Abstract
OBJECTIVE
Cerebral white matter (WM) injury is common after cardiac surgery in neonates and young infants who have brain immaturity and genetic abnormalities. In order to understand better the mechanisms associated with WM injury, we tested the adequacy of a novel ex-vivo brain slice model, with a particular focus on how the maturational stage modulates the injury.
METHODS
To replicate conditions of cardiopulmonary bypass living brain slices were transferred to a closed chamber perfused by artificial cerebrospinal-fluid under controlled temperature and oxygenation. Oxygen-glucose deprivation (OGD) simulated circulatory arrest. The effects of maturation were investigated in 7 and 21-day-old mice (P7, P21) that are equivalent in maturation stage to the human fetus and young adult.
RESULTS
There were no morphological changes in axons after 60 min OGD at 15°C in both P7 and P21 WM. Higher temperature and longer duration of OGD were associated with significantly greater WM axonal damage, suggesting that the model replicates the injury seen after hypothermic circulatory arrest. The axonal damage at P7 was significantly less than at P21 demonstrating that immature axons are more resistant than mature axons. Conversely, a significant increase in caspase3+ oligodendrocytes in P7 mice was identified relative to P21, indicating that oligodendrocytes in immature WM are more vulnerable than oligodendrocytes in mature WM.
CONCLUSIONS
Neuroprotective strategies for immature WM may need to focus on reducing oligodendrocyte injury. The brain slice model will be helpful in understanding the effects of cardiac surgery on the immature brain and the brain with genetic abnormalities.
Both prospective clinical trials and retrospective clinical studies have documented significant neurodevelopmental deficits in children with congenital heart disease (CHD)1–3. Recent studies using magnetic resonance imaging have identified evidence of white matter (WM) injury in CHD newborns and young infants4–6. Major clinical correlates of WM injury are gross and fine motor deficits, which are the most common neurological deficits seen in children after cardiac surgery7–9. In addition, recent advances in the field of neuroscience illuminate an important role of WM structure in specific cognitive functions, including reading, verbal function, executive decision making, working memory, and learning complex skill10. Interestingly, impairments of these functions are largely observed in CHD school age children and adolescents following cardiac surgery2, 8, 11. Therefore, understanding the cellular and molecular events that result in such WM injury is of crucial importance in order to develop targeted therapies and conditions which will minimize the risk of neurological deficits in CHD patients12.
Cellular and molecular processes underlying WM injury and its repair have been extensively explored in rodent animal models based on the tremendous power of transgenic and gene knockout technologies13. This approach has assisted in establishing novel therapeutic strategies for WM disorders such as multiple sclerosis14. We have recently introduced cutting-edge neuroscience techniques to study WM injury in the porcine model15; however, transgenic and gene knockout technologies are still extremely limited in large animals. Thus, for further investigation of WM injury and the development of novel treatment approaches, it is imperative to explore new animal models that replicate pathological conditions to which the brain is exposed under CHD and subsequent cardiac surgery.
Clinical studies have reported a significant high incidence (25% to 55%) of newly-developed WM injury after cardiac surgery4–6. The major brain insults during surgery includes cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA)8, 16. DHCA is a unique and specific pathological condition in patients undergoing cardiac surgery, which causes in ischemia-reperfusion/reoxygenation under hypothermia. In order to reproduce conditions of CPB and DHCA, we developed a unique brain slice model in which living brain slices are transferred to a closed circulation system perfused by artificial cerebrospinal fluid (aCSF) under controlled temperature, pH and oxygenation. In the present study, we examined the adequacy of our brain slice model for the investigation of WM injury associated with DHCA. We assessed WM injury due to the hypothermic ischemia-reperfusion/reoxygenation using two transgenic mice strains with a focus on axons and oligodendrocytes which are major cellular components in the WM13, 14. Since recent clinical studies identified brain maturation as an important factor in WM injury after cardiac surgery5, we studied how maturation stage modulates the damage in WM axons and oligodendrocytes.
METHODS
Animals
Two lines of transgenic mice were studied. In thy1-yellow fluorescent protein-16 (C57BL/6) mice, yellow fluorescent proteins are selectively expressed in neuronal bodies and axons. In 2',3'-cyclic nucleotide 3'phosphodiesterase (CNP) mice, human enhanced green fluorescent protein is overexpressed in the oligodendrocyte lineage under the CNP promoter.
Brain maturation in CHD newborns is delayed approximately one month17, 18. Therefore the effects of brain maturation on WM injury were investigated in both 7 and 21-day-old mice (P7, P21) which have WM maturation equivalent to the human fetus (35–36 weeks of gestation) and 10-year-old child, respectively19. We performed all experiments in compliance with the NIH Guide for the Care and Use of Laboratory animals. The study was approved by the Animal Care and Use Committee of the Children’s National Medical Center.
Brain slice preparation
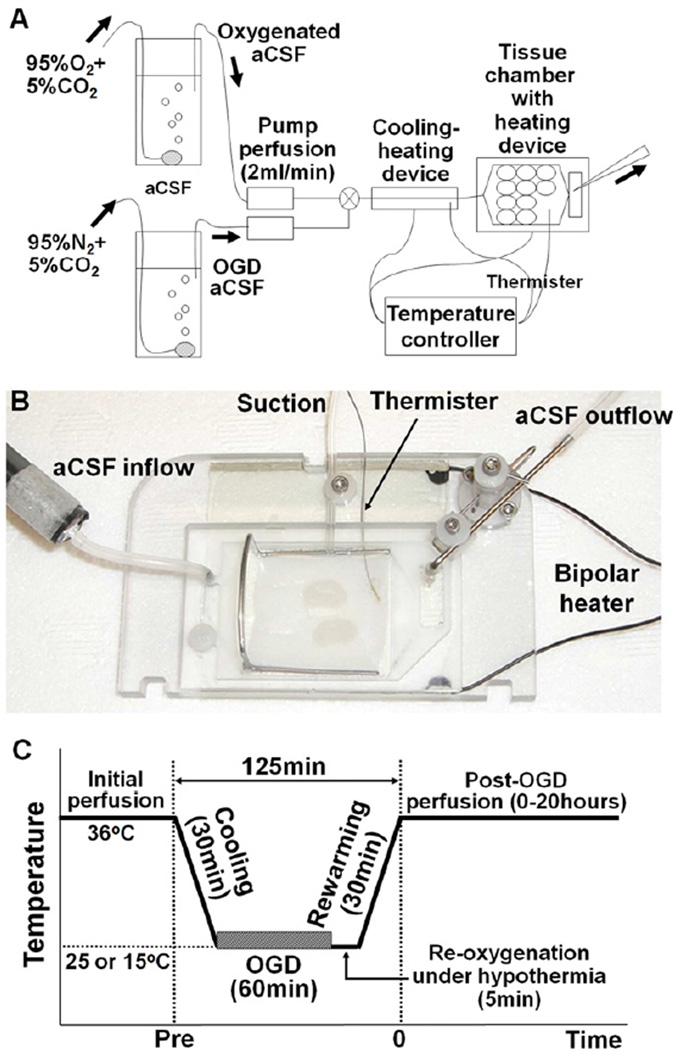
The brains were collected from two transgenic mice strains at P7 and P21 and were dissected out into ice-cold 95%-oxygenated modified aCSF slicing solution composed of the following (in mM): 87 NaCl, 2.5 KCl, 3 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, and 75 sucrose, pH 7.4. Coronal sections, 400 um, were cut using a vibratome (Leica 1200 VT, Leica Microsystme Inc. Buffalo Grove, IL). Slices were allowed to recover at 22°C in the slicing solution for 1 hour. Perfusate temperature was then gradually increased up to 36°C over 2 hours as is the practice for electrophysiological studies using murine brain slices20. Only slices containing corpus callosum, which is a major WM structure in the mouse brain, were included in the experiments (two–three per animal). The slices from three littermates (6–9 per experiment) were transferred to a customized tissue chamber (CellMicro, Norfork, VA; Figure 1, A and B).
Figure 1.
A, Schematic image of ex-vivo brain slice culture system. B, Tissue chamber for rodent brain slices with controlled perfusion and temperature. C, Perfusion protocol of the oxygen-glucose deprivation under the hypothermia to replicate brain conditions under deep hypothermic circulatory arrest.
In the closed chamber system, brain slices were perfused (2 ml/min) with aCSF composed of the following (in mM): 126 NaCl, 3.5 KCl, 1.3 MgCl2, 2 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 10 glucose, and pH 7.4; saturated with 95% O2/5% CO2. A heating/cooling device (CellMicro, Norfork, VA) was connected with the system to control the temperature of the aCSF. The bottom of the chamber itself also contained the temperature-control device (CellMicro, Norfork, VA). This bipolar temperature controller allows precise temperature control in slices being studied. Brain slice preparation and perfusion methods were modified from previous reports20, 21.
Oxygen-glucose deprivation
In order to replicate DHCA slices were exposed to oxygen-glucose deprivation (OGD) (Figure 1, A). OGD was achieved by changing the perfusion solution from aCSF saturated with 95% O2/5% CO2 to glucose-free aCSF (supplemented with 10 mM sucrose to maintain osmolarity) saturated with 95% N2/5% CO2. The slice was reperfused with glucose-containing aCSF saturated with 95% O2/5% CO2 after the period of OGD, providing ischemia-reperfusion/reoxygenation to the brain slices. This technique has been widely used to investigate ischemia-induced brain injury in culture systems21, 22. In the present study, the OGD was performed under three hypothermic conditions using the temperature controlled circulation system described above. The contents of oxygenated aCSF and aCSF under OGD are shown in Table E1.
Perfusion protocol
Each experiment was assigned to one of three groups with different temperature of 60 min OGD (15°C, 25°C, and 36°C-OGD). After initial recovery period following the slice procedure, brain slices were cooled to a temperature of 15, 25, or 36°C for 30 min according to the protocol. The OGD was then performed for 60 min under three temperature settings. After the OGD, slices were reperfused with glucose containing aCSF saturated with 95% O2/5% CO2 in 5 min, followed by 30 min rewarming period (Figure 1, C). This replicates DHCA-induced hypothermic ischemia-reperfusion/reoxygenation. Damage to WM axons and OLs in brain slices was assessed at 0 to 20 hours after rewarming. Control slices which were perfused at 36°C with aCSF saturated with 95% O2/5% CO2 for the same duration (no-OGD) were used for comparison with the OGD groups.
Tissue preparation and immunohistochemistry
At the conclusion of each experiment, slices were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) for 1 hour at room temperature. Each slice was then cryoprotected for 48 hours in 30% sucrose in PBS. All slices were stored at −80°C until further processing. For assessment of WM axonal and oligodendrocyte injury, 20-µm sections were developed from each 400 um brain slice using a cryotome. Sections from the outer 100 um of each slice were excluded from the assessment to avoid tissues that may have been damaged during slice preparation. For immunohistochemistry, 20-µm sections were incubated for 1 hour at room temperature with blocking solution (10% normal goat serum, 1% bovine serum albumin, and 0.3% Tween 20 in PBS, pH 7.4) and then incubated at 4°C overnight with primary antibody and carrier solution (1% normal goat serum, 1% bovine serum albumin, and 0.3% Tween 20 in PBS, pH 7.4). Sections were washed with PBS and then incubated for 1 hour at room temperature with secondary antibody and carrier solution15, 20. An antibody to cleaved-Caspase3 was utilized to identify apoptosis in the WM15.
Assessments of WM cellular damage
A Zeiss LSM 510 confocal laser scanning microscopic system (Carl Zeiss Microimaging LLC, Thornwood, NY) was used for the analysis of fluorescence. Three different laser lines were used to image localization of FITC (488 nm excitation; 522/35 emission filter), Cy3 (560 nm excitation; 605/32 emission filter), and DAPI (400 nm excitation). Data acquisition and processing were controlled by LSM software. Analysis of immunofluoresence was performed on confocal z-stacks. Confocal Assistant 4.02 and NIH Image J software (http://rsb.info.nih.gov) were used to merge images for analysis. Merged images were processed in Photoshop 7.0 (Adobe Systems; San Jose, CA) with minimal manipulation of contrast as previously described15, 20.
For the assessment of WM axonal damage, a total of 3 optical images 45×45×2 µm (X, Y, Z planes) were collected in the z-axis from a single microscopic field in the midline of the corpus callosum of each section using a 100× oil immersion objective lens. The images including all tested groups were randomized and renamed by Ant Renamer 2.09.1. Randomized images were divided into a 5×5 grid (a total of 25 grids in a single image), and blindly scored by a single observer using following scoring system: 0, no damage; 1, axon swelling and/or beading; 2, axon fragmentation and/or loss of fluorescence, as previously described23. The total score for a single image was 0–50 (50 = 2 × 25 grids). The number from three images were averaged and used as damage score of a single section. WM oligodendrocyte injury was assessed by counting CNP+caspase-3+ cell numbers in 225×225×20 µm (X, Y, Z planes) for cell/volume quantifications. To determine cell density, the antibody positive cells were quantified in 3 microscopic fields from the corpus callosum of each brain slice as previously described15, 20.
Statistical analysis
One-way ANOVA with Bonferroni post-hoc comparisons was used to detect differences in the axonal damage score and caspase3+CNP3+ cell number in the experimental groups. Two-group comparisons in the OGD conditions and ages were performed by unpaired Student t-tests. Two-way ANOVA with Bonferroni comparisons was used to evaluate changes in axonal damage score over time between OGD groups. The Spearman rank correlation coefficient (rho) was used as a measure of the association between cellular damage and OGD temperature and OGD duration. All data are expressed as mean ± SEM. Reported P values are two-tailed. Analysis of the data was performed using SPSS version 19.0 (SPSS Inc./IBM, Chicago, IL).
RESULTS
Ex-vivo Brain Slice Model Replicates DHCA-induced WM Injury in a Rodent Animal
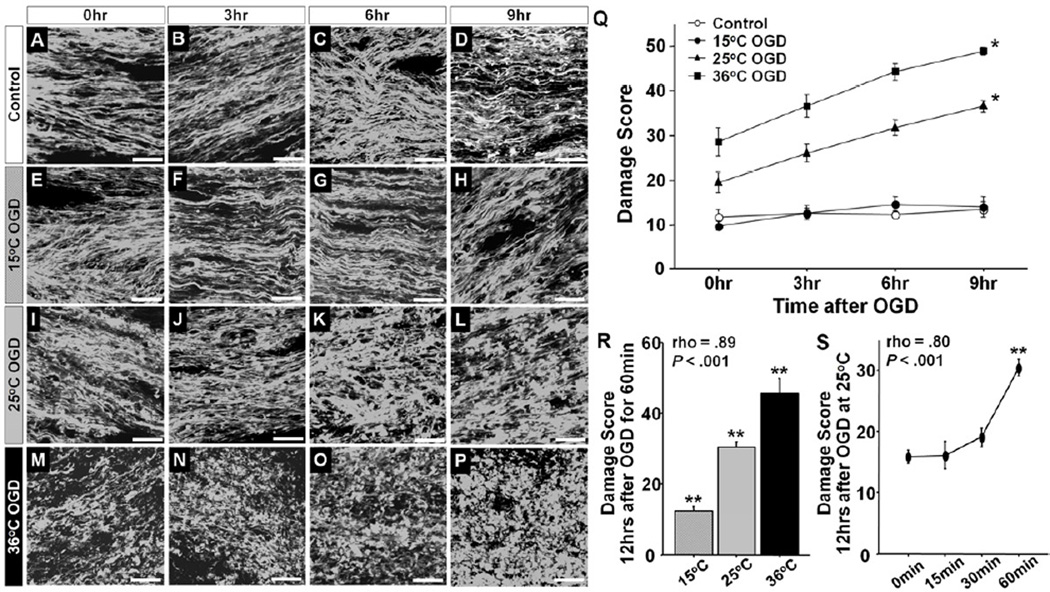
To test the adequacy of brain slice model for investigation of DHCA-induced WM injury, we first assessed WM axonal injury using thy1–YFP mice. Morphological changes in the axons including beading and fragmentation are sensitive markers of WM axonal injury23. In our model, WM axons in P21 Control slices maintained normal morphological structures until the end of experiment (Figure 2A–D). There was no significant increase in damage score in the Control slices over time (Figure 2Q), indicating that the aCSF circulation system is able to maintain the morphological structure of WM axons. On the other hand, 25°C- and 36°C-OGD induced significant alterations including diffuse swelling, beading, and fragmentation (Figure 2, I–P). The morphological alterations and damage score after 25°C- and 36°C-OGD significantly increased over time (Figure 2Q). Damage observed with 60 min OGD, however, were not observed with OGD under the deep hypothermia at 15°C compared with Control (Figure 2, E–H, Q). The protective effects of hypothermia at 15°C have been noted previously in a porcine model of DHCA16. The OGD-induced WM injury in our ex-vivo model demonstrated a significant positive correlation with temperature during OGD (Figure 2R). Furthermore, longer OGD duration was associated with significantly higher WM damage score at 25°C (Figure 2S). These observations are consistent with the results of DHCA-induced brain injury in a large animal model16.
Figure 2.
A–P, Morphological changes of WM axons at 0, 3, 6, and 9 hours after experiments in Control (A–D), 15°C OGD (E–H), 25°C OGD (I–L), and 36°C OGD (M–P). Q, Damage scores at 0, 3, 6, and 9 hours after experiments in Control, 15°C, 25°C, and 36°C OGD. The scores differ according to the time after the OGD (F = 20.0, P < .001) and four OGD groups (F = 155.2, P < .001), independently (F = 4.0, P < .001; n=6–13 each). There are no significant differences of the score between Control and 15°C OGD (P = .84). R, The damage score at 12 hours after 60 min OGD at three temperature groups. There are significant differences between three temperature (F = 60.7, P < .001; n=12, 16, and 6 in 15, 25, and 36°C OGD, respectively). The scores are significantly correlated with temperature during OGD. S, The damage score at 12 hours after 25°C OGD at four duration groups. There are significant differences between 4 OGD durations (F = 26.3, P < .001; n=12, 6, 6, and 17 in 0, 15, 30, and 60 min, respectively). The scores are significantly correlated with the OGD duration. P < .001 vs. other three groups by two-way ANOVA with Bonferroni adjusted comparisons. *P < .001 vs. other groups by one-way ANOVA with Bonferroni comparisons. Scale bar = 10 µm, Data are shown as mean ± SEM.
Axons in Immature White Matter are More Resistant to Insults than Mature White Matter
Newborns with CHD born at full term have a delay in brain development of approximately one month17, 18. Furthermore brain immaturity is associated with WM injury after surgery5. Based on oligodendrocyte lineage progression and myelination, it has been confirmed that WM maturation in P7 mice is equivalent to the human fetus at 35–36 weeks gestation19. We therefore investigated the effects of WM maturation on DHCA-induced axonal injury using P7 and P21 murine brain slices.
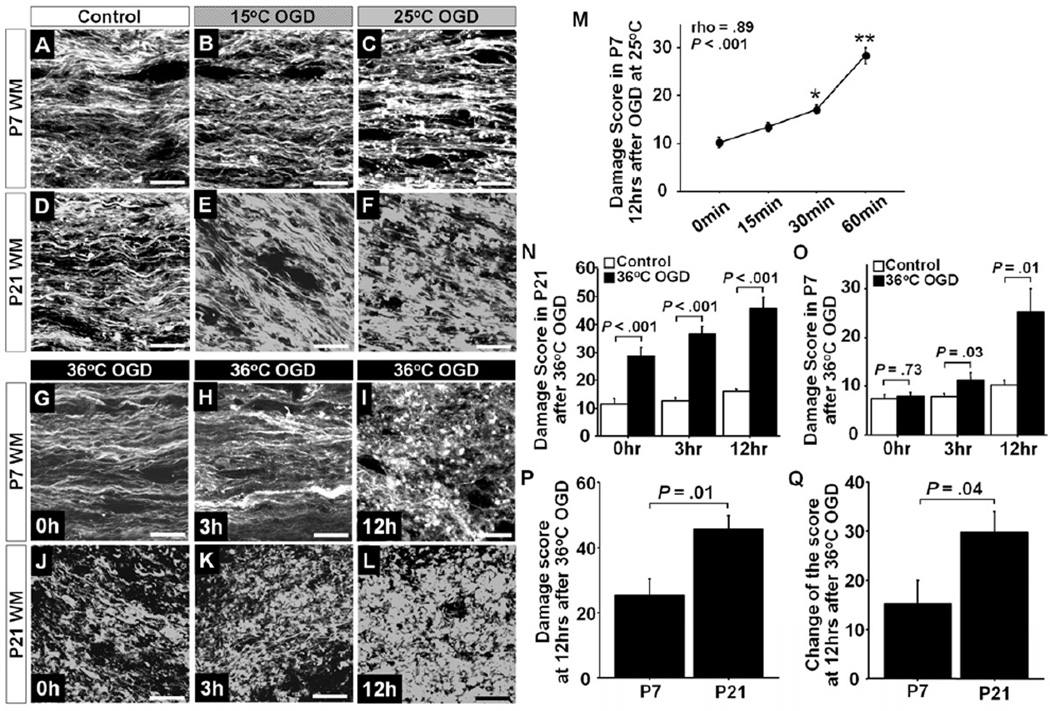
Consistent with the findings in P21-WM, significant degeneration of WM axons and an increase in damage score were observed at 12 hours after 25°C- and 36°C-OGD compared with Control, but not after 15°C-OGD (Figure 3 A–F,I,L and Table 1). Longer OGD duration at 25°C in P7 slices was significantly correlated with a higher damage score as observed in P21 slices (Figure 3M). In P7-WM, morphological degeneration due to OGD-induced insult was significantly less compared with in P21 slices for all temperature groups (Figure 3 A–L). In P21-WM, significant axonal beading and fragmentation and increase in the damage score were identified immediately after 36°C-OGD (Figure 3 G,H,N); however, these observations were not seen in P7-WM (Figure 3 J,K,O). At 12 hours after 36°C-OGD, there was extensive fragmentation in P21- WM (Figure 3 L). On the other hand significant alterations were not identified in P7-WM at 12 hours after 36°C-OGD (Figure 3 I). In this age group, beading and swelling were observed; however, the morphological structure of axons was still maintained (Figure 3 I). When the axonal damage score and the change from Control were compared between P7 and P21, a significant decrease in both parameters at 12 hours after 36°C-OGD was identified in P7-WM compared with P21 (Figure 3 P,Q). Overall these results indicate that the axons in immature WM are more resistant to ischemia-reperfusion/reoxygenation injury compared with mature WM.
Figure 3.
A–F, Morphological changes of WM axons between P7 (A–C) and P21 (D–F) at 12 hours after experiments in Control (A,D), 15°C OGD (B,E), and 25°C OGD (C,F). G–L, Morphological changes of WM axons at 0, 3, and 12 hours after 36°C OGD in P7 WM (G–I) and P21 WM (J–L). M, Axonal damage score in P7 WM at 12 hours after 25°C OGD between four OGD durations. There were significant differences between the 4 groups (F = 36.6, P < .001; n=6, 9, 9, and 11 at 0, 15, 30, and 60 minutes, respectively). The scores are significantly correlated with the OGD duration. N, Axonal damage score in P21 WM at 0, 3,12 hours after 36°C OGD and Control experiment (n=6–12 each in Control; n=6–9 each in 36°C OGD). O, Axonal damage score in P7 WM at 0, 3, 12 hours after 36°C OGD and Control experiment (n=6–12 each in Control; n=6 each in 36°C OGD). P, The axonal damage score after 36°C OGD between P7 and P21 WM (n=6 each).Q, The change in axonal damage score after 36°C OGD from Control between P7 and P21 WM (n=6 each). *P < .01 vs. 0 min, P < .0001 vs. 0, 15, and 30 min, ANOVA with Bonferroni adjusted comparisons. Scale bar = 10 µm, Data are shown as mean ± SEM.
Table 1.
Comparison of WM axonal and oligodendrocyte injury
| Control | 15°C OGD | 25°C OGD | 36°C OGD | F value | P value | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axonal damage score | ||||||||||||
| P7 | 10.2 ± 2.8 | 9.1 ± 2.4 | 28.4 ± 5.8 | 25.3 ± 11.8 | 16.8 | < .001 | .785 | < .001 | < .001 | < .001 | < .001 | .376 |
| P21 | 15.9 ± 3.6 | 12.3 ± 4.3 | 30.3 ± 5.9 | 45.7 ± 10.1 | 59.6 | < .001 | .134 | < .001 | < .001 | < .001 | < .001 | < .001 |
| Caspase3+CNP+ cell number per 106µm | ||||||||||||
| P7 | 8.6 ± 1.4 | 6.8 ± 4.0 | 8.7 ± 4.3 | 21.0 ± 6.7 | 26.5 | < .001 | .437 | .977 | < .001 | .330 | < .001 | < .001 |
| P21 | 7.3 ± 2.9 | 5.4 ± 1.7 | 9.7 ± 3.5 | 14.5 ± 4.3 | 18.3 | < .001 | .176 | .100 | < .001 | < .01 | < .001 | < .001 |
OGD, oxygen-glucose deprivation; P7, postnatal day 7; P21, postnatal day 21.
1, Control vs. 15°C OGD; 2, Control vs. 25°C OGD; 3, Control vs. 36°C OGD; 4, 15°C vs. 25°C OGD; 5, 15°C vs. 36°C OGD; 6, 25°C vs. 36°C OGD by ANOVA with Bonferroni comparisons.
Oligodendrocytes in Immature White Matter are More Vulnerable Compared with Oligodendrocytes in the Mature Brain
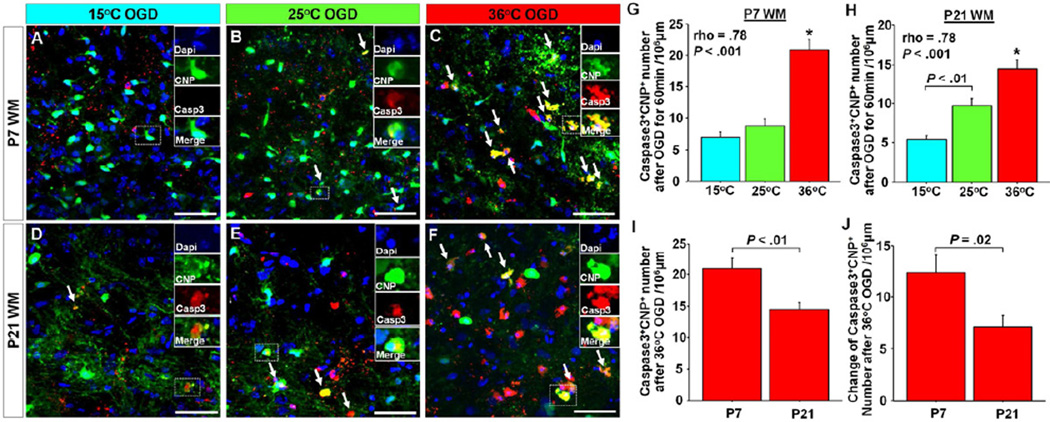
We next investigated WM oligodendrocyte injury after OGD-induced insults. Consistent with findings in WM axonal injury, there were significant positive correlations between CNP+caspase3+ cell number and temperatures during OGD in both P7- and P21-WM (Figure 4 A–H). CNP+caspase3+ cell numbers after 36°C-OGD were significantly greater compared with other groups at both P7 and P21, as was observed with WM axonal injury (Figure 4 G,H). There was a significant difference in the number of CNP+caspase3+ cells between 15°C and 25°C-OGD in P21 WM (Figure 4H); however, this was not seen in P7 slices (Figure 4G). This indicates that oligodendrocyte response to hypothermia varies according to WM maturational stage.
Figure 4.
A–F, Images of Caspase3+ oligodedrocytes in P7 and P21 WM between three OGD groups. G,H, Caspase3+CNP+ cell number in P7 and P21 WM after 60 min OGD between three temperature groups. There are significant differences among 3 groups in both P7 (F = 33.4, P < .001; n=15, 13, and 16 in 15, 25, and 36°C OGD, respectively) and P21 (F = 23.5, P < .001; n=12, 15, and 15 in 15, 25, and 36°C OGD, respectively). The numbers are significantly correlated with temperature during OGD. I, Caspase3+CNP+ cell number after 36°C OGD for 60 min between P7 and P21 WM (n=6 each). J, Changes of Caspase3+CNP+ cell number after 35°C OGD from Control between P7 and P21 WM (n=6 each). *P < .001 vs. other two groups by ANOVA with Bonferroni comparisons. Arrows indicate Caspase3+CNP+ cells. Scale bar = 50 µm, Data are shown as mean ± SEM.
To identify how maturational stages modulate oligodendrocyte injury, CNP+caspase3+ cell numbers after 36°C-OGD were compared between P7- and P21-WM. In WM axons, there was a significant decrease in the damage in P7-WM compared with in P21-WM (Figure 3 P,Q). Conversely a significant increase in the CNP+caspase3+ cell number as well as changes of the number from Control were demonstrated in P7 compared with in P21 (Figure 4 I,J), indicating that oligodendrocytes in the immature WM are more vulnerable compared with oligodendrocytes in the mature brain. These results suggest that oligodendrocytes should be the target cell population to develop novel protective strategies for immature WM.
DISCUSSION
This study is the first to describe an ex-vivo mouse model replicating DHCA under specific brain conditions including ischemia-reperfusion/reoxygenation under hypothermia. We demonstrated temperature-dependent WM injury similar to observations in large animal models of DHCA. Using two transgenic mouse lines our analysis has identified a likely cellular target, namely the oligodendrocyte, to reduce risks of WM injury in immature WM.
WM injury in neonates and infants with CHD has been the focus of several recent reports4–6, 12. Recent clinical studies suggest that brain maturation in CHD newborns is delayed approximately one month17, 18, and that immaturity increases susceptibility to WM injury after cardiac surgery5. In the present study, the effects of WM maturation stage on injury was studied using P7 and P21 mice WM, which are equivalent in the human to 35–36 weeks gestation and the 10-year-old child19. Interestingly our results demonstrate that axons in immature WM are more resistant to ischemia-reperfusion/reoxygenation injury compared with mature WM; in contrast oligodendrocytes are more susceptible in immature WM. Our previous study using a juvenile porcine model identified both WM oligodendrocyte injury and axonal damage after CPB-induced ischemia-reperfusion/reoxygenation injury15. There has been controversy whether neuro-axonal elements or oligodendrocyte injury are a primary cause of hypomyelination in WM injury24. However, we were not able to determine the primary cause of CPB-induced hypomyelination at 4 weeks after surgery in our porcine CPB model15. Our results in this study suggest that the primary cellular target to prevent CPB-induced WM injury in immature brain is oligodendrocyte lineage cells.
Oligodendrocyte lineages are the cells responsible for myelin synthesis and for axonal myelination, which increases conduction velocity up to 300 times, allowing fast and efficient communication between neurons and within neural circuits10, 13. Cellular alterations in the oligodendrocyte development include oligodendrocyte death, delayed oligodendrocyte differentiation, abnormal myelination, and hypomyelination13, 14. Clinical findings of molecular imaging such as higher average diffusivity and lower WM fractional anisotropy in CHD newborns with delayed brain maturation are associated with alterations in the oligodendrocyte development and myelination4, 10. Within different oligodendrocyte developmental stages, we already showed selective vulnerability of pre-oligodendrocytes to CPB-induced brain insults in porcine CPB model15. Thus, our understanding of cellular/molecular alterations of pre-oligodendrocytes resulted from many pathological conditions including ischemia-reperfusion, reoxygenation, hypothermia, inflammation, and anesthesia will contribute to the development of optimal management strategies and adjunctive pharmacological approaches to reduce risks of WM injury in neonates and young infants with CHD.
There is accumulating evidence that CHD patients requiring surgical correction using CPB and DHCA early in life are at significant risk of neurological deficits2, 5, 7. Causes of insults to the developing brain triggered by CPB and DHCA include: i) significant risk of ischemia-reperfusion injury resulting from air or particulate emboli, blood steal by aorto-pulmonary collateral vessels, and possible cerebral vessel abnormalities; ii) reoxygenation and hypothermia caused by DHCA or selective cerebral perfusion; and iii) systemic inflammatory response due to blood exposure to non-endothelial surfaces in the CPB system8, 16. Furthermore it has been demonstrated that brain injury after cardiac surgery is impacted by genetic abnormalities25, 26 and preoperative brain immaturity5 secondary to hypoxia in utero12, 27. Thus it is possible that CPB and DHCA management strategies that are optimal for the protection in the normally developing brain are damaging to the brain that has developed in the presence of genetic abnormalities, syndromes, and immaturities. Therefore for further and more complete refinement of cardiac surgery management it will be necessary to investigate the effects of CPB and DHCA-induced insults on the brain with genetic abnormalities or immaturity.
The results of the present study demonstrating temperature-dependent injury are similar to observations using a porcine DHCA model16, suggesting that this model is suitable for investigation of brain injury resulting from hypothermic circulatory arrest. Since the ex-vivo model uses rodents, it allows us to investigate the effect of DHCA on genetically manipulated brains and the immature brain. In the present study two lines of transgenic mice at two different ages either with or without brain immaturity were utilized to investigate the effects of ischemia-reperfusion/reoxygenation under different degrees of hypothermia. Within mechanisms of brain insults due to CPB and DHCA, we did not include effects of the inflammatory response in the present study. However, since cellular and molecular mechanisms of the brain inflammation have been investigated using a brain slice model28, we are anticipating that this model will be suitable for study of other mechanisms of brain injury related to cardiac surgery including inflammation. Our future study will investigate effects of inflammatory factors during and after OGD-induced ischemia-reperfusion and reoxygenation in this model. Rodent brain slice models have been used widely for the development of pharmacological therapies for neurological disorders29. Thus the model will also be useful for the study of optimal anesthetic strategy in cardiac surgery, the need for which has been suggested by population-based developmental testing in children following noncardiac surgery which has raised concern over optimal anesthetic strategies in children27.
The brain slice model is certainly different from CPB and DHCA, and is not suitable to investigate effects of hemodynamic changes in the cerebral circulation or blood-brain barrier disruption30, 31. Compared with brain conditions in neonates and young infants during CPB and DHCA, this ex-vivo system we described differs in extracellular volume or diffusion characteristics and constituents of extracellular brain volume. For investigation of these effects, an in-vivo model using a large animal such as the piglet is beneficial for laboratory study15. Mechanisms underlying brain insults during CPB and DHCA are complex and multietiological8, 16. In contrast to an in-vivo model, the brain slice model allows us to study each factor individually and helps in our understanding of complex and multifactorial events on the developing brain, such as our investigation of ischemia-reperfusion/reoxygenation under hypothermia in this study. Future studies using both rodent brain slice model and an in-vivo large animal model will provide novel insights of cellular and molecular mechanisms underlying neurological deficits after CPB and DHCA, and allow for the design of targeted therapies and conditions which will minimize the risk of neurodevelopmental deficits in CHD patients.
In conclusion, efforts to improve WM protection in individuals with an immature brain undergoing cardiac surgery should focus on reducing oligodendrocyte injury. Future studies using our ex-vivo slice model will provide novel insights of the possible additive effects of genetic abnormalities and brain immaturity on CPB and DHCA-induced brain insults, and allow for the design of targeted therapies to improve neurodevelopmental outcomes in patients undergoing cardiac surgery.
Supplementary Material
Acknowledgements
We thank Dr. David Zurakowski for assistance with statistical analysis. We are thankful to Dr. Jean-Marie Mangin for assistance in the preparation of the brain slice culture and to Joseph Scafidi for critical discussions on model development.
Sources of Funding: NIH R01HL060922 (R.A.J.), R01HL104173 (R.A.J.), R01NS045702 (V.G.), P30HD040677 (V.G.), P01 NS0626860 (V.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- 1.Kaltman JR, Andropoulos DB, Checchia PA, Gaynor JW, Hoffman TM, Laussen PC, Ohye RG, Pearson GD, Pigula F, Tweddell J, Wernovsky G, Del Nido P. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation. 2010;121:2766–2772. doi: 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, Rappaport LA, Wernovsky G, Jonas RA, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Jr, Li J, Smith SE, Bellinger DC, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the american heart association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 4.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 5.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD., Jr Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Barnes PD, Holmes GL, Hickey PR, Strand RD, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 8.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 9.Jonas RA, Wypij D, Roth SJ, Bellinger DC, Visconti KJ, du Plessis AJ, Goodkin H, Laussen PC, Farrell DM, Bartlett J, McGrath E, Rappaport LJ, Bacha EA, Forbess JM, del Nido PJ, Mayer JE, Jr, Newburger JW. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: Results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–1774. doi: 10.1016/j.jtcvs.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The boston circulatory arrest trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 12.McQuillen PS, Miller SP. Congenital heart disease and brain development. Ann N Y Acad Sci. 2010;1184:68–86. doi: 10.1111/j.1749-6632.2009.05116.x. [DOI] [PubMed] [Google Scholar]
- 13.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 14.Franklin RJ, Ffrench-Constant C. Remyelination in the cns: From biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi N, Scafidi J, Murata A, Korotcova L, Zurakowski D, Gallo V, Jonas RA. White matter protection in congenital heart surgery. Circulation. 2012;125:859–871. doi: 10.1161/CIRCULATIONAHA.111.048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas RA. Hypothermia, reduced flow and circulatory arrest. London: A Hodder Arnold Publication; 2004. [Google Scholar]
- 17.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig A, Ling Luo N, Beardsley DJ, Wingate-Pearse N, Walker DW, Hohimer AR, Back SA. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 20.Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of ng2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15:1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable ampa/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarran WJ, Goldberg MP. White matter axon vulnerability to ampa/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaynor JW, Nord AS, Wernovsky G, Bernbaum J, Solot CB, Burnham N, Zackai E, Heagerty PJ, Clancy RR, Nicolson SC, Jarvik GP, Gerdes M. Apolipoprotein e genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124:241–250. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnham N, Ittenbach RF, Stallings VA, Gerdes M, Zackai E, Bernbaum J, Clancy RR, Gaynor JW. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. 2010;140:144–149. doi: 10.1016/j.jtcvs.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 28.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: A crucial role of p2×7 receptor-mediated il-1beta release. J Neurochem. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 29.Pena F. Organotypic cultures as tool to test long-term effects of chemicals on the nervous system. Curr Med Chem. 2010;17:987–1001. doi: 10.2174/092986710790820679. [DOI] [PubMed] [Google Scholar]
- 30.Anttila V, Hagino I, Zurakowski D, Lidov HG, Jonas RA. Higher bypass temperature correlates with increased white cell activation in the cerebral microcirculation. J Thorac Cardiovasc Surg. 2004;127:1781–1788. doi: 10.1016/j.jtcvs.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi N, Iwata Y, Okamura T, Zurakowski D, Lidov HG, Jonas RA. Differential neuronal vulnerability varies according to specific cardiopulmonary bypass insult in a porcine survival model. J Thorac Cardiovasc Surg. 2010;140:1408–1415. doi: 10.1016/j.jtcvs.2010.03.008. e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.