. 2009;30:1789–1795. doi: 10.1093/carcin/bgp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet Cytogenet. 2003;145:1–30. doi: 10.1016/S0165-4608(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L. et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- Xu S, De BA, De RH, Van CB, Vanderkerken K, Van R. I: In vitro expanded bone marrow-derived murine (C57Bl/KaLwRij) mesenchymal stem cells can acquire CD34 expression and induce sarcoma formation in vivo. Biochem Biophys Res Commun. 2012;424:391–397. doi: 10.1016/j.bbrc.2012.06.118. [DOI] [PubMed] [Google Scholar]
- Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Hisha H, Takaki T, Adachi Y, Li M, Song C. et al. Transformation potential of bone marrow stromal cells into undifferentiated high-grade pleomorphic sarcoma. J Cancer Res Clin Oncol. 2010;136:829–838. doi: 10.1007/s00432-009-0723-0. [DOI] [PubMed] [Google Scholar]
- Josse C, Schoemans R, Niessen NA, Delgaudine M, Hellin AC, Herens C. et al. Systematic chromosomal aberrations found in murine bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1167–1173. doi: 10.1089/scd.2009.0264. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Rubio R, Masip M, Catalina P, Nieto A, De la Cueva T. et al. Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells. Neoplasia. 2009;11:397–U106. doi: 10.1593/neo.81620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R. et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- Ruther U, Komitowski D, Schubert FR, Wagner EF. c-fos expression induces bone tumors in transgenic mice. Oncogene. 1989;4:861–865. [PubMed] [Google Scholar]
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F. et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- de Andrea CE, Reijnders CM, Kroon HM, De JD, Hogendoorn PC, Szuhai K. et al. Secondary peripheral chondrosarcoma evolving from osteochondroma as a result of outgrowth of cells with functional EXT. Oncogene. 2012;31:1095–1104. doi: 10.1038/onc.2011.311. [DOI] [PubMed] [Google Scholar]
- Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer. 2010;10:481–488. doi: 10.1038/nrc2869. [DOI] [PubMed] [Google Scholar]
- Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J. et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Tsumura H, Yoshida T, Saito H, Imanaka-Yoshida K, Suzuki N. Cooperation of oncogenic K-ras and p53 deficiency in pleomorphic rhabdomyosarcoma development in adult mice. Oncogene. 2006;25:7673–7679. doi: 10.1038/sj.onc.1209749. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP. et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC. et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- de la Fuente R, Bernad A, Garcia-Castro J, Martin MC, Cigudosa JC. Spontaneous human adult stem cell transformation (retraction of vol 65, pg 3035, 2005) Cancer Res. 2010;70:6682. doi: 10.1158/0008-5472.CAN-10-2451. [DOI] [PubMed] [Google Scholar]
- Fletcher CDM, Unni K, Mertens F. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARCPress; 2002. pp. 264–270. [Google Scholar]
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yang R, Zhang W, Dorfman H, Rao P, Gorlick R. Genetically transforming human mesenchymal stem cells to sarcomas: changes in cellular phenotype and multilineage differentiation potential. Cancer. 2009;115:4795–4806. doi: 10.1002/cncr.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrea CE, Hogendoorn PCW. Epiphyseal growth plate and secondary peripheral chondrosarcoma: the neighbours matter. J Pathol. 2012;226:219–228. doi: 10.1002/path.3003. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Losada J, Sanchez-Martin M, Rodriguez-Garcia MA, Perez-Mancera PA, Pintado B, Flores T. et al. Liposarcoma initiated by FUS/TLS-CHOP: the FUS/TLS domain plays a critical pole in the pathogenesis of liposarcoma. Oncogene. 2000;19:6015–6022. doi: 10.1038/sj.onc.1204018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J. et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- Torsvik A, Rosland GV, Svendsen A, Molven A, Immervoll H, McCormack E. et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Res. 2010;70:6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- Berger M, Muraro M, Fagioli F, Ferrari S. Osteosarcoma derived from donor stem cells carrying the Norrie's disease gene. N Engl J Med. 2008;359:2502–2504. doi: 10.1056/NEJMc0807172. [DOI] [PubMed] [Google Scholar]
- Bielack SS, Rerin JS, Dickerhoff R, Dilloo D, Kremens B, Von SA. et al. Osteosarcoma after allogeneic bone marrow transplantation: a report of four cases from the cooperative osteosarcoma study group (COSS) Bone Marrow Transplant. 2003;31:353–359. doi: 10.1038/sj.bmt.1703864. [DOI] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L. et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T. et al. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- Shima Y, Okamoto T, Aoyama T, Yasura K, Ishibe T, Nishijo K. et al. In vitro transformation of mesenchymal stem cells by oncogenic H-ras(VaI12) Biochem Biophys Res Commun. 2007;353:60–66. doi: 10.1016/j.bbrc.2006.11.137. [DOI] [PubMed] [Google Scholar]
- Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C. et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. PNAS. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. the biology of cancer. Garland Science; 2007. Eternal life: cell immortalization and tumorigenesis; pp. 357–398. [Google Scholar]
- Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Boeuf S, Kunz P, Hennig T, Lehner B, Hogendoorn P, Bovee J. et al. A chondrogenic gene expression signature in mesenchymal stem cells is a classifier of conventional central chondrosarcoma. J Pathol. 2008;216:158–166. doi: 10.1002/path.2389. [DOI] [PubMed] [Google Scholar]
- Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA. et al. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–3257. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC. et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. PNAS. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI. et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V. et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- Maheshwari AV, Cheng EY. Ewing sarcoma family of tumors. J Am Acad Orthop Surg. 2010;18:94–107. doi: 10.5435/00124635-201002000-00004. [DOI] [PubMed] [Google Scholar]
- Riggi N, Cironi L, Suva ML, Stamenkovic I. Sarcomas: genetics, signalling, and cellular origins. Part 1: the fellowship of TET. J Pathol. 2007;213:4–20. doi: 10.1002/path.2209. [DOI] [PubMed] [Google Scholar]
- Italiano A, Sung YS, Zhang L, Singer S, Maki RG, Coindre JM. et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer. 2012;51:207–218. doi: 10.1002/gcc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuhai K, Ijszenga M, De JD, Karseladze A, Tanke HJ, Hogendoorn PC. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009;15:2259–2268. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- Yeny CT, Eliazer S, Xiang LL, Richardson JA, Ilaria RL. Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells results in EWS/FLI-1-dependent, Ewing sarcoma-like tumors. Cancer Res. 2005;65:8698–8705. doi: 10.1158/0008-5472.CAN-05-1704. [DOI] [PubMed] [Google Scholar]
- Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C. et al. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- Miyagawa Y, Okita H, Nakaijima H, Horiuchi Y, Sato B, Taguchi T. et al. Inducible expression of chimeric EWS/ETS proteins confers Ewing's family tumor-like phenotypes to human mesenchymal progenitor cells. Mol Cell Biol. 2008;28:2125–2137. doi: 10.1128/MCB.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PP, Pandey MK, Jin FH, Xiong SB, Deavers N, Parant JM. et al. EWS-FLI1 induces developmental abnormalities and accelerates sarcoma formation in a transgenic mouse model. Cancer Res. 2008;68:8968–8975. doi: 10.1158/0008-5472.CAN-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. 2005;19:501. doi: 10.1016/j.hoc.2005.03.004. vii. [DOI] [PubMed] [Google Scholar]
- Haldar M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell. 2007;11:375–388. doi: 10.1016/j.ccr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Naka N, Takenaka S, Araki N, Miwa T, Hashimoto N, Yoshioka K. et al. Synovial sarcoma is a stem cell malignancy. Stem Cells. 2010;28:1119–1131. doi: 10.1002/stem.452. [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Bermejo-Rodriguez C, Sanchez-Martin M, Abollo-Jimenez F, Pintado B, Sanchez-Garcia I. FUS-DDIT3 prevents the development of adipocytic precursors in liposarcoma by repressing PPAR gamma and C/EBP alpha and activating eIF4E. PLoS One. 2008;3:e2569. doi: 10.1371/journal.pone.0002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatley ME, Tang W, Garcia MR, Finkelstein D, Millay DP, Liu N. et al. A mouse model of rhabdomyosarcoma originating from the adipocyte lineage. Cancer Cell. 2012;22:536–546. doi: 10.1016/j.ccr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straessler KM, Jones KB, Hu H, Jin H, van de Rijn M, Capecchi MR. Modeling clear cell sarcomagenesis in the mouse: cell of origin differentiation state impacts tumor characteristics. Cancer Cell. 2013;23:215–227. doi: 10.1016/j.ccr.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013;75:645–668. doi: 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- Berniakovich I, Giorgio M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int J Mol Sci. 2013;14:2119–2134. doi: 10.3390/ijms14012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbut E, Ptak-Belowska A, Brzozowski T. Mechanisms promoting physiological cells progression into tumorigenesis. J Physiol Pharmacol. 2012;63:565–570. [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]