Abstract
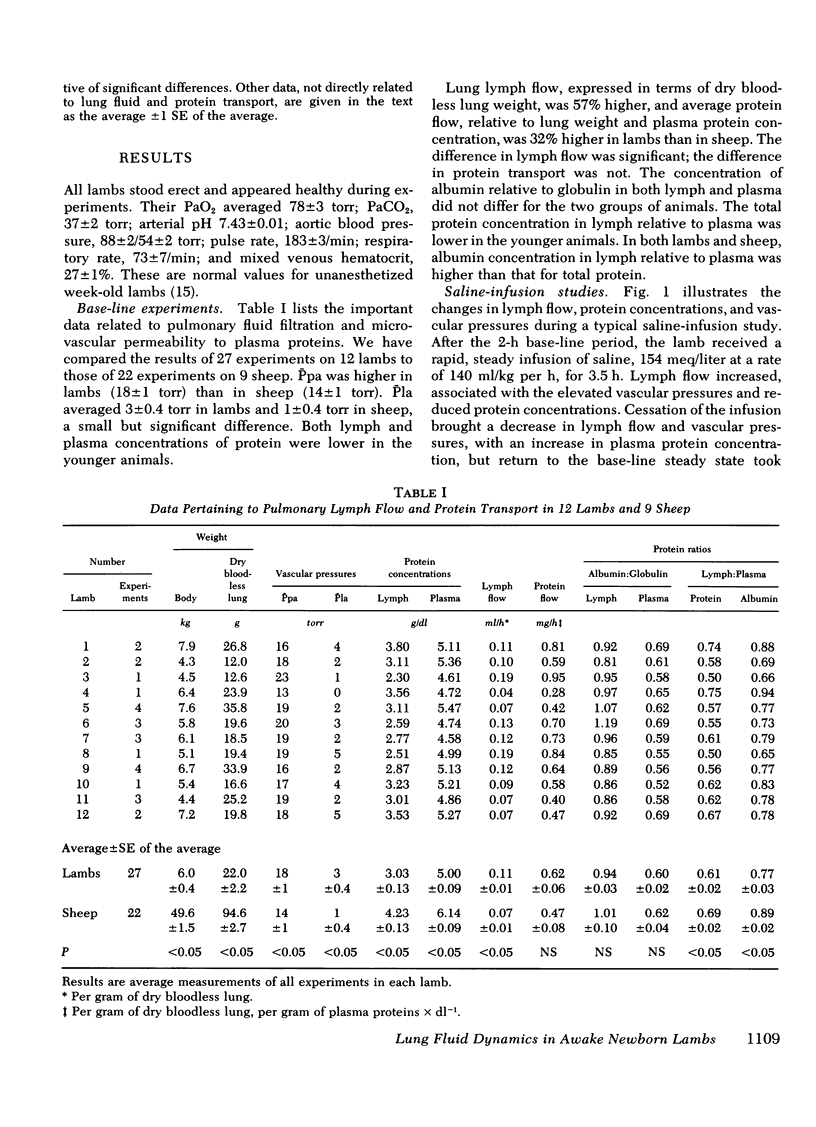
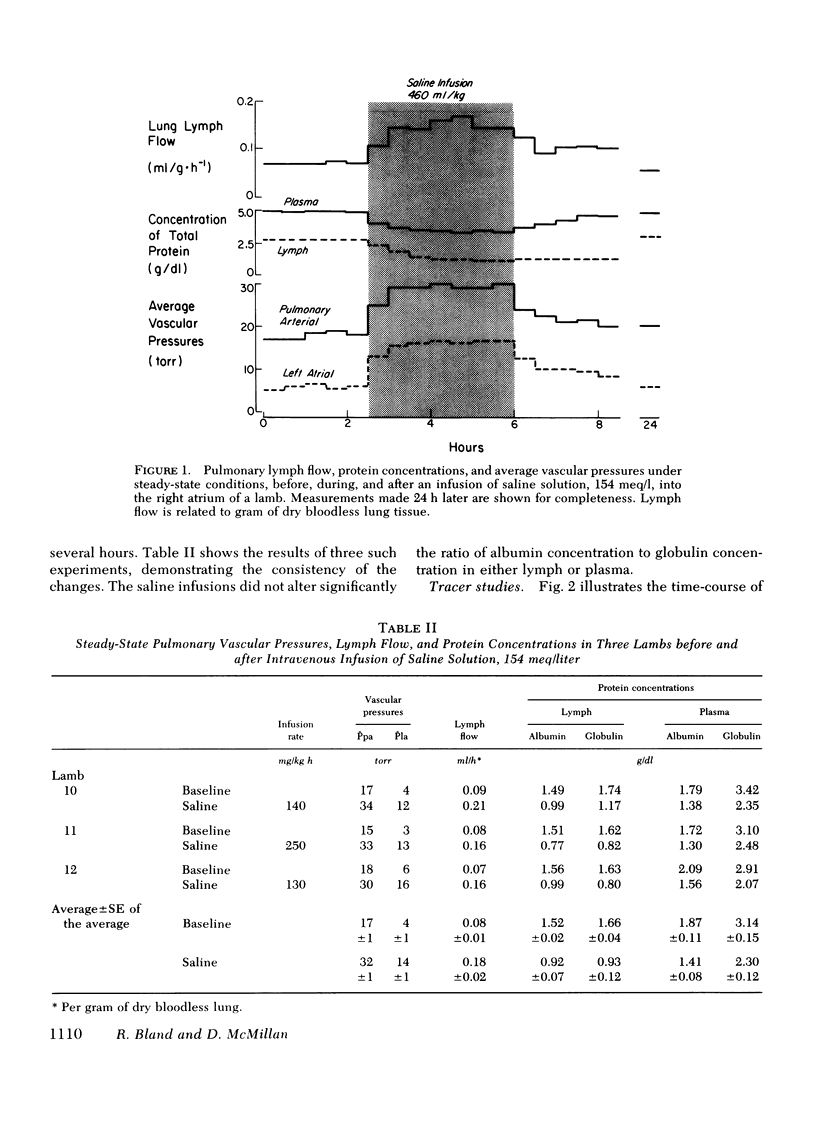
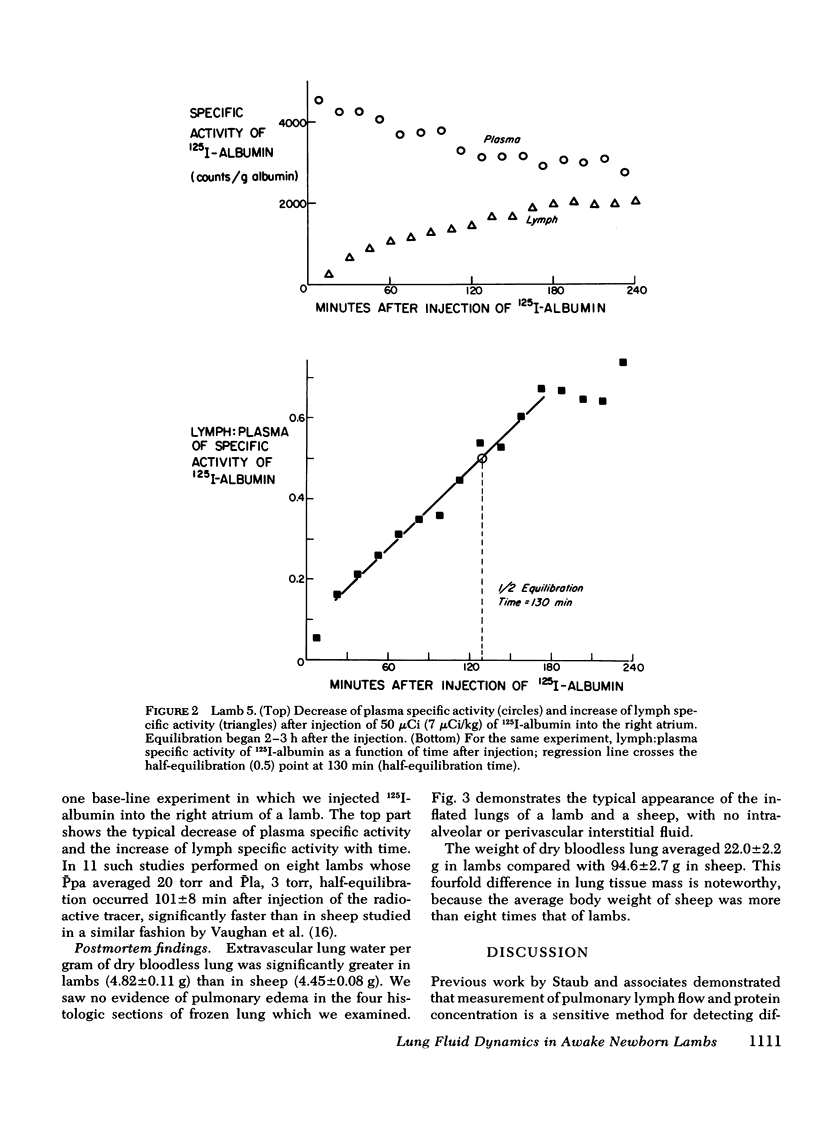
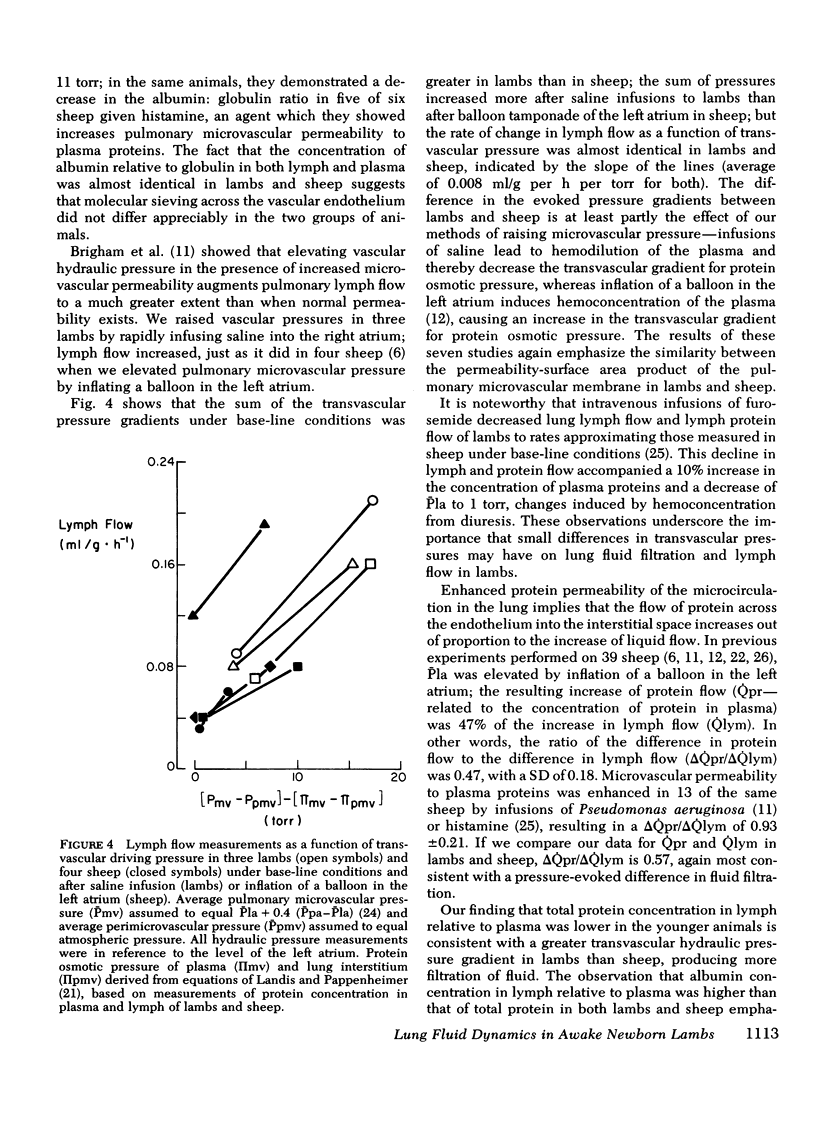
We measured steady-state lung lymph flow, lymph protein flow, and simultaneous pulmonary vascular pressures in 12 1-wk-old unanesthetized lambs and compared these measurements to those of previous studies, performed under similar conditions, on nine awake adult sheep. The purpose of these experiments was to compare newborn and adult sheep with respect to transvascular filtration of fluid and microvascular permeability to plasma proteins. We prepared the lambs surgically to isolate and collect lung lymph and measure average pulmonary arterial and left atrial pressures, allowing at least 2 days for the lambs to recover from surgery before studies began. Lambs had higher pulmonary arterial and left atrial pressures, lower lymph and plasma protein concentrations, and 57% more lymph flow per gram of dry bloodless lung than sheep; the difference in protein flow between lambs and sheep was not significant. Protein concentration in lymph relative to that in plasma was significantly lower in lambs than in sheep; but the ratio of albumin concentration to globulin concentration in both lymph and plasma was almost identical in the two groups of animals. Extravascular lung water per gram of dry bloodless lung was greater in lambs (4.82±0.11 g) than in sheep (4.45±0.08 g), but there was no histologic evidence of pulmonary edema in either group of animals. These findings suggest that lambs have more transvascular filtration of fluid per unit lung mass than sheep, but that microvascular sites for protein exchange do not differ appreciably in lambs and sheep. To test this conclusion, we measured steady-state lymph flow in three lambs before and after raising pulmonary microvascular pressure by rapid intravenous infusion of saline. Lymph flow increased as a function of the net transvascular driving pressure (hydraulic pressure gradient—protein osmotic pressure gradient). This response was almost identical to that of four sheep with pulmonary microvascular pressure augmented by inflation of a balloon in the left atrium. In eight lambs we measured the time for intravenously injected 125I-albumin to equilibrate in lymph at half the specific activity of plasma: the protein tag equilibrated faster than in sheep. This difference could be explained partly by the higher pulmonary arterial and left atrial pressures of lambs than sheep, and possibly by the presence of more microvascular sites for protein exchange relative to the volume of distribution of protein in the lung of the younger animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOSTON R. W., HUMPHREYS P. W., REYNOLDS E. O., STRANG L. B. LYMPH-FLOW AND CLEARANCE OF LIQUID FROM THE LUNGS OF THE FOETAL LAMB. Lancet. 1965 Sep 4;2(7410):473–474. doi: 10.1016/s0140-6736(65)91428-5. [DOI] [PubMed] [Google Scholar]
- Bland R. D., Demling R. H., Selinger S. L., Staub N. C. Effects of alveolar hypoxia on lung fluid and protein transport in unanesthetized sheep. Circ Res. 1977 Mar;40(3):269–274. doi: 10.1161/01.res.40.3.269. [DOI] [PubMed] [Google Scholar]
- Body R. D., Hill J. R., Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Permeability of lung capillaries to macromolecules in foetal and new-born lambs and sheep. J Physiol. 1969 May;201(3):567–588. doi: 10.1113/jphysiol.1969.sp008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Owen P. J. Increased sheep lung vascular permeability caused by histamine. Circ Res. 1975 Nov;37(5):647–657. doi: 10.1161/01.res.37.5.647. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Owen P. J. Mechanism of the serotonin effect on lung transvascular fluid and protein movement in awake sheep. Circ Res. 1975 Jun;36(6):761–770. doi: 10.1161/01.res.36.6.761. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri P. H., Dbaly J., Weibel E. R. The postnatal growth of the rat lung. I. Morphometry. Anat Rec. 1974 Apr;178(4):711–730. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- Erdmann A. J., 3rd, Vaughan T. R., Jr, Brigham K. L., Woolverton W. C., Staub N. C. Effect of increased vascular pressure on lung fluid balance in unanesthetized sheep. Circ Res. 1975 Sep;37(3):271–284. doi: 10.1161/01.res.37.3.271. [DOI] [PubMed] [Google Scholar]
- GUYTON A. C., LINDSEY A. W. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959 Jul;7(4):649–657. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- Gaar K. A., Jr, Taylor A. E., Owens L. J., Guyton A. C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol. 1967 Oct;213(4):910–914. doi: 10.1152/ajplegacy.1967.213.4.910. [DOI] [PubMed] [Google Scholar]
- Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Pulmonary lymph flow and the uptake of liquid from the lungs of the lamb at the start of breathing. J Physiol. 1967 Nov;193(1):1–29. doi: 10.1113/jphysiol.1967.sp008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine O. R., Rodriguez-Martinez F., Mellins R. B. Fluid filtration in the lung of the intact puppy. J Appl Physiol. 1973 May;34(5):683–686. doi: 10.1152/jappl.1973.34.5.683. [DOI] [PubMed] [Google Scholar]
- Nicolaysen G., Nicolaysen A., Staub N. C. A quantitative radioautographic comparison of albumin concentration in different dized lymph vessels in normal mouse lungs. Microvasc Res. 1975 Sep;10(2):138–152. doi: 10.1016/0026-2862(75)90002-3. [DOI] [PubMed] [Google Scholar]
- PEARCE M. L., YAMASHITA J., BEAZELL J. MEASUREMENT OF PULMONARY EDEMA. Circ Res. 1965 May;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Steady state pulmonary transvascular water filtration in unanesthetized sheep. Circ Res. 1971 Jan;28(Suppl):135–139. [PubMed] [Google Scholar]
- Taylor P. M., Boonyaprakob U., Waterman V., Watson D., Lopata E. Clearances of plasma proteins from pulmonary vascular beds of adult dogs and pups. Am J Physiol. 1967 Aug;213(2):441–449. doi: 10.1152/ajplegacy.1967.213.2.441. [DOI] [PubMed] [Google Scholar]
- Thurlbeck W. M. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975 Jun;111(6):803–844. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- Vreim C. R., Snashall P. D., Demling R. H., Staub N. C. Lung lymph and free interstitial fluid protein composition in sheep with edema. Am J Physiol. 1976 Jun;230(6):1650–1653. doi: 10.1152/ajplegacy.1976.230.6.1650. [DOI] [PubMed] [Google Scholar]



